Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Advancements in Monomolecular Multimodal Platforms for Cancer Theranostics
*Corresponding author: prof. Dr. Nasser Thallaj Pharmaceutical chemistry and drug quality control Department, Faculty of Pharmacy, Al-Rachid privet University, Damascus, Syria. ORCID ID: 0000-0002-6279-768X.
Received: April 03, 2025; Published: June 20, 2025
DOI: 10.34297/AJBSR.2025.27.003569
Abstract
Cancer remains a leading cause of death globally, necessitating innovative approaches in diagnosis and treatment. This thesis investigates the development of monomolecular Multimodal Platforms (MOMIPs) for cancer theranostics, focusing particularly on prostate cancer. Theranostics merges therapeutic and diagnostic functionalities, enabling real-time monitoring of disease progression and personalized treatment strategies. Central to this approach is the use of nanoparticles, which exploit the Enhanced Permeability and Retention (EPR) effect for selective drug accumulation in tumor tissues. The work explores various targeted ligands, including small molecules and monoclonal antibodies, to enhance specificity in therapeutic applications. Additionally, advancements in chelation strategies and conjugation chemistries are examined to ensure the stability of radiometals used in imaging and therapy. This research also highlights the integration of imaging modalities such as PET, SPECT, and MRI to provide comprehensive insights into tumor biology. By leveraging the unique properties of gold and gadolinium nanoparticles, this study aims to create advanced theranostic tools that improve cancer detection and treatment outcomes. Ultimately, the findings contribute to the growing field of personalized medicine, emphasizing the importance of interdisciplinary collaboration in developing effective cancer management strategies. The potential clinical applications underscore the significance of these innovative platforms in enhancing patient care and outcomes in oncology.
Keywords: Theranostics, Nanoparticles, Cancer, Imaging, Targeted ligands, Personalized medicine, Radiotherapy
Introduction
Cancer continues to be one of the leading causes of mortality worldwide, highlighting the urgent need for advancements in both diagnostic and therapeutic strategies. Traditional modalities, including surgery, chemotherapy, and radiotherapy, present limitations such as systemic toxicity and the development of resistance [1,2]. Recently, theranostics-a paradigm that integrates therapy and diagnostics-has emerged as a transformative approach to cancer management. This integrated strategy facilitates real-time monitoring of disease progression, drug delivery, and therapeutic efficacy, thereby promoting personalized medicine [3]. A cornerstone of theranostics is the utilization of multifunctional platforms that combine imaging agents and therapeutic payloads within a single system [4,5]. Nanoparticles, due to their tunable physicochemical properties, play a pivotal role in this domain [6]. They leverage the Enhanced Permeability and Retention (EPR) effect, allowing for passive accumulation in tumor tissues. Further refinement can be achieved through active targeting, wherein ligands such as peptides, antibodies, or small molecules bind to overexpressed receptors on cancer cells, enhancing specificity and therapeutic efficacy [7,8,9]. Nanoparticles have been extensively investigated for drug delivery, enhancement of radiotherapy, and multimodal imaging [10]. For instance, inorganic nanoparticles such as gold (Au) and Gadolinium (Gd)-based systems serve as radiosensitizers due to their high atomic numbers, which amplify the effects of ionizing radiation. Similarly, Superparamagnetic Iron Oxide Nanoparticles (SPIONs) and Gd chelates are widely employed as contrast agents in Magnetic Resonance Imaging (MRI) [11]. The integration of these functionalities into a single platform holds significant promise for improving diagnostic accuracy and therapeutic outcomes [12]. Imaging modalities such as Positron Emission Tomography (PET), Single- Photon Emission Computed Tomography (SPECT), and optical imaging provide complementary information [13].
