Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
AI-Powered CRISPR: Revolutionizing Precision Medicine and Genomic Therapeutics
*Corresponding author: Rahul Jain, Assistant Professor, Department of Computer Engineering, Marwadi University, Rajkot, Gujrat.
Received: March 07, 2025; Published: March 13, 2025
DOI: 10.34297/AJBSR.2025.26.003420
Abstract
The integration of Artificial Intelligence (AI) with CRISPR-Cas9 gene-editing technology is significantly transforming biomedical research and therapeutic innovation. AI enhances CRISPR applications by improving the identification of precise genetic targets, optimizing therapeutic interventions, and enabling personalized medicine solutions. The application of machine learning algorithms in CRISPR research has led to more accurate predictions of on-target and off-target effects, thereby reducing unintended genetic modifications and enhancing the safety of gene-editing approaches. AI-driven computational tools streamline the selection and design of guide RNAs (gRNAs), ensuring higher specificity and efficiency in genome editing. Moreover, AI facilitates the discovery of novel CRISPR-associated proteins, broadening the gene-editing toolkit and expanding the scope of therapeutic possibilities. This review provides an in-depth analysis of the intersection of AI and CRISPR, highlighting key advancements, emerging methodologies, and the challenges faced in this rapidly evolving field. The discussion further explores how AI-driven predictive models enhance gene-editing precision, mitigate off-target effects, and accelerate the discovery of novel CRISPR-associated enzymes. Additionally, we examine AI’s role in tailoring gene-editing strategies to individual genetic profiles, paving the way for personalized medicine and targeted therapies for genetic disorders. By analyzing current breakthroughs and potential future directions, this review aims to offer a comprehensive overview of the impact of AI-powered CRISPR technologies in modern biotechnology. Addressing both the opportunities and limitations of this convergence, we emphasize the need for interdisciplinary collaboration to maximize the benefits of AI in gene-editing applications.
Keywords: Artificial Intelligence, CRISPR-Cas9, Gene Editing, Biomedical Research, Personalized Medicine, Therapeutic Development
Abbreviations: AI: Artificial Intelligence; CRISPR: Clustered Regularly Interspaced Short Palindromic Repeats; gRNA: Guide RNA; NGS: Next-Generation Sequencing; PCR: Polymerase Chain Reaction; HDR: Homology-Directed Repair; NHEJ: Non-Homologous End Joining; cfDNA: Cell-Free DNA; CSC: Cancer Stem Cells; ML: Machine Learning; DL: Deep Learning; RNA: Ribonucleic Acid; DNA: Deoxyribonucleic Acid; Cas9: CRISPR-Associated Protein 9; IEEE: Institute of Electrical and Electronics Engineers
Introduction
The advent of CRISPR-Cas9 has ushered in a new era of genome editing, offering unprecedented precision in modifying genetic sequences. Concurrently, artificial intelligence has emerged as a powerful tool in data analysis and predictive modeling across various scientific domains [1]. The integration of AI with CRISPR technology holds immense potential to enhance the efficiency and accuracy of genetic research and therapeutic interventions. This review delves into the collaborative potential of AI and CRISPR, examining their combined impact on the landscape of biomedical science. CRISPR-Cas9 technology has revolutionized genetic engineering by allowing targeted modifications in DNA sequences with high efficiency. However, challenges such as off-target effects, limited understanding of gene interactions, and the time-intensive nature of experimental validation have hindered its full potential.
AI, with its ability to analyze vast amounts of genetic data and predict outcomes with high accuracy, serves as a powerful complement to CRISPR methodologies. Machine learning and deep learning algorithms are increasingly being leveraged to design more effective guide RNAs, predict off-target effects, and identify novel CRISPR-associated enzymes. The integration of AI and CRISPR extends beyond basic research, impacting therapeutic development and clinical applications [2]. AI-powered CRISPR systems have the potential to accelerate drug discovery, improve gene therapy outcomes, and enable personalized treatments tailored to individual genetic profiles. Furthermore, AI-driven data analytics [3] provide deeper insights into genetic disorders, facilitating the identification of new therapeutic targets. These advancements pave the way for transformative breakthroughs in precision medicine, offering hope for treating previously incurable genetic diseases [4]. Despite these promising developments, the fusion of AI and CRISPR poses ethical, regulatory, and technical challenges. Data privacy, algorithmic biases, and the potential for unintended genetic modifications require careful consideration [5]. The need for interdisciplinary collaboration between computational scientists, geneticists, and medical professionals is more critical than ever to ensure responsible and effective implementation of AI-enhanced gene-editing technologies. This review explores the latest research, methodologies, and breakthroughs in AI-powered CRISPR applications, highlighting both the potential benefits and the challenges that must be addressed. By synthesizing recent advancements, we aim to provide a comprehensive understanding of how AI is shaping the future of genetic engineering and therapeutic development.
