Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Studying The Effects of Microvascular Transplantation on Tissue in A Limb Ischemia Model
*Corresponding author: Yanbo Qi, MNUMS, School of Medicine, Department of Surgery in Ulaanbaatar, the capital of Mongolia.
Received: March 24, 2025; Published: March 28, 2025
DOI: 10.34297/AJBSR.2025.26.003448
Abstract
Vascular occlusion-induced ischemia can occur in any part of the body, causing oxygen deprivation and microvascular dysfunction, which restricts blood flow to muscles, tissues, and organs. The severity of ischemia depends on whether the vessel is partially or completely occluded, with symptoms varying based on the degree of blood flow restriction.
Objectives: To study the effects of microvascular grafting surgery on tissue in a limb ischemia model.
Methods: Forty male Wistar rats (Rattus norvegicus, body weight: 200-300g, age: 8weeks) were obtained from the Biomedical Institute and Experimental Animal Center of the National Institute of Medical Sciences. The rats were randomly divided into two groups: Control Group (n=10): Underwent direct end-to-end vascular grafting of a 0.3-0.5mm diameter artery in the right femur. Experimental Group (n=30): Underwent end-to-end microvascular grafting using donor vessels preserved for 3 days and 7 days.
Results: In the limb ischemia model, in the end-to-end anastomosis of allogeneic vascular transplantation, the tissue cell ischemia- reperfusion injury recovery ability of the vascular anastomosis preserved for 3 days was better than that of the vascular anastomosis preserved for 7 days.
Conclusion: In the limb ischemia model, microvascular transplantation surgery showed different degrees of tissue edema, uneven cell distribution, tissue necrosis and other phenomena, which were more obvious in the 7-day ischemia group. When selecting vascular transplantation anastomosis, through our comparative study, it is best to choose blood vessels with a shelf life of less than 3 days.
Introduction
Microscopic vascular grafting represents a significant advancement in modern surgical technology, with microvascular anastomosis under a microscope forming its foundation [1].
Vascular occlusion-induced ischemia can occur in any part of the body, causing oxygen deprivation [2] and microvascular dysfunction, which restricts blood flow to muscles, tissues, and organs. The severity of ischemia depends on whether the vessel is partially or completely occluded, with symptoms varying based on the degree of blood flow restriction [3]. Peripheral arterial disease (PAD) is prevalent among individuals over the age of 50 and often presents with subtle or asymptomatic signs [4]. In 2015, over 236 million people worldwide were estimated to have PAD, marking a 23.5% increase since 2000. This rise is attributed to an aging global population and an increased prevalence of risk factors such as diabetes mellitus (DM) [5]. PAD is characterized by a sudden decrease in blood supply to a specific limb region, presenting with symptoms such as pain, pallor, loss of palpable pulses, cold extremities, and paralysis. These symptoms can lead to severe complications, including acute multi-organ failure, hyperkalemia, and metabolic acidosis [6-7]. The annual risk of limb amputation due to PAD is approximately 10-15%, or 1.5 per 10,000 people, with a 30-day mortality rate ranging from 15-25% [8].
In recent years, innovative treatments such as microscopic vascular grafting, artificial vascular replacement, and endovascular surgery have been introduced to address ischemia caused by vascular occlusion. However, despite these advancements, amputation rates remain high, and studies examining the outcomes of microvascular grafting surgery and its effects on tissue regeneration remain limited. This study aims to address this research gap by evaluating the outcomes of microvascular grafting surgery in a controlled limb ischemia model.
Material and Methods
Animal Model and Grouping
Forty male Wistar rats (Rattus norvegicus, body weight: 200- 300g, age: 8weeks) were obtained from the Biomedical Institute and Experimental Animal Center of the National Institute of Medical Sciences. The rats were randomly divided into two groups:
i. Control Group (n=10): Underwent direct end-to-end vascular grafting of a 0.6-0.8mm diameter artery in the right femur.
ii. Experimental Group (n=30): Underwent end-to-end microvascular grafting using donor vessels preserved for 3 days and 7 days.
All procedures adhered to the following standards:
i. MNS 6871:2020: General hygiene requirements for laboratory animal housing
ii. MNS 6872:2020: General methods for restraining laboratory animals
iii. MNS 6873:2020: General methods for blood collection from laboratory animals
iv. MNS 6874:2020: General methods for administering drugs to laboratory animals.
