Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Clinical Management of COVID-19: Pharmacotherapeutic Options
*Corresponding author: Dr. Jun Ren, Department of Cardiology, Zhongshan Hospital Fudan University, Shanghai 200032 China and National Clinical Research Center for Interventional Medicine, Shanghai, 200032, China.
Received: February 19, 2025; Published: February 25, 2025
DOI: 10.34297/AJBSR.2025.26.003391
Abstract
First reported and identified in Wuhan, China, the SARS-CoV-2 infection immediately spread throughout the world, claiming more than 580 million infections and over 6.42 million deaths. Given the severity of this pandemic, scientists and physicians are strived to develop antiviral strategies to combat this disease. Vaccines, such as inactivated vaccines, nucleic acid-based vaccines, and vector vaccines, have already entered clinical trials. Although vaccines serve as a vital public health tool to lower the COVID-19 burden, management of this disease will still heavily depend on pharmacotherapy. Several specific FDA-approved therapeutics have already been repurposed for COVID-19 treatment, including antiviral drugs, immunotherapy, and traditional Chinese medicine. This review will systemically discuss on the efficacy of these medications drugs comprising clinical trials, combination therapies, other treatment strategy and future drug development. Currently, the efficacy and safety of COVID-19 drugs must be closely monitored, as their long-term efficacy can only be recognized after several years of clinical trials.
Introduction
SARS-CoV-2, classified as a beta coronavirus, is an enveloped, sizable virus with a single-stranded RNA genome, exhibiting genomic plasticity owing to its high mutation rate, genetic diversity, and adaptability to multiple hosts [1]. It is estimated that the main route of transmission of SARS-CoV-2 is infection by respiratory droplets via aerosol contact, Contaminated surfaces are also a route of infection because all three structural proteins (E, M and S) play a vital role in the replication cycle of SARS-CoV-2, with infection initiated when the spiked glycoprotein binds to human angiotensin- converting enzyme 2 [2].
Featuring variable clinical severity, the clinical image of SARSCoV- 2 infection ranges from asymptomatic to acute respiratory distress syndrome (ARDS), even death [3]. Propelled by clinical evidence, dysregulated and excessive proinflammatory cytokines result in ARDS, which is common in severe cases in health deterioration [4].
For therapeutics and management of SARS-CoV-2 infection, three vaccines are used in the clinics including two mRNA vaccines and the Janssen viral vector vaccine. In the meantime, pharmacotherapy constitutes a significant component to alleviate disease burden. Therefore, this review is aimed to offer some insights to wards the pharmacotherapy being evaluated, repurposed or newly developed in the management of COVID-19.
The Role of Immunotherapy in the Treatment of COVID-19
Convalescent Plasma
Case reports show that the use of convalescent plasma is useful and effective in the treatment of COVID-19 [48]. In the case of convalescent plasma therapy, plasma collected from a convalescent patient is transfused to a symptomatic patient [49]. This type of convalescent plasma provides passive immunity in actively infected patients [50]. It was reported that convalescent plasma may execute a novel role in the inhibition of progression to noninvasive or high-flow oxygen, invasive mechanical ventilation or ECMO, ultimately mortality [51].
Compared with other therapeutics for COVID-19, convalescent plasma was well tolerated, making it a potential therapy without serious transfusion-related adverse effects [52]. Moreover, convalescent plasma administered with favipiravir improved clinical outcomes and patients were typically discharged two weeks [53].
Although convalescent plasma may provide clinical benefits in the treatment of COVID-19, the efficacy of recovery plasma for all patients has not been demonstrated, resulting in largely inconclusive consensus [54]. Furthermore, due to varying donor titer amount and dosing, standardization is warranted to arouse great practical awareness [55]. Since convalescent plasma is often used in combination with other therapies, it is hard to attribute efficacy solely to convalescent plasma [56].
