Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
The ARP2/3 Complex: A Potential Mediator between Pathogenic Microorganisms and Hosts
*Corresponding author: Juan Wang, Department of Biology, College of Chemistry and Life Science, Beijing University of Technology, 100124, Beijing, China.
Received: March 08, 2025; Published: March 26, 2025
DOI: 10.34297/AJBSR.2025.26.003444
Abstract
The ARP2/3 complex, a protein complex composed of seven subunits, regulates critical cellular processes such as cell motility, morphology maintenance, and intracellular trafficking by catalyzing actin nucleation and forming branched actin filament networks. Some pathogenic microorganisms differentially exploit the ARP2/3 complex-mediated actin polymerization machinery to facilitate their dissemination within hosts, leading to infectious diseases. Additionally, emerging evidence links the ARP2/3 complex to non-infectious disorders, including embryonic developmental abnormalities, fibrosis, and cartilage-related pathologies. This review synthesizes recent advances in understanding the pleiotropic functions of the ARP2/3 complex, providing a theoretical foundation for elucidating disease mechanisms and developing targeted therapies.
Keywords: ARP2/3 complex, Fungal infection, Viral infections
Abbreviations: ARP2/3: Complex -Actin; Related protein 2/3 complex; AtFH1: Arabidopsis Thaliana Formin Homology 1; EBOV: Ebola Virus; HIV-1: Human Immunodeficiency Virus-1; HSV-1: Herpes Simplex Virus Type 1; mRNA: Messenger Ribonucleic Acid; NCLS: Nucleocapsid-Like Structure; NPF: Nucleation-Promoting Factor; N-WASP: Neuronal Wiskott-Aldrich Syndrome Protein; PICK1: Protein Interacting With Protein Kinase C Alpha 1; RSV: Respiratory Syncytial Virus; SIV: Simian Immunodeficiency Virus; TNTs: Tunneling Nanotubes; TonEBP: Tonicity-Responsive Enhancer Binding Protein; t-SNARE: Target-Soluble NSF (N-Ethylmaleimide- Sensitive Factor) Attachment Protein Receptor; VIGS: Virus-Induced Gene Silencing; WAVE/SCAR: Wiskott-Aldrich Syndrome Protein and Verprolin Homology/Scar; WASH: Wiskott - Aldrich Syndrome protein and SCAR homolog; WHAMM: WASP Homolog Associated with Actin, Membranes, and Microtubules; W/SRC: Wave/Scar Regulatory Complex.
Introduction
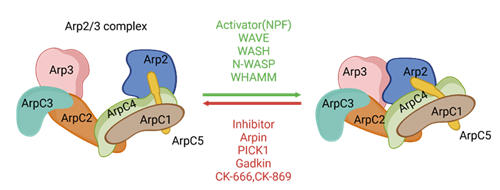
The ARP2/3 complex was initially discovered in Acanthamoeba and has since been extensively studied in yeast and mammalian cells [1]. This complex is a flat ellipsoid measuring approximately 15 nm in length, 14 nm in width, and 7 to 10 nm in thickness, composed of seven subunits: Arp2, Arp3, ArpC1, ArpC2, ArpC3, ArpC4, and ArpC5, with a total molecular weight of around 250 kDa [2]. Functionally, the ArpC1 to ArpC5 subunits correspond to the Arc15, Arc18, Arc19, Arc35, and Arc40 subunits in yeast. Among these, Arp2 and Arp3 are actin-related proteins that serve as core subunits with actin-like folding structures, although they lack nucleotide- binding capabilities. ArpC1 contains a β-helical insertion point comprised of seven blades, which associates with the sides of actin filaments. ArpC2 and ArpC4 share similar N-terminal α/β-folded regions and are interconnected at the center of the complex through a long C-terminal α-helix, forming a “C”-shaped clamp that envelops the Arp2 and Arp3 subunits and constitutes the core of the complex. ArpC3 and ArpC5 are globular α-helical subunits located at the edges of the complex, playing a crucial role in maintaining the structural integrity of the entire complex [2-4] (Figure 1).