Study Design
Body weight, arterial caliber, vascular grafting time, and free blood flow were analyzed before surgery and on days 3, 7, and 21 post-surgeries. Both groups underwent the same vascular grafting procedure, with differences limited to the suture techniques and vessel preservation duration. In the experimental group, the femoral artery was ligated to induce ischemia. The control group underwent artery exposure without ligation. Research conditions, including the surgical environment, equipment, and rat diet (food and water), were consistent across all groups.
Anesthesia and Preoperative Preparation
i. Body weight was measured for each rat.
ii. Anesthesia was administered via intraperitoneal injection of 0.5ml/100g pentobarbital sodium (500mg) +sodium chloride (0.9%,50ml solution).
Surgical Procedure: Isolation of the Femoral Artery: A longitudinal incision was made along the anterior right thigh, extending from the greater trochanter to the lateral femoral condyle. Subcutaneous tissue was carefully dissected to expose the femoral neurovascular bundle. 2% lidocaine was applied to prevent vasoconstriction [9]. The femoral artery’s diameter was measured using a digital caliper. Using fine forceps, the connective tissue surrounding the vessels was gently separated vertically, carefully isolating the arteries, veins, and nerves to prevent nerve damage.
Rat Femoral Artery Ligation and Microvascular Grafting Method
In the experimental group, the femoral artery of each rat was carefully isolated, and a 9-0 suture was used to create a model of blood supply deficiency, maintained for 3 and 7 days, respectively. In the control group, the femoral artery was also isolated, but no ligation was performed. Following ligation in the experimental group, the vessel and surrounding soft tissues were returned to their normal anatomical positions and the wound was carefully sutured to ensure proper closure.
During the microvascular grafting procedure, the two ends of the artery were isolated with minimal vascular tension to prevent unnecessary stress on the vessel. Blood flow status was then assessed by washing the damaged area along the vascular resection margin. Active blood flow at the proximal end of the artery was confirmed, as any lack of free bleeding indicated proximal blockage requiring a secondary incision. In cases of excessive bleeding, a vascular clamp was applied to control blood flow. To verify arterial reflux, the distal vascular clamp was temporarily opened. If a thrombus was detected at the distal site, it was carefully treated before proceeding with the anastomosis to ensure unobstructed blood flow. Next, the outer membrane of the vessel was delicately removed, and the ends of the artery were adjusted to align accurately, avoiding twisting or narrowing. During anastomosis, the inner membrane of one vessel end was precisely connected to the inner membrane of the other, and the muscle layers were aligned appropriately to maintain vascular integrity. Fine vascular forceps were used to clamp the damaged vessel ends while the outer membrane was trimmed carefully to prevent thrombosis during suturing. Throughout this process, care was taken to avoid damaging the vessel’s inner wall with surgical scissors, ensuring a smooth and secure anastomosis.
Irrigation and Assessment of Graft Status
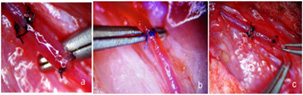
After both ends of the graft were evenly cut, the vessel lumen was irrigated with a 0.1% heparin saline solution to prevent thrombosis during the grafting procedure. Alternatively, 0.5% procaine or 3.8% sodium citrate solution could also be used for irrigation to ensure optimal blood flow and reduce the risk of clot formation within the vessel. Following the completion of the end-to-end vascular grafting procedure in the experimental rat model, the Acland test was performed to assess the patency and functionality of the graft (Figure 1). This test evaluated blood flow through the grafted vessel, ensuring the anastomosis was secure and unobstructed [10- 11].
In the figure A and C are the graft segments, and B is the end-toend graft of the vessel.
Donor Vessel Preservation and Experimental Assessments
The femoral arteries of experimental rats were carefully dissected under a microscope according to their anatomical structure, with each segment measuring 1-2 cm in length. The vascular cavities were thoroughly washed with 0.1% heparin saline solution, with alternatives including 0.5% procaine or 3.8% sodium citrate solution, to remove residual blood and soft tissue. The cleaned arteries were then preserved in 50 ml of Custodiol Cardioplegia solution at 4°C under both static and dynamic conditions for 3 and 7 days, depending on the ischemic group requirements.