Monoclonal Antibodies in Monotherapy
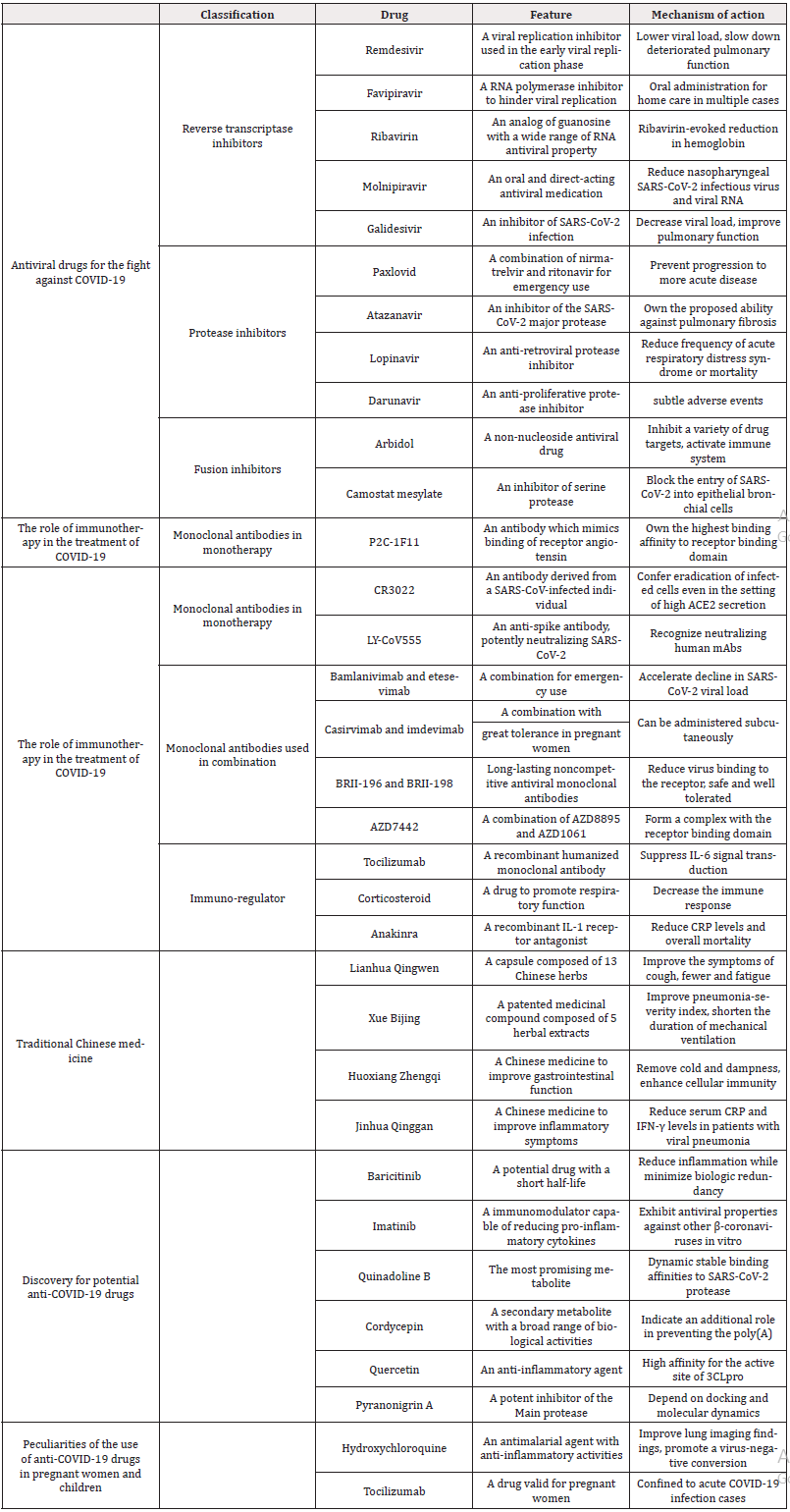
Ample evidence has suggested the utility of antibody neutralization of SARS-CoV-2 for effective therapeutics and vaccine development. Many monoclonal antibodies concluded in Table 1, were isolated from SARS-CoV-2-infected individuals, with a number of promising antibodies. According to a corresponding enzyme study, P2C-1F11, P2B-2F6 and P2C-1A3 were the most potent live SARSCoV-2 inhibitors [53]. Among them, P2C-1F11, an antibody which mimics binding of receptor angiotensin converting enzyme 2 in cellular models, is highlighted [57] and displays potent neutralizing activity in vitro to confer protection against SARS-CoV-2 infection [58]. Meanwhile, it occupies the largest binding surface, demonstrating the highest binding affinity to receptor binding domain. Distinct from other antibodies, P2C-1F11 possesses unique ability to trigger immediate and robust shedding of S1 from cell-surface expressed spike glycoprotein in cellular models [59].
To investigate the potential immunoprotective and immunopathological role of antibodies, the CR3022 antibody derived from a human infected with SARS-CoV has attracted considerable attention [60]. CR3022, which targets a conserved epitope in the receptor binding domain, was evaluated for its functional potential as a SARS-responsive antibody to investigate the role of Fc activity in immunity against SARS-CoV-2 [61]. This allowed us to secure the broadly active effector function of the antibody for critical immune clearance [62]. Moreover, CR3022 has the potential to ensure eradication of infected cells even in the presence of high ACE2 secretion [63]. Although the ability of antibodies to target infected cells through Fc interaction is crucial for virus elimination, antibody competence is affected by different functional profiles of Fc, suggesting mechanisms of antibody action that may not be acceptable in the development of antiviral antibody therapeutics [64].
LY-CoV555 is derived from a convalescent COVID-19 patient to treat other patients and acts as an anti-spirate antibody that potently neutralizes SARS-CoV-2 [65]. It is able to protect the upper and lower respiratory tract of non-human primates against SARS-CoV-2 infection, reinforcing the promise of LY-CoV555 in the clinical assessment for the treatment and prevention of COVID-19 [66]. Courtesy of its identification and characterization, LY-CoV555 has the ability to rapidly recognize neutralizing human mAbs [67]. Therefore, it could play a role in the early stages of addressing an evolving pandemic, supplementing widespread vaccination efforts by offering immediate passive immunity and safeguarding vulnerable populations [68].
Monoclonal Antibodies Used in Combination
The recent boost in monoclonal antibodies revealed the benefit of combination therapy, in addition to monotherapy. The following drugs have been summarized in Table 1. For example, combination of bamlanivimab and etesevimab is indicated for emergency use because the combination not only resulted in a reduced incidence of COVID-19-related hospitalizations and deaths, but also accelerated the decline in SARS-CoV-2 viral load [69]. Bamlanivimab and etesevimab should be administered immediately after a positive SARSCoV-2 test and within 10 days of the onset of COVID-19 symptoms [70]. It is recommended that patients be treated in a center with staff and equipment for the treatment of anaphylaxis [71]. It is also recommended to monitor patients for hypersensitivity reactions during drug administration for at least 1 hour after infusion [72].
Casirvimab and imdevimab, administered intravenously or subcutaneously, are approved for the combination treatment of mild-to-moderate COVID-19 in individuals over 12 years of age and weighing no more than 40 kg [10]. If intravenous infusion is not available or there is a delay in treatment, this combination may be administered subcutaneously [73]. Conversely, casirvimab and imdevimab may worsen outcomes if administered to COVID-19 patients who are hospitalised or require high flow oxygen or mechanical ventilation [13]. Although anaphylaxis reactions are very rare, they have occasionally been reported with this combination. Moreover, casirvimab and imdevimab have shown great tolerance in pregnant women [74].