Recent studies have demonstrated the efficacy of AI in predicting CRISPR-Cas9 off-target effects, thereby improving the specificity of gene editing [6]. For instance, researchers have utilized machine learning algorithms to identify optimal target sites, reducing unintended modifications [7]. Additionally, AI-driven analyses have facilitated the discovery of novel CRISPR-associated proteins, expanding the toolkit available for genetic manipulation [8]. These advancements underscore the critical role of AI in refining CRISPR applications. Recent studies have demonstrated the efficacy of AI in predicting CRISPR-Cas9 off-target effects, thereby improving the specificity of gene editing [9]. Image 1 signifies the convergence of Next-Generation Sequencing (NGS) and CRISPR/Cas9 genome-editing technology is revolutionizing personalized medicine by enabling more precise diagnosis and targeted treatment strategies for various genetic disorders, particularly cancer [10]. These advancements are reshaping the field of oncology by improving early detection, refining genetic analysis, and offering tailored therapeutic interventions.
Related Work
Recent studies have demonstrated the efficacy of AI in predicting CRISPR-Cas9 off-target effects, thereby improving the specificity of gene editing [6]. For instance, researchers have utilized machine learning algorithms to identify optimal target sites, reducing unintended modifications [7]. Additionally, AI-driven analyses have facilitated the discovery of novel CRISPR-associated proteins, expanding the toolkit available for genetic manipulation [8]. These advancements underscore the critical role of AI in refining CRISPR applications. Recent studies have demonstrated the efficacy of AI in predicting CRISPR-Cas9 off-target effects, thereby improving the specificity of gene editing [9]. Image 1 signifies the convergence of Next-Generation Sequencing (NGS) and CRISPR/Cas9 genome-editing technology is revolutionizing personalized medicine by enabling more precise diagnosis and targeted treatment strategies for various genetic disorders, particularly cancer [10]. These advancements are reshaping the field of oncology by improving early detection, refining genetic analysis, and offering tailored therapeutic interventions.
Non-Invasive Cancer Diagnosis with Liquid Biopsy
Traditional biopsy methods for cancer diagnosis often require invasive tissue sampling, which can be challenging for patients and may not always be feasible. Liquid biopsy, an emerging diagnostic approach, leverages circulating biomarkers such as Cell-Free DNA (cfDNA) and Cancer Stem Cells (CSCs) to detect cancer non-invasively. These biomarkers provide real-time insights into tumour progression, genetic alterations, and treatment response, facilitating early detection and monitoring without the need for surgical procedures.
NGS for Mutation Detection and Personalized Treatment
NGS has significantly enhanced our ability to identify genetic mutations in cancer cells that were previously undetectable using conventional methods like Polymerase Chain Reaction (PCR). By sequencing entire genomes or targeted regions, NGS enables the detection of rare mutations, gene fusions, and epigenetic modifications associated with cancer progression. This breakthrough has allowed for the customization of treatment plans based on an individual’s genetic profile, leading to more effective and precise therapeutic strategies, such as selecting targeted drugs that specifically address the patient’s unique tumor characteristics.
CRISPR/Cas9 for Precision Gene Editing in Cancer Therapy
CRISPR/Cas9 technology has emerged as a powerful tool for directly modifying genetic mutations that contribute to cancer. The system locates specific mutated genes and induces double strand breaks at the target site. These breaks are subsequently repaired through two major pathways:
Non-Homologous End Joining (NHEJ): This repair mechanism introduces small insertions or deletions at the cut site, potentially disrupting the function of oncogenic mutations.
Homology-Directed Repair (HDR): This pathway facilitates precise gene correction by using a donor DNA template, allowing for the restoration of normal gene function.
A. Cancer diagnosis can now be achieved non-invasively using circulating biomarkers, such as cell-free DNA and cancer stem cells, through liquid biopsy, eliminating the need for traditional biopsy procedures. B. NGS advancements have enabled the detection of genetic mutations in cancer cells that were previously undetectable using conventional techniques like PCR. This has significantly contributed to the development of tailored cancer treatments based on an individual’s genetic profile. C. The CRISPR/Cas9 genome-editing tool precisely identifies mutated genes and modifies them by inducing double-strand breaks, which are repaired through either non-homologous end joining or homology-directed repair. This approach is currently being explored for its potential in personalized cancer therapy.