Experimental Results
Weight Analysis in the Control Group
The body weight of mice in the control group was compared before surgery and 21 days post-vascular anastomosis. The average weight before surgery was 230.09 g, with a standard deviation of 22.16, and a 95% confidence interval ranging from 214.24 to 245.95 g. By 21 days post-surgery, the average weight increased to 240.24 g, with a standard deviation of 25.77 and a confidence interval ranging from 221.81 to 258.67 g. The paired T-test revealed a statistically significant difference (t = -5.8419, P = 0.0002).
Analysis of Body Weight in the Ischemic Group on the 3rd Day of the Experiment: The body weights of the mice in the ischemic group were compared between the 3rd day of the experiment and 21 days after vascular anastomosis. Before surgery, the average body weight was 240.49 g, with a standard deviation of 25.25 and a 95% confidence interval ranging from 231.06 to 249.91 g. By the 21st day post-surgery, the average body weight increased to 256.73 g, with a standard deviation of 24.27 and a 95% confidence interval ranging from 247.67 to 265.79 g. A paired T-test revealed a statistically significant difference between the preoperative and postoperative weights (t = -11.2249, P = 0.0000).
Analysis of the body weight of the ischemic group on day 7 of the experiment: The weight of the mice in the ischemic group on day 7 of the experiment was compared before surgery and 21 days after vascular anastomosis. The average body weight before surgery was 253.86g, the standard deviation was 25.26, the lower limit of the confidence interval was 244.43, and the upper limit was 263.30. The body weight 21 days after surgery was 264.24g, the standard deviation was 24.11, the lower limit of the confidence interval was 255.24, and the upper limit was 273.25. The T test compared the body weight before surgery and 21 days after surgery (-13.9642), which was statistically significant (P= 0.0000).
By comparing the weights of mice in the control group and the experimental group, there was no weight gain after 3 days and 7 days of ischemia (the weight of the 7-day ischemia group was significantly reduced). After vascular anastomosis, the weight of both groups increased significantly, but the weight gain after 7 days of ischemia was not as obvious as that after 3 days of ischemia, this result suggests a recovery trend and an improvement in the overall health condition of the animals after surgical intervention and the increase in the control group was particularly obvious.
Comparison Of Total Vascular Anastomosis Time Between The Two Groups
The time required for vascular anastomosis was compared between the control group and the experimental groups (3 day and 7 day ischemia). The results demonstrated that increased ischemia duration was directly correlated with a longer anastomosis time. In the control group, the average anastomosis time was 24.51±2.29minutes, with a maximum time of 28.15 minutes. In contrast, the 3-day ischemia group had an average anastomosis time of 47.16±8.36 minutes, with a maximum time of 61.05 minutes. The 7 day ischemia group exhibited the longest average anastomosis time at 60.04±15.21minutes, with a maximum time of 85.15 minutes. These findings align with previous research by Jiri Dostal [12], Pavel Klein, and colleagues, who reported vascular anastomosis times ranging from 22 to 70 minutes, with an average of 46 minutes. The extended anastomosis times observed in our experimental groups can be attributed to anatomical changes in the stored vascular tissue and the impact of prolonged ischemia on vessel integrity.
Comparison Of Vascular Anastomosis Time Between Two Groups
The average vascular anastomosis time and efficiency were compared between the control group, 3-day ischemia group, and 7-day ischemia group. In the control group, the average vascular anastomosis time was 10.48 ± 1.23 minutes, with a maximum time of 12.39 minutes. This result closely aligns with findings from Rui Sergio Monteiro de Barros [13], Rafael Aquino Leal, and colleagues, who reported an average anastomosis time of 11.97 minutes for interrupted suture techniques. In contrast, the 3-day ischemia group had a significantly prolonged anastomosis time of 37.07±8.31 minutes, with a maximum of 50.3 minutes. The 7-day ischemia group required even longer, with an average time of 47.99±13.76 minutes and a maximum of 70.4 minutes.
Comparison Of Blood Loss During Vascular Anastomosis
Blood loss during vascular anastomosis was also evaluated across the groups. In the control group, the average blood loss was 0.27±0.12ml. In comparison, the 3-day ischemia group exhibited significantly higher blood loss, with an average of 1.19±0.69ml and a maximum of 2.55ml. The 7-day ischemia group experienced the highest blood loss, averaging 2.57±1.14 ml, with a maximum recorded loss of 5.15 ml.