The left side illustrates the inactive conformation, where Arp2 and Arp3 are spaced far apart. The right side shows the active conformation, where the ARP2/3 complex rearranges upon activation, allowing it to bind to the sides of actin filaments. This conformation enables the ARP2/3 complex to recruit actin monomers, initiating the assembly and growth of new actin filaments from the sides of existing ones. The regulatory factors are indicated above and below the arrows. CK-666 and CK-869 are two small-molecule inhibitors that activate the ARP2/3 complex [6].
The primary function of the ARP2/3 complex is to activate actin nucleation in response to extracellular regulatory signals, promoting filament branching and subsequently regulating cytoskeletal remodeling, lamellipodia extension, and intracellular transport [7- 9]. The specific functions of each subunit are detailed in Table 1. When the ARP2/3 complex is in its inactive conformation, Arp2 is positioned far away from Arp3 within the scaffold structure formed by ArpC1-5. Upon activation, Arp2 and Arp3 transition from a distant state to a closely associated state, providing nucleation sites for actin and facilitating the polymerization of actin to form filaments. ArpC2 and ArpC4 can bind to actin filaments, while ArpC1, ArpC3, and ArpC5 promote actin activation and assembly by coupling with extracellular signals [10-13] (Table 1).
Research has shown that the ARP2/3 complex influences the morphological changes, motility, and interactions of tumor cells with the extracellular matrix through dynamic regulation of the actin cytoskeleton, thereby participating in the processes of tumor cell invasion and metastasis. In recent years, an increasing number of studies have revealed that the role of the ARP2/3 complex extends beyond the realm of cancer, playing a critical role in the pathogenesis and progression of various other diseases. Therefore, to gain a more comprehensive understanding of the functions of the ARP2/3 complex in disease, this review aims to systematically summarize its roles in diseases other than cancer. By synthesizing and integrating these research findings, we hope to provide a more thorough theoretical basis for the prevention and treatment of related diseases.
The ARP2/3 Complex Is Hijacked by Pathogenic Microorganisms to Assist in their Invasion of the Human Body
Fungal
Aspergillus fumigatus is an opportunistic fungal pathogen that can cause severe infections, with Airway Epithelial Cells (AECs) playing a critical role in the internalization of conidia during the early stages of infection. A study by Culibrk, et al., [16] found that knocking down WIPF2 (an upstream regulator of the ARP2/3 complex) significantly reduced the internalization of conidia into airway epithelial cells. Treatment of cells with the ARP2/3 small molecule inhibitor CK-666 also resulted in a 43% decrease in conidia internalization, suggesting that the ARP2/3 complex promotes the invasion of Aspergillus fumigatus into human airway epithelial cells by regulating actin remodeling.
Similarly, Candida albicans is a common opportunistic fungal pathogen, and its biofilm formation is one of the key factors contributing to the difficulty of treating infections. Nobile, et al., [17] utilized functional genomic approaches to identify that the Arc18 gene, which encodes a member of the ARP2/3 complex, is critical for cellular adhesion. When Arc18 transcription is suppressed, the function of the ARP2/3 complex is impaired, leading to altered hydrophobicity of the cell surface and increased exposure of chitin and β-glucan in the cell wall. This disruption damages cell wall integrity and activates Rho1-mediated cell wall stress responses, resulting in cell wall remodeling that severely affects the adhesion capability of Candida albicans to solid surfaces, thereby hindering biofilm formation. These findings provide new insights into the mechanisms of human fungal infections and suggest potential directions for therapeutic target research.