BRII-196 and BRII-198 are non-competitive, long-acting monoclonal antibodies against SARS-CoV-2 that reduce the binding of the virus to the receptor [75]. This combination has been shown to be safe and well tolerated, with a significant reduction in the risk of hospitalization and death among adults with mild to moderate COVID-19 and at high risk of progression to severe disease [76]. Meanwhile, adverse events are rarely observed among patients on BRII-196 and BRII-198.
AZD7442 is a combination of two monoclonal antibodies, the long-acting AZD8895 (zigagevimab) and AZD1061 (zilgavimab), which bind simultaneously to different non-translocated epitopes [77]. Both have potent neutralising activity against SARS-CoV-2 and antigenic variants, forming a complex with receptor binding domains. AZD7442 did not improve SARS-CoV-2 post-exposure prophylaxis [78]. However, in pre-exposure prophylaxis, AZD7442 plays a significant role in reducing the risk of developing symptomatic COVID-19, maintaining for up to 12 months [79].
Immuno-Regulator
Clinical benefits were observed with different immuno-regulators, among which the following drugs concluded in table 1 carry the most prominent efficacy.
Tocilizumab is a recombinant humanized monoclonal antibody bound to IL-6 receptor to suppress IL-6 signal transduction [80]. It is clinically used for the treatment of certain forms of juvenile arthritis because it can result in a significant reduction of invasive mechanical ventilation and death [81]. Although tocilizumab is not effective in preventing intubation or death in moderately ill hospitalised patients with COVID-19, patients treated with tocilizumab had fewer serious infection compared with other drugs [82]. Comparable effects were observed with intravenous and subcutaneous administration of tocilizumab.
The ability of corticosteroid to decrease the immune response offers advantage in COVID-19 therapy. Although the effect of corticosteroid varied significantly according to respiratory support, patients treated with corticosteroid exhibited a distinct recovery of respiratory function [83]. Furthermore, a lower clinical outcome score, a trend towards decreased mortality, shorter hospital stay and a reduced need for respiratory support were observed in patients after treatment [84]. When corticosteroid is used in combination with tocilizumab, an additive effect was noted, contributing to mortality reduction by one-third up to 50% depending on the the severity of oxygen support [85].
Since SARS-CoV-2 induces a pro-inflammatory syndrome, the recombinant IL-1 receptor antagonist anakinra exhibited benefit in reducing CRP levels and overall mortality [86]. Moreover, anakinra treatment was accompanied with improvements in respiratory function and a rapid reduction of serum CRP level. These results suggest that anakinra may be beneficial in patients with very high serum CRP levels [87]. It is noteworthy that anakinra should be administered early, as its use in patients with mechanical ventilation has not been effective [88].
Traditional Chinese Medicine
As an ancient system of alternative medicine, traditional Chinese medicine plays an active role in the prevention of COVID-19 and the following drugs are summarized in Table 1. It improves the clinical symptoms of patients, effectively relieving the operating pressure on the national medical system during critical conditions [89].
Composed of 13 Chinese herbs, Lianhua Qingwen capsules are able to improve the symptoms of cough, fewer and fatigue, leading to the shorter median time to symptom recovery [90]. Besides, it is reported that Lianhua Qingwen could not only affect the viral morphology in vivo, but also suppress the replication of SARS-CoV-2 [91]. Therefore, Lianhua Qingwen prevented mild to moderate cases of COVID-19 from deteriorating into severe forms [92]. Meanwhile, it reliably delivers the desired efficacy in the treatment of COVID-19 due to its ability to clear the lungs, clear away heat, and have functionality in treating viral infection and inflammatory response [93].
Developed and marketed for SARS treatment in China, Xue Bijing injection is a patented Chinese medicinal compound composed of 5 herbal extracts, including Saffron, Paeonia lactiflora, Rhizoma Ligustici Chuanxiong, Salvia miltiorrhiza Angelica Sinensis [94]. Combination of Xuebijing injection and a classical anti-infective drug is proved to improve their pneumonia-severity index, shortening the duration of mechanical ventilation and length of ICU stay reduce the mortality in patients with severe pneumonia and [95].
Based on dispelling cold and dampness, Huoxiang Zhengqi enhances cellular immunity and improves gastrointestinal function, composed of Rhizoma Atractylodis Macrocephalae, Pericarpium Citri Reticulatae, Ginger, Angelica Dahurica and Poria [96]. It is recommended that Huoxiang Zhengqi can be given to COVID-19 patients in case of fatigue and diarrhea under medical supervision [97]. Furthermore, it is used to relieve abdominal distention and pain, vomiting and diarrhea caused by exogenous wind-cold and endogenous moisture stagnation [98].
Mainly composed of Golden Honeysuckle, Gypsum, Scutellaria Baicalensis and Forsythia, Jinhua Qinggang granules can reduce serum CRP and IFN-γlevels, improve inflammatory symptoms and regulate immunity in patients with viral pneumonia [99]. Therefore, due to its thermoremediation and detoxification functions, it may not only improve patients’ general clinical symptoms but also alleviate their psychological distress. It is reported that Jinhua Qinggang granules have good efficacy and clinical safety in the windheat invasion syndrome of influenza lung invasion [100]. Additionally, it effectively shortens the detection time of nucleic acids and promotes the absorption of inflammatory exudate in pneumonia without obvious side effects [101].
Discovery for Potential anti-COVID-19 Drugs
To fight against COVID-19, it is pertinent to identify potential therapies with antiviral and/or anti-inflammatory properties.
With a short half-life, Baricitinib reduces inflammation while minimizing biologic redundancy with antiviral and less immunosuppressive properties [102]. Despite concerns for immunosuppression and secondary infections, addition of baricitinib was not associated with an overtly higher incidence of adverse or thromboembolic events [103]. Moreover, among patients receiving high oxygen flow or non-invasive ventilation, baricitinib in combination with remdesivir is more effective than remdesivir alone in terms of recovery time and clinical improvement in patients with COVID-19 [104].