PET and SPECT offer high sensitivity and quantitative data but are limited by spatial resolution, while MRI provides excellent anatomical detail but lower sensitivity [14]. Multimodal imaging, which combines these techniques, addresses their individual limitations and enables comprehensive disease characterization [15]. For example, PET/MRI hybrids merge metabolic and anatomical information, while optical imaging allows for real-time visualization during surgical procedures. Targeted ligands are essential for the success of theranostic platforms [16]. Small molecules such as folic acid and cyclic RGD peptides bind to receptors that are overexpressed in tumors, including folate receptors and integrins [12- 16]. Monoclonal Antibodies (mAbs) provide high specificity but encounter challenges such as poor tissue penetration and immunogenicity. Advances in antibody engineering, including the development of fragments and conjugates, aim to mitigate these limitations. Chelators are another critical component, ensuring the stable complexation of radiometals (e.g., ^64Cu, ^68Ga, ^177Lu) for imaging or therapeutic purposes [17,18]. Macrocyclic chelators such as DOTA and NOTA offer superior stability compared to acyclic analogs, thereby minimizing off-target effects [19]. Additionally, fluorescent probes, including cyanine dyes (e.g., Cy5, IR-783), enable optical imaging with high sensitivity and minimal background interference [20]. Conjugation chemistry is vital for assembling these multifunctional systems [21]. Strategies such as maleimide-thiol coupling, click chemistry, and silanization facilitate the attachment of targeting ligands, chelators, and imaging probes to nanoparticles or antibodies [22]. These reactions must be efficient, site-specific, and biocompatible to ensure the functionality of the final construct [23]. This thesis explores the development of monomolecular multimodal platforms (MOMIPs) for cancer theranostics, with a focus on prostate cancer as a model [24]. It investigates the synthesis of targeted ligands for PET, SPECT, and optical imaging, as well as their application in nanoparticle-based systems [25]. By leveraging the unique properties of gold and gadolinium nanoparticles, this work aims to create advanced theranostic tools for enhanced cancer detection and treatment [26]. The findings contribute to the growing body of research on personalized medicine, offering potential clinical applications for improved patient outcomes [27,28].
The integration of nanotechnology, targeted ligands, and multimodal imaging holds immense potential for revolutionizing cancer theranostics [29]. This research aligns with global efforts to develop precise, effective, and minimally invasive strategies for cancer management, paving the way for future innovations in the field [30].
Cancer theranosis: Approaches and avenues
The term theranostics-a fusion of “therapy” and “diagnostics”- refers to agents or systems capable of simultaneously diagnosing, treating, and monitoring diseases within a single platform. Coined by John Funkhouser in 1998, this concept has since evolved into a cornerstone of precision medicine Figure 1.
Theranostic agents are designed to integrate diagnostic imaging with targeted therapy in a synergistic manner, enabling real-time visualization of pathological sites, assessment of drug distribution (pharmacokinetics), and evaluation of therapeutic response (pharmacodynamics). The overarching objective is to refine therapeutic regimens with unprecedented precision, thereby enhancing efficacy while minimizing off-target effects [31,32].
Cancer Therapy
As illustrated in Figure 1, contemporary cancer management relies on four primary therapeutic modalities: surgical resection, radiotherapy, chemotherapy, and immunotherapy. These strategies may be applied independently or in combination, tailored to tumor type, stage, and molecular profile. While localized benign tumors are often amenable to curative surgical excision, malignant cancers typically necessitate multimodal interventions [33-34].
Radiotherapy employs ionizing radiation to ablate tumor tissues. This can be administered as external beam radiation (e.g., X-rays) or as brachytherapy, where radioactive sources are placed directly at or near the tumor site. An advanced variant involves the systemic delivery of radionuclides such as ^177Lu or ^90Y, conjugated to targeting moieties that selectively bind tumor-specific receptors. Chelating agents are used to stabilize these isotopes and facilitate their targeted delivery. One notable example is Lutathera® (lutetium-177 dotatate), approved by the FDA in 2018 for peptide receptor radionuclide therapy (PRRT) in neuroendocrine tumors. Similarly, radioimmunotherapy, using Monoclonal Antibodies (e.g., Zevalin® and Bexxar®), combines immunological specificity with radiotoxicity to target hematologic malignancies [34].
Chemotherapy remains a foundational treatment in oncology, employing cytotoxic agents-either synthetic or semisynthetic-to disrupt critical cellular processes such as DNA replication and mitosis. Despite its widespread use, traditional chemotherapy is limited by poor selectivity, often damaging healthy tissues and leading to severe side effects. Current research focuses on enhancing the therapeutic index through drug modification and the development of delivery systems that preferentially target malignant cells. Nanotechnology plays a central role in this pursuit, offering nanoparticle- based carriers designed to deliver chemotherapeutics directly to tumor sites, thereby reducing systemic toxicity [35].