Virus
Viral infections pose a significant threat to human health, causing various diseases and even global pandemics, with profound societal impacts. Understanding the mechanisms of viral infections is therefore critical for developing effective prevention and treatment strategies. In recent years, the ARP2/3 complex has been increasingly implicated in these processes across multiple studies. Ohkawa, et al., [18] found that during baculovirus infection, ARP2/3 complex- mediated actin polymerization drives the movement of the virus within the nucleus and cytoplasm. Additionally, the ARP2/3 complex participates in disrupting nuclear membrane integrity, facilitating viral egress from the nucleus. In Respiratory Syncytial Virus (RSV) infection, the ARP2/3 complex drives actin polymerization to induce the formation of filopodia-like structures, promoting viral budding and intercellular spread. This process plays a crucial role in the dissemination of RSV between cells [19-21]. Komano, et al., [22] demonstrated that the infection efficiency of numerous viruses is influenced by the ARP2/3 complex. In studies on primate lentiviruses (HIV-1 and SIV), the expression of ARP2/3 complex inhibitors or knockdown of Arp2 significantly reduced the efficiency of viral infection in host cells. Similarly, Grikscheit, et al., [23] showed that treating cells with the ARP2/3 inhibitor CK666 or using siRNA to knock down ARP2/3-related proteins inhibited the long-distance transport of Nucleocapsid-Like Structures (NCLS) of the Ebola Virus (EBOV) and reduced actin tail formation. Herpes Simplex Virus type 1 (HSV-1) infection induces the formation of Tunneling Nanotubes (TNTs), which facilitate intercellular viral spread. The ARP2/3 complex is involved in the formation of TNTs, and treatment with CK666 reduced the number of TNTs, thereby weakening HSV-1’s ability to spread between cells [24]. In summary, the ARP2/3 complex plays an indispensable role in various stages of viral infections, including intracellular viral movement, nuclear egress, and intercellular spread. Its involvement in these critical processes highlights the ARP2/3 complex as an important potential target for future antiviral research and provides a theoretical foundation for the development of novel antiviral strategies.
The ARP2/3 Complex Helps Plants Resist the Invasion of Fungal Pathogens
Golovinomyces orontii is one of the pathogens responsible for powdery mildew in tomatoes and can parasitize various plants. Sun, et al., [25] used Virus-Induced Gene Silencing (VIGS) to knock down ArpC3, which resulted in increased susceptibility of tomatoes to the powdery mildew pathogen. This was accompanied by a reduction in defense responses, such as decreased Hypersensitive Reaction (HR), reduced accumulation of hydrogen peroxide (H₂O₂), and downregulation of salicylic acid signaling pathway-related gene expression. Conversely, overexpression of ArpC3 in Arabidopsis thaliana enhanced resistance to the powdery mildew pathogen. Qin, et al. [26] employed live-cell imaging techniques and discovered that early infection of Arabidopsis by the powdery mildew pathogen leads to the formation of actin patches beneath the fungal invasion sites, mediated by the ARP2/3 complex and its activator, the WAVE/SCAR-regulated complex (W/SRC). The knockout of W/ SRC-ARP2/3 pathway subunits compromised the ability of Arabidopsis to penetrate non-adapted powdery mildew, affecting the endocytic recycling and transport of the defense-related t-SNARE protein PEN1 to cell wall deposits, while also partially disrupting the formation of actin patches. Additionally, the knockout of Arp3 and Type 1 proteins homologous to AtFH1 in Arabidopsis severely impaired actin patch formation and deposition of cell wall materials, facilitating the entry of the powdery mildew pathogen into host cells.This indicates that the ARP2/3 complex and AtFH1 cooperate to enhance the penetration resistance of Arabidopsis against fungal invasion, playing a significant role in plant immune defense.Similar findings were observed in wheat rust disease caused by Puccinia triticina, where Qi, et al., [27] used VIGS technology to knock down ArpC3. They found that wheat resistance to the stripe rust fungus was diminished, accompanied by disorganization of the actin cytoskeleton, decreased accumulation of Reactive Oxygen Species (ROS), suppression of hypersensitive responses, and enhanced pathogen growth. These studies reveal that the ARP2/3 complex participates in plant defense responses against fungal diseases through various mechanisms, providing an important theoretical foundation and potential genetic targets for the prevention and treatment of fungal diseases in plants.