Comparison Of Tissue Analysis of Rats from Two Study Groups: (as shown in Figures 2-5)
(Figure 2) In the figure A is a tissue analysis of thigh muscles from control rats, muscle tissue, connective tissue, muscle fiber, muscle bundles and cell tissue are clearly defined, and the shape is normal. B is a tissue analysis of thigh muscles from 3 days of ischemia rats, some connective tissue, muscle fibers, muscle bundles and cell tissue are disordered, some have been broken. C is a tissue analysis of thigh muscles from 7 days of ischemia rats,most of the connective tissue, muscle fibers, muscle bundles and cell tissue are disordered, and the break is particularly obvious.
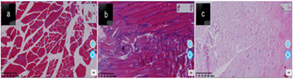
Figure 2: Assessment of the effect of emergency radiation therapy on patients, an effect on overall survival (OS).
(Figure 3) In the figure A is 3 days donor graft 21 days femoral tissue analysis, Most of the connective tissue, muscle fibers, muscle bundles and cell tissue boundaries become clear, and the broken parts gradually return to normal. b is 7 days donor graft 21 days femoral tissue analysis, Most of the connective tissue, muscle fibers, muscle bundles and cell tissue are not particularly clean, and most of the broken tissue is not yet healed. C is a tissue analysis of thigh muscles from control rats, muscle tissue, connective tissue, muscle fiber, muscle bundles and cell tissue are clearly defined, and the shape is normal.
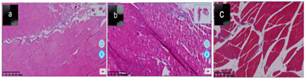
Figure 3: Image of Ischemia 3 days group rat femoral tissue analysis after 21 days of vascular end-to-end grafting.
(Figure 4) In the figure A is 7 days donor graft 21 days femoral tissue analysis, Most of the connective tissue, muscle fibers, muscle bundles and cell tissue boundaries become clear, and the broken parts gradually return to normal, not as good as ischemia 3 days 3 days donor group. b is 7 days donor graft 21 days femoral tissue analysis, Most of the connective tissue, muscle fibers, muscle bundles and cell tissue are not particularly clean, and most of the broken tissue is not yet healed. C is a tissue analysis of thigh muscles from control rats, muscle tissue, connective tissue, muscle fiber, muscle bundles and cell tissue are clearly defined, and the shape is normal.
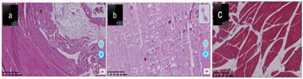
Figure 4: Image of Ischemia 7 days group rat femoral tissue analysis after 21 days of vascular end-to-end grafting.
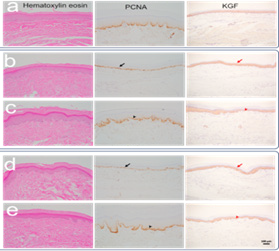
(Figure 5) In the figure A is a control group. b is Ischemia 3 days group. c is Ischemia 3 days group 21 days of vascular end-to-end grafting. d is Ischemia 7 days group. e is Ischemia 7 days group 21 days of vascular end-to-end grafting.The skin tissue of the paws was stained with hematoxylin and eosin to evaluate the morphological structure. At 3/7 days of ischemia, the squamous cell layer decreased and the epithelium became thinner. At 21 days after vascular grafting, skin regeneration was activated. Epithelial thickening was observed due to squamous epithelial hyperplasia. To confirm the above changes, immunohistochemical analysis of PCNA protein revealed a significant decrease in the number of PCNA-positive cells in the squamous epithelium at 3/7 days of ischemia (black arrows). The longer the duration of ischemia, or at 7 days of ischemia, the more the number of regenerating cells decreased. At 21 days after vascular grafting, the number of PCNA-positive cells increased, indicating that the regeneration of the paw skin was intensified (black arrows). PCNA-positive cells increased, but the staining intensity was weaker than that of the control group. KGF, a protein that promotes skin regeneration, was similarly expressed in the squamous epithelium of the paws of control rats, at 3/7 days of ischemia, and at 21 days after vascular grafting (red arrows). This suggests that KGF is secreted by squamous cells to promote regeneration during hypoxia.