Immunotherapy represents a paradigm shift in cancer treatment, leveraging the immune system to combat malignancy. At the forefront of this field are Monoclonal Antibodies (mAbs), initially developed by Milstein and Köhler, whose pioneering work earned them the Nobel Prize in 1984. mAbs exhibit high specificity due to their extensive binding interfaces, resulting in fewer off-target effects compared to small-molecule drugs. Advances in protein engineering and molecular immunology have led to the development of highly stable, efficacious antibody-based therapeutics for a range of cancers.
Mechanistically, mAbs exert antitumor effects via multiple pathways: antigen crosslinking leading to apoptosis, inhibition of pro-survival signalling, Antibody-Dependent Cellular Cytotoxicity (ADCC), Complement-Mediated Cytotoxicity (CMC), and immune modulation via cytokine milieu alterations. As of 2024, over 25 monoclonal antibodies have received regulatory approval for oncology indications [36,37].
Further extending their utility, mAbs serve as carriers in Antibody- Drug Conjugates (ADCs)-a class of biopharmaceuticals that combine the specificity of antibodies with the potency of cytotoxic drugs. With four ADCs currently approved and more than 50 candidates in clinical trials, this platform holds considerable promise for targeted cancer therapy (www.adcreview.com).
Nanoparticles in Cancer Therapy
Cancerous tissues are characterized by aberrant vasculature and impaired lymphatic drainage, giving rise to the Enhanced Permeability and Retention (EPR) effect. This phenomenon facilitates the passive accumulation of macromolecules, including nanoparticles, within the tumor microenvironment. The EPR effect is driven by a confluence of factors such as abnormal angiogenesis, vascular leakiness, heterogeneity in tumor architecture, and inefficient lymphatic clearance [38].
Once localized at the tumor site, nanoparticles can release therapeutic agents in a controlled manner, either passively or in response to external stimuli (e.g., heat, pH, or radiation). Some nanoparticles also enhance radiation effects, acting as radiosensitizers [39].
A wide array of nanoparticle systems has been engineered for both diagnostic and therapeutic purposes. These include:
i. Lipid-based carriers (e.g., liposomes),
ii. Polymeric nanoparticles,
iii. Inorganic nanostructures (e.g., gold, silica),
iv. Macromolecular conjugates,
v. Viral nanoparticles.
These systems offer multifunctional capabilities including targeted drug delivery, real-time imaging, and stimuli-responsive release- making them ideal candidates for theranostic applications in oncology Figure 2.
Lipid-based nanocarriers, such as liposomes, Solid Lipid Nanoparticles (SLNs), and Self-Microemulsifying Drug Delivery Systems (SMEDDS), are composed of fatty acid derivatives and have shown significant utility in oncology. Several liposomal formulations have been approved globally, including Doxil®, Myocet®, Mepact ®, Marqibo®, and DaunoXome®, particularly for chemotherapeutic delivery due to their enhanced circulation time and tumor accumulation via the EPR effect [40].
Polymeric nanoparticles comprise systems such as matrix-type particles formed from synthetic polymers (e.g., poly (lactic-co-glycolic acid), PLGA), polymeric micelles assembled from amphiphilic block copolymers (e.g., polycaprolactone-polyglutamic acid), and polymer-bound drug formulations like Abraxane® (albumin-bound paclitaxel nanoparticles), which leverage endogenous transport pathways to enhance drug delivery to tumors [41].
Conjugated nanocarriers, including polymer-drug conjugates and Antibody-Drug Conjugates (ADCs), have emerged as leading platforms for active targeting in cancer therapy. ADCs, in particular, are a rapidly advancing class in clinical oncology, combining the specificity of monoclonal antibodies with the potency of cytotoxic agents, with numerous candidates currently undergoing clinical trials [42].
Virus-Like Particles (VLPs) are another innovative class, composed of self-assembling viral capsid proteins that lack genetic material, rendering them non-infectious. These particles can encapsulate various payloads, including small molecules, nucleic acids, and imaging agents, and have applications in targeted therapy, molecular imaging, and vaccine development [43].