The Functions of the ARP2/3 Complex in Non - Infectious Diseases
Recent studies have found that the ARP2/3 complex is also associated with some non-infectious diseases. Sindram, et al., [28] generated Arpc5 knockout mice and found that homozygous deletion resulted in embryonic lethality. Embryos exhibited developmental defects such as incomplete neural tube closure, abnormal heart development, and pharyngeal arch malformations at 8.5-9.5 days post-fertilization. This study was the first to report a human syndrome caused by ARPC5 deficiency and demonstrated its vital role in embryonic development. Tessier, et al., [29] investigated the role of the ARP2/3 complex in cartilage tissue and found that it regulates cartilage health by modulating cell-extracellular matrix interactions and TonEBP-mediated osmoregulation. Impaired ARP2/3 function reduced the ability of chondrocytes to spread on the matrix, disrupted cell proliferation and differentiation, and altered extracellular matrix composition. These changes ultimately led to cartilage degeneration and contributed to the development of diseases such as intervertebral disc degeneration and osteoarthritis. Mergault, et al., [30] found that both in vivo and in vitro stimulation of fibrosis increased the mRNA expression of ARP2/3 complex subunits. In a mouse model, treatment with the ARP2/3 inhibitor CK666 alleviated bleomycin-induced pulmonary fibrosis by reducing levels of insoluble collagen and procollagen-1 in the lungs.
Interestingly, this antifibrotic effect was achieved without affecting lung weight, inflammation, or the activity of collagen metabolism- related enzymes, suggesting that ARP2/3 inhibition primarily reduces collagen synthesis to exert its effects. In addition, many studies have shown that mutations in the subunits of the ARP2/3 complex can lead to impaired maintenance of cytotoxic T lymphocytes and cytolytic activity, as well as severe inflammation and immunodeficiency [31-38].This underscores the importance of in-depth research into the mechanisms of the ARP2/3 complex, not only to enhance our comprehensive understanding of the pathogenesis and progression of these diseases but also to provide essential theoretical foundations and potential targets for the future diagnosis, treatment, and drug development of related diseases.
Discussion
Although there has been considerable progress in understanding the role of the ARP2/3 complex in various diseases, many areas remain to be explored further. At the molecular level, while it is known that the ARP2/3 complex is involved in numerous disease processes, how it precisely responds to upstream signals in different cellular environments and disease contexts, and how it coordinates with other intracellular signaling pathways to regulate disease progression, remain unclear. For example, in the context of fungal pathogen infections in humans versus plant defenses against fungal infections, the upstream signals and downstream regulatory targets of the ARP2/3 complex may differ significantly. Studying and comparing these differences in depth could help us better understand the complexity of host-pathogen interactions.
In addition, the development of targeted therapies based on the ARP2/3 complex holds great promise but faces significant challenges. On one hand, it is crucial to enhance the specificity of drugs targeting the ARP2/3 complex, minimizing negative impacts on normal cellular physiological functions. On the other hand, optimizing drug delivery systems to ensure efficient and precise targeting of the ARP2/3 complex in diseased cells is another hurdle that needs to be addressed. With the rapid advancement of technologies like single-cell sequencing and gene editing, future research offers the opportunity to investigate the role of the ARP2/3 complex at the single-cell level and in an individualized manner. This could provide a deeper understanding of its functions in various diseases and support the development of personalized diagnostic and therapeutic strategies. As research continues to progress, the ARP2/3 complex is expected to open new avenues and provide significant breakthroughs for the treatment of cancer, fungal infections, and other challenging diseases.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 32370805 and 31970044).
Conflict of Interest
The author declares that he has no known competing financial interests or personal relationships that could have appeared to in fluence the work reported in this paper.
References
- Welch M D and Mullins R D (2002) Cellular control of actin nucleation. Annu Rev Cell Dev Biol 18: 247-288.