In vitro investigations, has antiviral properties against other β-coronaviruses in vitro and inhibits the early stages of the viral life cycle [105]. Unfortunately, owing to conflicting results from preclinical studies, the exact efficacy of imatinib against SARS-CoV-2 viability remains controversial [106]. Additionally, according to preclinical models, it is suggested that Imatinib serves as a potential immunomodulator that can reduce pro-inflammatory cytokines and vascular adhesion molecules [107]. However, the molecular mechanisms are not completely understood and warrants further elucidation. Meanwhile, observed in some inflammatory conditions, imatinib is associated with the prevention of pulmonary endothelial barrier dysfunction, leading to a reduction in pulmonary capillary leakage [108].
Reviewing the history, fungal metabolites have served as a boon for the mankind from antibiotics to food preservatives [109]. Recently, as antiviral agents in fighting against COVID-19, the potentials of fungal metabolites are explored, and the success is promising.
Screening of fungal metabolites with antiviral activity has shown that quinadoline B is the most promising metabolite with dynamic stable binding to SARS-CoV-2 protease, RNA-directed RNA polymerase, non-structural protein 15 and S-protein [110]. Moreover, quinadoline B possesses an outstanding pharmacokinetic profile such as oral bioavailability, high drug likeness and lack of toxicity [111].
Cordycepin is a secondary metabolite with a wide range of biological effects, including antiviral activity. Molecular interaction modeling has shown its high affinity for binding to both major protease and spike protein binding sites [112]. Furthermore, this molecule indicates an additional role in preventing the poly(A) predictions also place great emphasis on the significant potential of cordyceps in various biological pathways linked to viral infections and point to it as a promising drug candidate for the treatment of COVID-19 [114].
Quercetin is another prospective candidate for drug development which is important for SARS-CoV-2 [113]. Pharmacology network against COVID-19. It certified high affinity not only for the active site of 3CLpro, but also for the potential targets involved in viral inhibition [115]. Used for clinical applications, Quercetin offers significant advantages in drug development due to its pharmacokinetic and ADMET properties [116]. Attention has been drawn to the potential impact of quercetin as an anti-inflammatory agent to protect patients from severe inflammation caused by SARS-CoV-2 infection [117].
It is proposed that pyranonigrin A could interact with the Main protease, which is one of the significant target proteins of SARSCoV- 2 [118]. Identified as a potent inhibitor of the Main protease, pyranonigrin A depends on docking and molecular dynamics to block the function of the Main protease. Therefore, it served as a promising drug candidate against COVID-19 [119].
The above-mentioned candidate metabolites cover a wide range of antiviral activities and are concluded in table 1, encouraging continuous endeavor to explore the potential of this chemical library in drug research programs [120].
Peculiarities of the use of anti-COVID-19 Drugs in Pregnant Women and Children
Since some drugs have been empirically used in pregnant women and children, obstetricians should consider whether the same therapies used in the general population are appropriate for pregnant women or children with serious illnesses, according to their safety profile [121].
The use of tocilizumab is confined to acute COVID-19 infection cases in which the patients experienced cytokine storm leading to multiorgan failure [122]. Fortunately, those pregnant women started or maintained the medications due to clinical necessity, delivered healthy newborns [122]. Moreover, direct association cannot be established between the use of tocilizumab and maternal and fetal complications [123]. Therefore, it is recommended that tocilizumab could be maintained along the pregnancy if its benefits exceed the potential risks [124].
Currently, inadequate evidence is readily available for the recommended routine use of monoclonal antibody medications in children with COVID-19, even for those at higher risk of disease severity or hospitalization [125]. At this time, neither bamlanivimab nor casirivimab combined with imdevimab should be considered as the standard care in any pediatric population, even in those meeting high-risk criteria [126].
The above peculiarities have been summarized in Table 1. It is worth noting that the significance of implementing recommended prevention strategies should be emphasized in order to reduce risk for severe acute respiratory syndrome and inform clinical care for pregnant women and children [127].
Conclusion
New evidence gradually emerges from ongoing clinical trials testing COVID-19 drugs and guidelines will be updated periodically. This review summarizes the efficacy and safety on repurposing medications towards treatment for SARS-CoV-2. Based on their mode of action, COVID-19 medications are grouped into antiviral drugs composed of different inhibitors, immunotherapy especially convalescent plasma, traditional Chinese medicine and novel anti-COVID-19 drugs with promises for SARS-CoV-2. Also, the peculiarities of anti-COVID-19 drugs in pregnant women and children are discussed. These findings should help to broaden the therapeutic arsenal against COVID-19, although they should still be interpreted with caution.
Many local and international research centers are strived to test potential drugs for COVID-19 disease in diverse stages of clinical research. With more intricate in vitro and in vivo examinations, these candidate drugs might become rational therapeutics against SARS-CoV-2. The effectiveness and safety of existing COVID-19 therapeutics must be closely monitored, recognizing that the longterm effects of drugs can only be evaluated through several years of clinical practice.
Acknowledgements
(Yiran E. Li and Yuqi Luo contributed equally to this work).
References
- Jackson CB, Farzan M, Chen B, Choe H (2022) Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol 23(1): 3-20.
- V'Kovski P, Kratzel A, Steiner S, Stalder H, Thiel V (2021) Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol 19(3): 155-170.
- Ramadori GP (2022) SARS-CoV-2-Infection (COVID-19): Clinical Course, Viral Acute Respiratory Distress Syndrome (ARDS) and Cause(s) of Death. Med Sci (Basel) 10(4): 58.
- Zawilska JB, Lagodzinski A, Berezinska M (2021) COVID-19: from the structure and replication cycle of SARS-CoV-2 to its disease symptoms and treatment. J Physiol Pharmacol 72(4).