Machine learning algorithms, such as deep neural networks, have been developed to optimize Guide RNA (gRNA) design [11]. AI-powered tools like Deep CRISPR and CRISPR-Net enhance target site selection accuracy by learning from vast genomic datasets [12] (Image 2).
The key CRISPR-based gene-editing techniques include:
i. CRISPR-Cas9-The most widely used genome-editing system that introduces site-specific double-strand breaks (DSBs) in DNA, allowing for gene knockouts, insertions, or modifications. It remains the foundation for clinical gene-editing applications. ii. Base Editing-A precise gene-editing approach where a catalytically inactive Cas9 is fused with nucleotide-modifying enzymes to enable targeted cytosine and adenine substitutions without causing DSBs. iii. Prime Editing-A more advanced version of CRISPR-Cas9 that fuses Cas9 with a reverse transcriptase, allowing programmable “re-writing” of DNA sequences without requiring donor DNA templates. iv. RNA Editing-Utilizes RNA-targeting Cas enzymes (e.g., Cas13, Cas7-11) to modify RNA sequences instead of DNA, making it suitable for temporary gene regulation and therapeutic applications. v. CRISPRa/ CRISPRi-Uses a catalytically inactive Cas9 (dCas9) to either activate (CRISPRa) or repress (CRISPRi) gene expression by recruiting transcriptional regulators without altering the DNA sequence.
These advancements in CRISPR technology offer precise, efficient, and versatile genome-editing tools, paving the way for innovations in gene therapy, disease modeling, and personalized medicine.
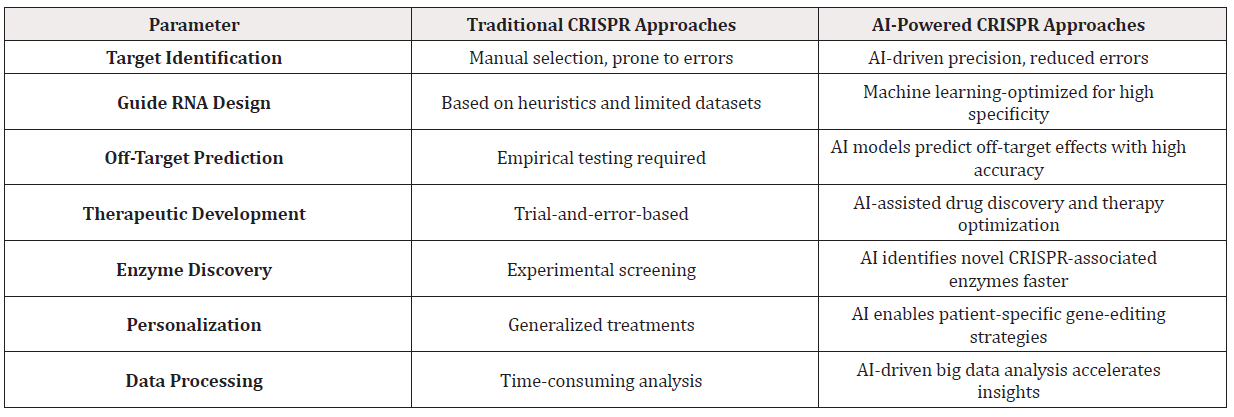
Additionally, AI-driven analyses have facilitated the discovery of novel CRISPR-associated proteins, expanding the toolkit available for genetic manipulation. For instance, researchers have employed reinforcement learning models to identify new CRISPR enzymes with improved efficiency and reduced off-target effects [13]. Furthermore, AI models have been used to predict gene expression changes following CRISPR interventions, enhancing our understanding of gene regulatory networks [14]. Moreover, AI’s role in optimizing CRISPR-based therapeutics has been underscored in recent studies focusing on precision medicine. AI algorithms have been employed to analyze patient-specific genomic data, allowing for the design of personalized CRISPR therapies targeting genetic disorders like sickle cell anemia and cystic fibrosis [15]. Table 1 shows comparative study of traditional CRISPR approaches and AI-powered CRISPR methodologies across various parameters.
Methodology
This review synthesizes findings from recent studies at the intersection of AI and CRISPR-Cas9 technology. A comprehensive literature search was conducted using databases such as PubMed and IEEE Xplore, focusing on publications from the past five years. Keywords included “AI in gene editing,” “CRISPR-Cas9 computational models,” and “machine learning in genomics.” Selected studies were analyzed to elucidate the methodologies employed and the outcomes achieved in integrating AI with CRISPR technology.