- Robinson RC, Turbedsky K, Kaiser DA, JB Marchand, HN Higgs, et al. (200) Crystal Structure of Arp2/3 Complex. Science 294: 1679-1684.
- Pollard T D, Beltzner CC (2002) Structure and function of the Arp2/3 complex. Curr Opin Struct Biol 12(6): 768-774.
- Amann KJ, Pollard TD (2001) The Arp2/3 complex nucleates actin filament branches from the sides of pre-existing filaments. Nat Cell Biol 3: 306-310.
- Dyche Mullins R, Pollard TD (1999) Structure and function of the Arp2/3 complex. Current Opinion in Structural Biology 9: 244-249.
- Hetrick B, Han Min S, Helgeson Luke A (2013) Small Molecules CK-666 and CK-869 Inhibit Actin-Related Protein 2/3 Complex by Blocking an Activating Conformational Change. Chemistry & Biology 20: 701-712.
- Nolen BJ, Pollard TD (2008) Structure and biochemical properties of fission yeast Arp2/3 complex lacking the Arp2 subunit. J Biol Chem 283: 26490-8.
- Higgs HN, Pollard TD (2001) Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Annu Rev Biochem 70: 649-676.
- Maritzen T, Zech T, Schmidt MR (2012) Gadkin negatively regulates cell spreading and motility via sequestration of the actin-nucleating ARP2/3 complex. Proc Natl Acad Sci U S A 109: 10382-10387.
- Dalhaimer P, Pollard TD (2010) Molecular dynamics simulations of Arp2/3 complex activation. Biophys J 99: 2568-2576.
- Rouiller I, Xu XP, Amann KJ (2008) The structural basis of actin filament branching by the Arp2/3 complex. J Cell Biol 180: 887-895.
- Liu SL, May JR, Helgeson LA (2013) Insertions within the actin core of actin-related protein 3 (Arp3) modulate branching nucleation by Arp2/3 complex. J Biol Chem 288: 487-497.
- Pfaendtner J, Volkmann N, Hanein D (2012) Key structural features of the actin filament Arp2/3 complex branch junction revealed by molecular simulation. J Mol Biol 416: 148-161.
- Winter DC, Choe EY, Li R (1999) Genetic dissection of the budding yeast Arp2/3 complex: A comparison of the in vivo and structural roles of individual subunits. Proceedings of the National Academy of Sciences 96: 7288-7293.
- Pollard T (2002) Structure and function of the Arp2/3 complex. Current Opinion in Structural Biology 12: 768-774.
- Culibrk L, Croft CA, Toor A (2019) Phagocytosis of Aspergillus fumigatus by Human Bronchial Epithelial Cells Is Mediated by the Arp2/3 Complex and WIPF2. Front Cell Infect Microbiol 9: 16.
- Nobile C, Lee JA, Robbins N (2016) Functional Genomic Analysis of Candida albicans Adherence Reveals a Key Role for the Arp2/3 Complex in Cell Wall Remodelling and Biofilm Formation. PLOS Genetics 12.
- Ohkawa T, Welch MD (2018) Baculovirus Actin-Based Motility Drives Nuclear Envelope Disruption and Nuclear Egress. Current Biology 28: 2153-2159.e4.
- Paluck A, Osan J, Hollingsworth L (2021) Role of ARP2/3 Complex-Driven Actin Polymerization in RSV Infection. Pathogens 11(1): 26.
- Mehedi M, Collins PL, Buchholz UJ (2017) A novel host factor for human respiratory syncytial virus. Communicative & Integrative Biology 10(3): e1319025.
- Schnell MJ, Mehedi M, McCarty T (2016) Actin-Related Protein 2 (ARP2) and Virus-Induced Filopodia Facilitate Human Respiratory Syncytial Virus Spread. PLOS Pathogens 12(12): e1006062.
- Komano J, Miyauchi K, Matsuda Z (2004) Inhibiting the Arp2/3 Complex Limits Infection of Both Intracellular Mature Vaccinia Virus and Primate Lentiviruses. Molecular Biology of the Cell 15: 5197-5207.