Conclusion
In the limb ischemia model, microvascular transplantation surgery resulted in tissue edema, uneven cell distribution, and tissue necrosis, which were more obvious in the 7-day ischemia group. At 3 and 7 days of ischemia, the skin tissue of the thigh and paw showed thinning of connective tissue due to ischemia. In the limb ischemia model, in the end-to-end anastomosis of allogeneic vascular transplantation, the tissue cell recovery ability of the vascular anastomosis preserved for 3 days was better than that of the vascular anastomosis preserved for 7 days. Therefore, when selecting the anastomosis for vascular transplantation, through our comparative study, it is best to choose blood vessels with a storage period of less than 3 days.
Discussion
Ischemia is the deprivation of oxygen and metabolites necessary for cellular metabolism caused by restricted blood supply to a tissue. Although restoration of blood flow is essential to prevent irreversible tissue and cellular damage, reperfusion can exacerbate tissue damage caused by ischemia and cause multi-organ dysfunction, a process known as “ischemia-reperfusion.” [14] Limb tissue is relatively resistant to ischemia compared to other tissues, and acute limb ischemia/reperfusion caused by various mechanisms is an important cause of mortality and morbidity, which is associated with oxidative stress [15].
Ischemia-reperfusion injury (IRI) is a phenomenon that occurs as a result of the re-oxygenation of tissues that have previously been exposed to anoxic conditions. The restoration of blood flow triggers a cascade of biochemical processes, including the generation of reactive oxygen species, the activation of cytokines, and changes in capillary permeability, which exacerbate the cellular dysfunctions that occurred during the ischemic process, and which are most commonly seen in limb muscle tissue [16]. Acute limb ischemia is one of the most common peripheral vascular events and is associated with significant morbidity, mortality, and costs. Despite the availability of surgical and pharmacological revascularization options, the level of treatment to reduce postoperative complications has not significantly improved, with mortality rates exceeding 25% and a major amputation risk of 20% [17].
Comparative study of postoperative complications between the two groups: In the anastomosis of our two groups, different degrees of anastomotic vascular stenosis occurred after the operation. That is, in the control group, 10 rats had end-to-end femoral artery anastomosis, and1 cases of anastomotic vascular stenosis occurred, while in the 3-day ischemia group, 4 cases occurred among 15 cases, and 6 cases occurred among 15 cases after 7-day ischemia, accounting for 10%, 26.67%, and 40%, respectively. This is related to the mechanism of ischemia-reperfusion injury and changes in the tissue structure of the storage vessels. In the 15 cases of endto- end anastomosis of allogeneic vascular transplantation in the 3-day ischemia group, the postoperative blood flow patency rate and success rate reached 100%. During the anastomosis process, one case of transplanted blood vessel failed to anastomose for the first time, and another transplanted blood vessel was replaced and re-anastomosed to achieve satisfactory results. It can be inferred that its effective success rate is 93.33%, which is similar to the research results of scholars Chiu YenHao, Chang DunHao [18-19], etc., that the effective success rate of transplanting free flap tissue is 90- 95%, and only 25% achieve satisfactory treatment effects due to complications after surgery. Personally, I believe that the increased postoperative failure rate is mostly due to improper intraoperative operation and long-term tissue ischemia. However, among the 15 cases of end-to-end anastomosis of allogeneic transplanted blood vessels in the 7-day ischemia group, 8 cases failed in the first anastomosis, and the second reselection of transplanted blood vessels was considered to be successfully completed. The remaining 7 cases achieved perfect results in the first anastomosis. It can be inferred that the anastomosis failure rate of stored blood vessels can be as high as 53.33%, and about 46.67% of the anastomosis can be completed in the first operation. This research result is consistent with the results of Ramadan Jashari, Vanessa [20] Bouzet and others, who found that 2.407 (34.1%) of 7066 free flap tissue anastomoses could not be used and 4659 (65.9%) could be used normally. In addition, among the 92 donated blood vessels, 44 (47.8%) were abandoned and 48 (52.2%) were selected for use. These data are different from our research data, which may be related to the different conditions and reagents for our selection of stored blood vessels. The reasons for these failures are summarized as follows: the failure rate is 32% in the research data of scholars Jashari, et al., of which 59% are tissue morphological changes and 31% are caused by contamination [21].