- Lin HXJ, Cho S, Meyyur Aravamudan V, Sanda HY, Palraj R, et al. (2019) Remdesivir in Coronavirus Disease 2019 (COVID-19) treatment: a review of evidence. Infection 49(3): 401-410.
- Agostini ML, Andres EL, Sims AC, Graham RL, et al. (2018) Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease mBio 9(2): e00221-18.
- Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, et al. (2017) Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med 9(396): eaal3653.
- Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, et al. (2020) Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809): 465-469.
- Ghasemnejad-Berenji M, Pashapour S (2021) Favipiravir and COVID-19: A Simplified Summary. Drug Res (Stuttg) 71(3): 166-170.
- Suzuki M, Imai T, Sakurai A, Komoto S, Ide T, et al. (2021) Virological and genomic analysis of SARS-CoV-2 from a favipiravir clinical trial cohort. J Infect Chemother 27(9): 1350-1556.
- Ashour NA, Abo Elmaaty A, Sarhan AA, Elkaeed EB, Moussa AM, et al. (2022) A Systematic Review of the Global Intervention for SARS-CoV-2 Combating: From Drugs Repurposing to Molnupiravir Approval. Drug Des Devel Ther. 16: 685-715.
- Korula P, Alexander H, John JS, Kirubakaran R, Singh B, et al. (2024) Favipiravir for treating COVID-19. Cochrane Database Syst Rev 2(2): Cd015219.
- Fischer W, Eron JJ, Holman W, Cohen MS, Fang L, et al. (2021) Molnupiravir, an Oral Antiviral Treatment for COVID-19. medRxiv.
- Zahraa Talib K, Shihab MS, Hamah-Ameen B (2021) Drugs that Might Be Possibly Used for Treatment of COVID-19 Patients. Russ J Bioorg Chem 47(4): 789-804.
- Agrawal U, Raju R, Udwadia ZF (2020) Favipiravir: A new and emerging antiviral option in COVID-19. Med J Armed Forces India 76(4): 370-376.
- Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, et al. (2013) Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res 100(2): 446-454.
- Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, et al. (2012) VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res 22(3): 568-576.
- Warren TK, Wells J, Panchal RG, Stuthman KS, Garza NL, et al. (2014) Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature 508(7496): 402-405.
- Shiraki K, Daikoku T (2020) Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol Ther 209: 107512.
- Pandey A, Nikam AN, Shreya AB, Mutalik SP, Gopalan D, et al. (2020) Potential therapeutic targets for combating SARS-CoV-2: Drug repurposing, clinical trials and recent advancements. Life Sci 256: 117883.
- Saravolatz LD, Depcinski S, Sharma M (2023) Molnupiravir and Nirmatrelvir-Ritonavir: Oral Coronavirus Disease 2019 Antiviral Drugs. Clin Infect Dis 76(1): 165-171.
- Julander JG, Demarest JF, Taylor R, Gowen BB, Walling DM, et al. (2021) An update on the progress of galidesivir (BCX4430), a broad-spectrum antiviral. Antiviral Res 195: 105180.
- Celik I, Erol M, Duzgun Z (2022) In silico evaluation of potential inhibitory activity of remdesivir, favipiravir, ribavirin and galidesivir active forms on SARS-CoV-2 RNA polymerase. Mol Divers 26(1): 279-292.
- Jean SS, Lee PI, Hsueh PR (2020) Treatment options for COVID-19: The reality and challenges. J Microbiol Immunol Infect 53(3): 436-443.
- Wang Z, Yang L (2022) In the age of Omicron variant: Paxlovid raises new hopes of COVID-19 recovery. J Med Virol 94(5): 1766-1767.
- Lange NW, Salerno DM, Jennings DL, Choe J, Hedvat J, et al. (2022) Nirmatrelvir/ritonavir use: Managing clinically significant drug-drug interactions with transplant immunosuppressants. Am J Transplant 22(7): 1925-1926.
- Amani B, Amani B (2023) Efficacy and safety of nirmatrelvir/ritonavir (Paxlovid) for COVID-19: A rapid review and meta-analysis. J Med Virol 95(2): e28441.
- Islam T, Hasan M, Rahman MS, Islam MR (2022) Comparative evaluation of authorized drugs for treating Covid-19 patients. Health Sci Rep 5(4): e671.
- Mazaherpour H, Sofian M, Farahani E, Abdi A, Mazaherpour S, et al. (2022) Comparing Outcomes of Two Antiviral Therapy Combinations among COVID-19 Patients. Biomed Res Int 2022: 1522426.
- Hoetelmans RM, Meenhorst PL, Mulder JW, Burger DM, Koks CH, et al. (1997) Clinical pharmacology of HIV protease inhibitors: focus on saquinavir, indinavir, and ritonavir. Pharm World Sci 19(4): 159-175.
- Wu C, Liu Y, Yang Y, Zhang P, Zhong W, et al. (2020) Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B 10(5): 766-788.
- Chu CM, Cheng VC, Hung IF, Wong MM, Chan KH, et al. (2004) Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax 59(3): 252-256.
- Ledford H (2022) Hundreds of COVID trials could provide a deluge of new drugs. Nature 603(7899): 25-27.
- Fintelman-Rodrigues N, Sacramento CQ, Ribeiro Lima C, Souza da Silva F, Ferreira AC, et al. (2020) Atazanavir, Alone or in Combination with Ritonavir, Inhibits SARS-CoV-2 Replication and Proinflammatory Cytokine Production. Antimicrob Agents Chemother 64(10): e00825-2.
- Huang C, Wang Y, Li X, Ren L, Zhao J, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223): 497-506.
- Agarwal S, Agarwal SK (2021) Lopinavir-Ritonavir in SARS-CoV-2 Infection and Drug-Drug Interactions with Cardioactive Medications. Cardiovasc Drugs Ther 35(3): 427-440.