- Grikscheit K, Dolnik O, Takamatsu Y (2020) Ebola Virus Nucleocapsid-Like Structures Utilize Arp2/3 Signaling for Intracellular Long-Distance Transport. Cells 9(7): 1728.
- Wang J, Shang KT, Ma QH (2023) Herpes Simplex Virus Type 1 Infection Induces the Formation of Tunneling Nanotubes. Microorganisms 11(8): 1916.
- Sun G, Feng C, Guo J (2019) The tomato Arp2/3 complex is required for resistance to the powdery mildew fungus Oidium neolycopersici. Plant Cell Environ 42: 2664-2680.
- Qin L, Liu L, Tu J (2021) The ARP2/3 complex, acting cooperatively with Class I formins, modulates penetration resistance in Arabidopsis against powdery mildew invasion. Plant Cell 33(9): 3151-3175.
- Tuo Qi, Juan Wang, Qixiong Sun,Brad Day, Jun Guo, et al. (2017) TaARPC3, Contributes to Wheat Resistance against the Stripe Rust Fungus. Front Plant Sci 8: 1245.
- Sindram E, Caballero Oteyza A, Kogata N, Shaina Chor Mei Huang, Zahra Alizadeh, et al. (2023) ARPC5 deficiency leads to severe early-onset systemic inflammation and mortality. Disease Models & Mechanisms 16(7): dmm050145.
- Tessier S, Doolittle AC, Sao K, Jeremy D Rotty, James E Bear, et al. (2020) Arp2/3 inactivation causes intervertebral disc and cartilage degeneration with dysregulated TonEBP-mediated osmoadaptation. JCI Insight 5(4): e131382.
- Mergault C, Lisée F, Tiroille V (2022) Inhibition of the Arp2/3 complex represses human lung myofibroblast differentiation and attenuates bleomycin‐induced pulmonary fibrosis. British Journal of Pharmacology 179: 125-140.
- Aiuti A, Gattorno M, Bustamante J (2018) T-cell defects in patients with ARPC1B germline mutations account for combined immunodeficiency. Blood 132:2362-2374.
- Kahr WHA, Pluthero FG, Elkadri A (2017) Loss of the Arp2/3 complex component ARPC1B causes platelet abnormalities and predisposes to inflammatory disease. Nature Communications 8: 14816.
- Kuijpers TW, Tool ATJ, van der Bijl I (2017) Combined immunodeficiency with severe inflammation and allergy caused by ARPC1B deficiency. Journal of Allergy and Clinical Immunology 140: 273-277.e10.
- Leung G, Zhou Y, Ostrowski P (2021) ARPC1B binds WASP to control actin polymerization and curtail tonic signaling in B cells. JCI Insight 6(23): e149376.
- Papadatou I, Marinakis N, Botsa E (2021) Case Report: A Novel Synonymous ARPC1B Gene Mutation Causes a Syndrome of Combined Immunodeficiency, Asthma, and Allergy With Significant Intrafamilial Clinical Heterogeneity. Frontiers in Immunology 12: :634313.
- Randzavola LO, Strege K, Juzans M, Yukako Asano, Jane C Stinchcombe, et al. (2019) Loss of ARPC1B impairs cytotoxic T lymphocyte maintenance and cytolytic activity. Journal of Clinical Investigation 129: 5600-5614.
- Somech R, Lev A, Lee YN, Amos J Simon, Ortal Barel, et al. (2017) Disruption of Thrombocyte and T Lymphocyte Development by a Mutation in ARPC1B. The Journal of Immunology 199: 4036-4045.
- Volpi S, Cicalese MP, Tuijnenburg P, Anton T J Tool, Eloy Cuadrado, et al. (2019) A combined immunodeficiency with severe infections, inflammation, and allergy caused by ARPC1B deficiency. Journal of Allergy and Clinical Immunology 143: 2296-2299.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.