The allogeneic vascular tissue in the 7-day storage group was more severely damaged than that in the 72-hour storage group, indicating that the longer the storage time, the longer the tissue cell damage, and even some tissue cells will be irreversibly damaged. These prolonged times in the ischemia groups are likely due to anatomical changes in the vascular tissue caused by ischemia and prolonged storage. Shorter anastomosis times are generally associated with reduced postoperative complications, including inflammation, vascular stenosis, and ischemia-reperfusion injury, highlighting the importance of minimizing surgical duration wherever possible. According to the results of the study of vascular end-to-end grafting by researcher Nupur Pruthi, et al. [22]: The overall vascular patency rate was 100% in the short term and 96.43% in the long term (27 out of 28), and the average vascular clamping time, suturing time, and single suture time were 65.48±16.93 minutes, 40.94±11.22 minutes, and 3.18±1.10 minutes, respectively. According to the study [12-13] by the operating time ranged from 22 to 70 minutes, with a mean of 46 minutes, while our operating time was 33.55±2.91 minutes in the control group, 39.50±3.03 minutes in the 3-day ischemic group, and 45.30±4.01 minutes in the 7-day ischemic group. The average time for vascular knot suturing was 11.97 minutes, while our end-to-end grafting time was 8.55±1.86 minutes in the control group, 14.50±1.97 minutes in the 3-day ischemic group, and 20.30±3.96 minutes in the 7-day ischemic group. This indicates that shortening the surgical time is an important factor in preventing tissue ischemia.
Since experimental rats tolerate very little bleeding, when blood is lost at the graft site during vascular grafting with floating sutures, the surgical time is increased depending on the suture. Careful surgical techniques and efficient anastomosis procedures are essential in minimizing intraoperative blood loss and ensuring surgical success. The only important reason for intraoperative bleeding is incomplete anastomosis. In our study, the total bleeding volume of the control group was 0.27±0.16 ml, while the bleeding volume of the ischemic 3-day and 7-day experimental groups using 3-day stored donor blood vessels and the bleeding volume of the ischemic 3-day group and the ischemic 7-day group using 7-day stored donor blood vessels were 0.60±0.60 ml and 1.64±0.43 ml, 1.78±0.45 ml and 3.50±0.82 ml, respectively. During the study, 4-5 ml of blood loss occurred in the ischemic 7-day group and the anastomosis group using 7-day stored allogeneic blood vessels, which was related to changes in the tissue structure of the stored donor blood vessels. These results suggest that prolonged ischemia and extended vessel preservation durations are associated with increased blood loss during vascular anastomosis. The structural and functional deterioration of the vascular tissue during extended ischemia likely contributes to these outcomes.
Ensuring the free blood flow of the graft vessel is an important factor for the success of microvascular surgery and is undoubtedly one of the most important stages in free tissue transplantation, limb transplantation and/or composite tissue transplantation. Also, during surgery, the anastomosis should be performed in a short time to protect the integrity of the tissue, prevent inflammatory reactions, vascular stenosis and thrombosis. It is also important to understand that the suturing technique should be minimal or no stripping of the outer membrane of the vessel, leaving a minimum of foreign material (sutures) in the vascular cavity, and using a small number of sutures should reduce necrosis and distortion of the inner part of the vessel edge. In the selection of transplanted blood vessels, it is best to choose blood vessels that have been stored for less than 3 days to avoid bleeding caused by damage to vascular tissue, which may lead to insufficient blood perfusion or anastomosis failure. The number of our experiments may not be sufficient. In future studies, we will further improve and deal with the related issues of complications after transplantation to provide a more complete experimental basis for lower limb ischemia-reperfusion injury and its tissue damage and repair in animal models.
References
- Yasargil MG (2010) Personal considerations on the history of microneurosurgery. Journal of neurosurgery 112(6): 1163-1175.
- Michael S, Andrew W, Philippe Kolh, et al. (2019) Global vascular guidelines on the management of chronic limb-threatening ischemia. Clinical practice guideline document J Vasc Surg 69: 3S-125S.
- Zhai Y, Petrowsky H, Johnny C Hong, Ronald W Busuttil, Jerzy W Kupiec Weglinski (2013) Ischaemia-reperfusion injury in liver transplantation-From bench to bedside. Nat Rev Gastroenterol Hepatol 10: 79-89.