- Wang D, Hu B, Hu C, Zhu F, Liu X, et al. (2020) Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. Jama 323(11): 1061-1069.
- Kim UJ, Won EJ, Kee SJ, Jung SI, Jang HC (2016) Combination therapy with lopinavir/ritonavir, ribavirin and interferon-α for Middle East respiratory syndrome. Antivir Ther 21(5): 455-459.
- Chavda VP, Gajjar N, Shah N, Dave DJ (2021) Darunavir ethanolate: Repurposing an anti-HIV drug in COVID-19 treatment. Eur J Med Chem Rep 3: 100013.
- Chen J, Xia L, Liu L, Xu Q, Ling Y, et al. (2020) Antiviral Activity and Safety of Darunavir/Cobicistat for the Treatment of COVID-19. Open Forum Infect Dis 7(7): ofaa241.
- Chen Y, Liu Q, Guo D (2020) Emerging coronaviruses: Genome structure, replication, and pathogenesis. J Med Virol 92(4): 418-423.
- Zhu Z, Lu Z, Xu T, Chen C, Yang G, et al. (2020) Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J Infect 81(1): e21-e3.
- Lu H (2020) Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci Trends 14(1): 69-71.
- Wang D, Li Z, Liu Y (2020) An overview of the safety, clinical application and antiviral research of the COVID-19 therapeutics. J Infect Public Health 13(10): 1405-1414.
- Zou L, Ruan F, Huang M, Liang L, Huang H, et al. (2020) SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med 382(12): 1177-1179.
- Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, et al. (2020) First Case of 2019 Novel Coronavirus in the United States. N Engl J Med 382(10): 929-936.
- Deeks ED (2018) Darunavir/Cobicistat/Emtricitabine/Tenofovir Alafenamide: A Review in HIV-1 Infection. Drugs 78(10): 1013-1024.
- Barone P, DeSimone RA (2020) Convalescent plasma to treat coronavirus disease 2019 (COVID-19): considerations for clinical trial design. Transfusion 60(6): 1123-1127.
- Casadevall A, Pirofski LA (2020) The convalescent sera option for containing COVID-19. J Clin Invest 130(4): 1545-1548.
- Salazar E, Perez KK, Ashraf M, Chen J, Castillo B, et al. (2020) Treatment of Coronavirus Disease 2019 (COVID-19) Patients with Convalescent Plasma. Am J Pathol 190(8): 1680-1690.
- Heustess AM, Allard MA, Thompson DK, Fasinu PS (2021) Clinical Management of COVID-19: A Review of Pharmacological Treatment Options. Pharmaceuticals (Basel) 14(6): 520.
- Libster R, Pérez Marc G, Wappner D, Coviello S, Bianchi A, et al. (2021) Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. N Engl J Med 384(7): 610-618.
- Joyner MJ, Carter RE, Senefeld JW, Klassen SA, Mills JR, et al. (2021) Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N Engl J Med 384(11): 1015-1027.
- Katz LM (2021) (A Little) Clarity on Convalescent Plasma for Covid-19. N Engl J Med 384(7): 666-668.
- Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, et al. (2021) REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N Engl J Med 384(3): 238-251.
- Avendaño-Solá C, Ramos-Martínez A, Muñez-Rubio E, Ruiz-Antorán B, Malo de Molina R, et al. (2021) A multicenter randomized open-label clinical trial for convalescent plasma in patients hospitalized with COVID-19 pneumonia. J Clin Invest 131(20): e152740.
- Salazar E, Christensen PA, Graviss EA, Nguyen DT, Castillo B, et al. (2020) Treatment of Coronavirus Disease 2019 Patients with Convalescent Plasma Reveals a Signal of Significantly Decreased Mortality. Am J Pathol 190(11): 2290-2303.
- Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, et al. (2021) A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. N Engl J Med 384(7): 619-629.
- Ge J, Wang R, Ju B, Zhang Q, Sun J, et al. (2021) Antibody neutralization of SARS-CoV-2 through ACE2 receptor mimicry. Nat Commun 12(1): 250.
- Ju B, Zhang Q, Ge J, Wang R, Sun J, et al. (2020) Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 584(7819): 115-119.
- Jones BE, Brown-Augsburger PL, Corbett KS, Westendorf K, Davies J, et al. (2020) LY-CoV555, a rapidly isolated potent neutralizing antibody, provides protection in a non-human primate model of SARS-CoV-2 infection. bioRxiv.
- Atyeo C, Slein MD, Fischinger S, Burke J, Schäfer A, et al. (2021) Dissecting strategies to tune the therapeutic potential of SARS-CoV-2-specific monoclonal antibody CR3022. JCI Insight 6(1):e143129.
- Shariatifar H, Farasat A (2023) Affinity enhancement of CR3022 binding to RBD; in silico site directed mutagenesis using molecular dynamics simulation approaches. J Biomol Struct Dyn 41(1): 81-90.
- Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, et al. (2010) Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 329(5993): 856-861.
- Jiang L, Wang N, Zuo T, Shi X, Poon KM, et al. (2014) Potent neutralization of MERS-CoV by human neutralizing monoclonal antibodies to the viral spike glycoprotein. Sci Transl Med 6(234): 234ra59.
- Zhang L, Zhang F, Yu W, He T, Yu J, et al. (2006) Antibody responses against SARS coronavirus are correlated with disease outcome of infected individuals. J Med Virol 78(1): 1-8.
- Wang N, Shi X, Jiang L, Zhang S, Wang D, et al. (2013) Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res 23(8): 986-993.
- Cohen SX, Ben Jelloul M, Long F, Vagin A, Knipscheer P, et al. (2008) ARP/wARP and molecular replacement: the next generation. Acta Crystallogr D Biol Crystallogr 64(Pt 1): 49-60.