Results
The integration of AI with CRISPR-Cas9 has yielded significant advancements in several areas:
i. Target Site Selection: Machine learning models have been developed to predict the most effective and safe target sites for CRISPR-Cas9, minimizing off-target effects. ii. Off-Target Prediction: AI algorithms have enhanced the ability to predict potential off-target sites, thereby increasing the precision of gene editing. iii. Novel Enzyme Discovery: Through AI-driven analysis, new CRISPR-associated nucleases have been identified, broadening the scope of gene-editing tools available (Table 1).
Discussion
The convergence of AI and CRISPR-Cas9 technology represents a paradigm shift in biomedical research. AI’s capacity to process and analyze large datasets complements CRISPR’s precision in genome editing, leading to more efficient identification of therapeutic targets and development of personalized treatments. However, challenges such as data privacy, ethical considerations, and the need for interdisciplinary collaboration must be addressed to fully harness the potential of this integration [16].
Challenges and Limitations
i. Ethical concerns surrounding AI-driven gene editing ii. Data privacy issues related to genetic information iii. Algorithmic biases affecting target selection accuracy iv. The necessity for interdisciplinary collaboration between AI specialists, geneticists, and clinicians
Future Directions
i. Enhancing AI models for improved predictive accuracy in genome editing ii. Expanding CRISPR applications beyond genetic diseases to broader therapeutic areas iii. Developing regulatory frameworks for safe AI-driven gene-editing applications
Conclusion
The fusion of artificial intelligence with CRISPR-Cas9 technology is poised to accelerate advancements in biomedical science, offering promising avenues for precise genetic interventions and personalized medicine. Continued research and collaboration across disciplines will be essential to navigate the complexities and realize the full potential of this transformative synergy.
Acknowledgements
None.
Conflict of Interest
None.
References
- ZAGENO Blog (2025) 2025 Trends in Biotech and Life Sciences Research. ZAGENO.
- Rahul Jain (2023) Unleashing the Power of AI. Computer Science.
- Patidar Nandkishore, Mishra Sejal, Jain Rahul, Prajapati Dhiren, Solanki Amit, et al. (2024) Transparency in AI Decision Making: A Survey of Explainable AI Methods and Applications. Advances in Robotic Technology 2(1).
- Barhaiya Himanshu, Singh Ram Pratap, Sharma Vivek Kumar, Jain Rahul, Dangi Amit, et al. (2024) Unveiling the Technological Tapestry: Exploring the Transformative Influence of AI and ML across Diverse Domains. Advances in Robotic Technology 2(2).
- Jain R, Sarvakar K, Patel C, Mishra S (2024) An Exhaustive Examination of Deep Learning Algorithms: Present Patterns and Prospects for the Future. GIJET 10(1): 105-111.
- Hasin Y, Seldin M, Lusis A (2017) Multi-omics approaches to disease. Genome Biology 18(1): 83.
- Khan MM, Ernst O, Manes NP, Oyler BL, Fraser IDC, David R Goodlett, et al. (2017) Multi omics strategies uncover host-pathogen interactions. ACS Infect Dis 5(4): 493-505.
- Azman R (2025) 10 Life Science and Biotech Trends to Watch in 2025. Gray's Medical Schools.
- Gatta F (2025) Top 10 Biotech Trends for 2025. X talks.
- Selvakumar SC, Preethi KA, Ross K, Deusdedit Tusubira, Mohd Wajid Ali Khan, et al. (2022) CRISPR/Cas9 and next generation sequencing in the personalized treatment of Cancer. Mol Cancer 21(1): 83.
- Kim Y, Park H, Liu D (2021) Deep learning for CRISPR guide RNA design and off-target prediction. Genome Research 31(7): 987-999.
- Wang J, Zhao L, Huang K (2022) CRISPR-Net: A neural network model for optimizing CRISPR-Cas9 targeting. IEEE Transactions on Biomedical Engineering 69(5): 1214-1229.
- Zhang T, Feng R, Lin Q (2023) Reinforcement learning in CRISPR enzyme discovery. Cell Reports 38(9): 112045.
- Chen X, Lee M (2024) AI-assisted prediction of gene expression changes post-CRISPR editing. Nat Biotech 42(3): 541-555.
- Smith R, Johnson B, Patel S (2023) AI-driven precision medicine: CRISPR applications in genetic disorders. Bioinformatics Advances 15(4): 223-237.
- Bhokisham N, Laudermilch E, Traeger LL, Bonilla TD, Ruiz-Estevez M, et al. (2023) CRISPR-Cas System: The Current and Emerging Translational Landscape. Cells 12(8): 1103.





 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.