- Violi F, Basili S, Berger JS, Hiatt WR (2012) Antiplatelet Therapy in Peripheral Artery Disease. Antiplatelet Agents Handbook of Experimental Pharmacology 210: 547-563.
- Caren G Solomon MD MPH Editer Iftikhar J, et al. (2016) Peripheral Artery Disease. N Engl J Med 374: 861-871.
- Smith David A, Lilie Craig J (2021) Acute Arterial Occlusion. StatPearls, Treasure Island (FL) StatPearls Publishing PMID 28722881, retrieved 10-27.
- Smith David A, Lilie Craig J (2023) Acute Arterial Occlusion. StatPearls [Internet] PMID: 28722881.
- Wang H (2016) Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388(10053): 1459-1544.
- Barros RS, Brito MV, Moura GP, Moura MP, Freitas LM, et al. (2011) Is it possible to do a microvascular anastomosis with an ordinary video camera? Experimental study. J Reconstr Microsurg 27(8): 503-508.
- Assersen K, Sørensen J (2015) Intravascular stenting in microvascular anastomoses. J Reconstr Microsurg. 31(2): 113-118.
- Leclère FM, Vogt P, Schoofs M, Delattre M, Mordon S (2016) Current laser applications in reconstructive microsurgery: a review of the literature. J Cosmet Laser Ther 18(3): 130-133.
- Jiri Dostal, Pavel Klein, Tereza Blassova, Vladimir Priban (2022) Comparison of the Microvascular Anastomosis Maturation in Continuous and Interrupted Suture Technique. Research square.
- Rui Sergio Monteiro de Barros, Rafael Aquino Leal, Renan Kleber Costa Teixeira, et al. (2017) Continuous versus interrupted suture technique in microvascular anastomosis in rats. Acta Cir Bras 32(9): 691-696.
- Onur Geldi MD, Emre Kubat MD, Celal Selçuk Ünal MD (2018) Acetaminophen Mitigates Myocardial Injury Induced by Lower Extremity Ischemia-Reperfusion in Rat Model. Brazilian Journal of Cardiovascular Surgery 33(3): 258-264.
- Kirişçi M, Güneri B, Seyithanoğlu M, Kazancı Ü, Doğaner A, Güneş H (2020) The protective effects of lycopene on ischemia/reperfusion injury in rat hind limb muscle model. Ulus Travma Acil Cerrahi Derg 26: 351-360.
- Cearra I, Herrero de la Parte B, Ruiz Montesinos I, Alonso Varona A, Moreno Franco DI, et al. (2021) Effects of Folinic Acid Administration on Lower Limb Ischemia/Reperfusion Injury in Rats. Antioxidants 10(12): 1887.
- Florian Dick, Jianhui Li, Marie-Noëlle Giraud, et al. (2008) Basic control of reperfusion effectively protects against reperfusion injury in a realistic rodent model of acute limb ischemia. Circulation 118(19): 1920-1928.
- Chiu YH, Chang DH, Perng CK (2017) Vascular Complications and Free Flap Salvage in Head and Neck Reconstructive Surgery: Analysis of 150 Cases of Reexploration. Annals of plastic surgery. 78: S83-S88.
- Egeler SA, de Jong T, Luijsterburg AJM, Mureau MAM (2018) Long-Term Patient-Reported Outcomes following Free Flap Lower Extremity Reconstruction for Traumatic Injuries. Plast Reconstr Surg 141(3): 773-783.
- Ramadan Jashari, Vanessa Bouzet, et al. (2023) Vascular allografts for clinical application in Europe: assessment of 30 years of experience with vascular tissue banking in Brussels. Cell Tissue Bank 24(3): 613-625.
- Gabriel Georges, Béatrice Allard, Mazen Dakkak, Ghislain Nourissat, et al. (2021) Appraising 5 years in activity of the largest public Canadian vascular graft bank. Journal of Vascular Surgery.74(3): 972-978.
- Nupur Pruthi, Gaurav Tyagi, Dhaval Gohil (2021) End-to-Side Microvascular Anastomosis on Rat Femoral Vessels Using Only 2-Throw Knot Interrupted Sututres - Evaluation of Feasibility and Patency Rates on Rat Femoral Vessels Model. Observational Study. World Neurosurg 148: e145-e150.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.