- Abd Elsalam S, Salama M, Soliman S, Naguib AM, Ibrahim IS, et al. (2021) RETRACTED: Remdesivir Efficacy in COVID-19 Treatment: A Randomized Controlled Trial. Am J Trop Med Hyg 106(3): 886-890.
- Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, et al. (2020) Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med 383(19): 1813-1826.
- Bavaro DF, Diella L, Solimando AG, Cicco S, Buonamico E, et al. (2022) Bamlanivimab and Etesevimab administered in an outpatient setting for SARS-CoV-2 infection. Pathog Glob Health 116(5): 297-304.
- Garcia Vidal C, Alonso R, Camon AM, Cardozo C, Albiach L, et al. (2021) Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother 76(12): 3296-3302.
- Solaymani Dodaran M, Ghanei M, Bagheri M, Qazvini A, Vahedi E, et al. (2021) Safety and efficacy of Favipiravir in moderate to severe SARS-CoV-2 pneumonia. Int Immunopharmacol 95: 107522.
- (2022) Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 399(10325): 665-676.
- Singh AK, Singh A, Singh R, Misra A (2021) Molnupiravir in COVID-19: A systematic review of literature. Diabetes Metab Syndr 15(6): 102329.
- (2022) Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial. Lancet Infect Dis22(5): 622-635.
- García Lledó A, Gómez Pavón J, González Del Castillo J, Hernández Sampelayo T, Martín Delgado MC, et al. (2022) Pharmacological treatment of COVID-19: an opinion paper. Rev Esp Quimioter 35(2): 115-130.
- Dong J, Zost SJ, Greaney AJ, Starr TN, Dingens AS, et al. (2021) Genetic and structural basis for SARS-CoV-2 variant neutralization by a two-antibody cocktail. Nat Microbiol 6(10): 1233-1244.
- Levin MJ, Ustianowski A, De Wit S, Launay O, Avila M, et al. (2022) Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for Prevention of Covid-19. N Engl J Med 386(23): 2188-2200.
- Mayer C, VanHise K, Caskey R, Naqvi M, Burwick RM, et al. (2021) Monoclonal Antibodies Casirivimab and Imdevimab in Pregnancy for Coronavirus Disease 2019 (COVID-19). Obstet Gynecol 138(6): 937-939.
- Fang X, Mei Q, Yang T, Li L, Wang Y, et al. (2020) Low-dose corticosteroid therapy does not delay viral clearance in patients with COVID-19. J Infect 81(1): 147-178.
- Chen RC, Tang XP, Tan SY, Liang BL, Wan ZY, et al. (2006) Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest 129(6): 1441-1452.
- Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. (2021) Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med 384(8): 693-704.
- Guo T, Fan Y, Chen M, Wu X, Zhang L, et al. (2020) Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 5(7): 811-818.
- Perez Moreiras JV, Gomez Reino JJ, Maneiro JR, Perez Pampin E, Romo Lopez A, et al. (2018) Efficacy of Tocilizumab in Patients With Moderate-to-Severe Corticosteroid-Resistant Graves Orbitopathy: A Randomized Clinical Trial. Am J Ophthalmol 195: 181-190.
- Brüssow H (2021) Host-modifying drugs against COVID-19: some successes, but not yet the breakthrough. Environ Microbiol 23(12): 7257-7270.
- Boglione L, Rostagno R, Poletti F, Moglia R, Bianchi B, et al. (2021) The proper use of corticosteroids for 2019-nCov pneumonia: Towards promising results? J Infect 82(1): e6-e7.
- Stone JH, Frigault MJ, Serling Boyd NJ, Fernandes AD, Harvey L, et al. (2020) Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N Engl J Med 383(24): 2333-2344.
- Dastan F, Nadji SA, Saffaei A, Marjani M, Moniri A, et al. (2020) Subcutaneous administration of interferon beta-1a for COVID-19: A non-controlled prospective trial. Int Immunopharmacol 85: 106688.
- Kharazmi AB, Moradi O, Haghighi M, Kouchek M, Manafi Rasi A, et al. (2022) A randomized controlled clinical trial on efficacy and safety of anakinra in patients with severe COVID-19. Immun Inflamm Dis 10(2): 201-208.
- Gasmi A, Tippairote T, Mujawdiya PK, Menzel A, Lysiuk R, et al. (2024) Traditional Chinese Medicine as the Preventive and Therapeutic Remedy for COVID-19. Curr Med Chem 31(21): 3118-3131.
- Harrison C (2020) Coronavirus puts drug repurposing on the fast track. Nat Biotechnol 38(4): 379-381.
- Gibert CL (2016) Treatment Guidelines for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents: An Update. Fed Pract 33(Suppl 3): 31s-36s.
- Huang J, Tao G, Liu J, Cai J, Huang Z, et al. (2020) Current Prevention of COVID-19: Natural Products and Herbal Medicine. Front Pharmacol 11: 588508.
- Liang W, Guan W, Chen R, Wang W, Li J, et al. (2020) Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 21(3): 335-337.
- Wang W, Xu Y, Gao R, Lu R, Han K, et al. (2020) Detection of SARS-CoV-2 in Different Types of Clinical Specimens. Jama 323(18): 1843-1844.
- Yongchen Z, Shen H, Wang X, Shi X, Li Y, et al. (2020) Different longitudinal patterns of nucleic acid and serology testing results based on disease severity of COVID-19 patients. Emerg Microbes Infect 9(1): 833-836.
- Xiao M, Tian J, Zhou Y, Xu X, Min X, et al. (2020) Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: A randomized controlled trial. Pharmacol Res 161: 105126.
- Tong T, Wu YQ, Ni WJ, Shen AZ, Liu Set al. (2020) The potential insights of Traditional Chinese Medicine on treatment of COVID-19. Chin Med 15: 51.
- Ren W, Liang P, Ma Y, Sun Q, Pu Q, et al. (2021) Research progress of traditional Chinese medicine against COVID-19. Biomed Pharmacother 137: 111310.
- Runfeng L, Yunlong H, Jicheng H, Weiqi P, Qinhai M, et al. (2020) Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol Res 156: 104761.
- Zhuang Z, Zhong X, Zhang H, Chen H, Huang B, et al. (2021) Exploring the Potential Mechanism of Shufeng Jiedu Capsule for Treating COVID-19 by Comprehensive Network Pharmacological Approaches and Molecular Docking Validation. Comb Chem High Throughput Screen 24(9): 1377-1394.
- Sutton D, Fuchs K, D Alton M, Goffman D (2020) Universal Screening for SARS-CoV-2 in Women Admitted for Delivery. N Engl J Med 382(22): 2163-2164.
- Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, et al. (2020) COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis 20(4): 400-402.
- Li Z, Peng M, Chen P, Liu C, Hu A, et al. (2022) Imatinib and methazolamide ameliorate COVID-19-induced metabolic complications via elevating ACE2 enzymatic activity and inhibiting viral entry. Cell Metab 34(3): 424-440.e7.
- Kalil AC (2020) Treating COVID-19-Off-Label Drug Use, Compassionate Use, and Randomized Clinical Trials During Pandemics. Jama 323(19): 1897-1898.
- Cantini F, Niccoli L, Matarrese D, Nicastri E, Stobbione P, et al. (2020) Baricitinib therapy in COVID-19: A pilot study on safety and clinical impact. J Infect 81(2): 318-356.
- Mei M, Tan X (2021) Current Strategies of Antiviral Drug Discovery for COVID-19. Front Mol Biosci 8: 671263.
- Han Y, Duan X, Yang L, Nilsson Payant BE, Wang P, et al. (2021) Identification of SARS-CoV-2 inhibitors using lung and colonic organoids. Nature 589(7841): 270-275.
- Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, et al. (2021) Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med 384(9): 795-807.
- Bernal Bello D, Morales Ortega A, Isabel Farfán Sedano A, de Tena JG, Martín López JVS, et al. (2021) Imatinib in COVID-19: hope and caution. Lancet Respir Med 9(9): 938-939.
- Gomes IS, Santana CA, Marcolino LS, Lima LHF, Melo Minardi RC, Dias RS, et al. (2022) Computational prediction of potential inhibitors for SARS-COV-2 main protease based on machine learning, docking, MM-PBSA calculations, and metadynamics. PLoS One 17(4): e0267471.
- Takahashi JA, Barbosa BVR, Lima M, Cardoso PG, Contigli C, (2021) Antiviral fungal metabolites and some insights into their contribution to the current COVID-19 pandemic. Bioorg Med Chem 46: 116366.
- Rangsinth P, Sillapachaiyaporn C, Nilkhet S, Tencomnao T, Ung AT, (2021) Mushroom-derived bioactive compounds potentially serve as the inhibitors of SARS-CoV-2 main protease: An in silico approach. J Tradit Complement Med 11(2): 158-172.
- Cheema HA, Sohail A, Fatima A, Shahid A, Shahzil M, et al. (2023) Quercetin for the treatment of COVID-19 patients: A systematic review and meta-analysis. Rev Med Virol 33(2): e2427.
- Rao P, Shukla A, Parmar P, Rawal RM, Patel BV, et al. (2022) Proposing a fungal metabolite-flaviolin as a potential inhibitor of 3CL (pro) of novel coronavirus SARS-CoV-2 identified using docking and molecular dynamics. J Biomol Struct Dyn 40(1): 348-360.
- Rao P, Shukla A, Parmar P, Rawal RM, Patel B, et al. (2020) Reckoning a fungal metabolite, Pyranonigrin A as a potential Main protease (M(pro)) inhibitor of novel SARS-CoV-2 virus identified using docking and molecular dynamics simulation. Biophys Chem 264: 106425.
- Tsuji M (2020) Potential anti-SARS-CoV-2 drug candidates identified through virtual screening of the ChEMBL database for compounds that target the main coronavirus protease. FEBS Open Bio 10(6): 995-1004.
- Tu YF, Chien CS, Yarmishyn AA, Lin YY, Luo YH, et al. (2020) A Review of SARS-CoV-2 and the Ongoing Clinical Trials. Int J Mol Sci 21(7): 2657.
- Rajagopal K, Varakumar P, Baliwada A, Byran G (2020) Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): an in silico approach. Futur J Pharm Sci 6(1): 104.
- Gogoi N, Chowdhury P, Goswami AK, Das A, Chetia D, et al. (2021) Computational guided identification of a citrus flavonoid as potential inhibitor of SARS-CoV-2 main protease. Mol Divers 25(3): 1745-1759.
- Martínez Sánchez N, De la Calle Fernández Miranda M, Bartha JL (2021) Safety profile of treatments administered in COVID 19 infection in pregnant women. Clin Invest Ginecol Obstet 48(3): 100663.
- Adhikari EH, Moreno W, Zofkie AC, MacDonald L, McIntire DD, et al. (2020) Pregnancy Outcomes Among Women With and Without Severe Acute Respiratory Syndrome Coronavirus 2 Infection. JAMA Netw Open 3(11): e2029256.
- Zambrano LD, Ellington S, Strid P, Galang RR, Oduyebo T, et al. (2020) Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep 69(44):1641-1647.
- Jering KS, Claggett BL, Cunningham JW, Rosenthal N, Vardeny O, et al. (2021) Clinical Characteristics and Outcomes of Hospitalized Women Giving Birth With and Without COVID-19. JAMA Intern Med 181(5): 714-717.
- Datta SD, Talwar A, Lee JT (2020) A Proposed Framework and Timeline of the Spectrum of Disease Due to SARS-CoV-2 Infection: Illness Beyond Acute Infection and Public Health Implications. Jama 324(22): 2251-2252.
- Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, et al. (2020) Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. Bmj 370: m3320.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.