Inorganic nanoparticles encompass a broad range of structures, including gold nanoparticles, iron oxide nanoparticles, silica nanoparticles, and quantum dots. These materials, often non-biodegradable, necessitate careful physicochemical optimization to mitigate potential toxicity and biodistribution concerns. Nonetheless, several have progressed to clinical evaluation-e.g., ferumoxtran and ferucarbotran-and others such as AGuIX® (gadolinium-based) and hafnium oxide nanoparticles are in early-phase clinical trials (e.g., Phase I/II), demonstrating translational potential in oncology (source: clinicaltrials.gov).
Passive vs. Active Targeting of Nanoparticles
Nanotechnology-based drug delivery systems in oncology exploit the unique pathophysiological characteristics of tumor microenvironments. Passive targeting relies on the Enhanced Permeability and Retention (EPR) effect, where nanoparticles within a defined size range preferentially accumulate in tumor tissues due to leaky vasculature and impaired lymphatic drainage. This approach does not require specific surface modifications [43].
In contrast, active targeting involves functionalizing nanoparticle surfaces with ligands-such as peptides, small molecules, antibodies, or antibody fragments-that recognize and bind to tumor-specific or overexpressed receptors (e.g., folate receptor, EGFR, HER2). While EPR-based accumulation can be highly variable across tumor types and patients, active targeting enables a more selective and personalized therapeutic strategy, aligning with the goals of precision medicine [44].
Nanoparticles in Radiotherapy
While advancements in targeted drugs have significantly improved cancer treatment outcomes, lifestyle, environmental factors, and an aging population continue to elevate cancer incidence rates. Data indicate that approximately 49% of cancer patients achieve remission through surgery, 40% through radiotherapy (alone or in combination), and 11% via chemotherapy, emphasizing the critical role of radiotherapy-particularly as a cost-effective modality for palliative and advanced-stage care [45].
Modern radiation techniques such as Image-Guided Radiotherapy (IGRT), Stereotactic Body Radiotherapy (SBRT), Intensity- Modulated Radiotherapy (IMRT), and proton beam therapy have enabled precise targeting of tumors with minimal damage to surrounding tissues.
Nanoparticles composed of high atomic number (Z > 50) elements, such as Gold (Au), Gadolinium (Gd), Platinum (Pt), and Iodine (I), have shown radiosensitizing capabilities due to their superior photoelectric absorption properties. These nanoparticles enhance radiation-induced damage through increased production of secondary electrons and free radicals upon irradiation, thereby improving therapeutic efficacy [46].
Mechanisms of Radiosensitization by Nanoparticles
Radiosensitization occurs through a sequence of physical, chemical, and biological phases:
i. Physical phase: Ionizing radiation interacts with high-Z elements in nanoparticles, resulting in photoelectric absorption, generation of secondary electrons, and molecular ionization.
ii. Chemical phase: Free radicals such as hydroxyl radicals (•OH) are generated and either neutralized by scavengers (e.g., glutathione) or cause oxidative damage (fixation) to macromolecules.
iii. Biological phase: These processes culminate in cellular outcomes described by the “5 Rs” of radiobiology-repair, reoxygenation, redistribution, repopulation, and radiosensitivity- which collectively influence treatment outcomes [46-49].
Among the leading candidates, gold nanoparticles are widely studied for their favorable biocompatibility and radiosensitization efficacy. Notably, collaborations under the ARGENT project (Advanced Radiotherapy using Gold and Gadolinium Nanoparticles) have advanced both gadolinium- and gold-based systems. For instance, the Dijon and Besançon groups in France, led by Prof. Stéphane Roux and involving institutions like Chematech, ICMUB, and UTINAM, have contributed significantly to the development of chelate-functionalized gold nanoparticles.
AGuIX® nanoparticles, gadolinium-based ultrasmall particles developed by Nano-H and TheraGuix (co-founded by Prof. Olivier Tillement), have demonstrated promise in preclinical models and are currently in Phase I clinical trials for brain metastases, offering dual functionality as radiosensitizers and MRI contrast agents.
This research project will focus on engineering novel synthetic ligands as molecular building blocks for next-generation multifunctional nanoparticles with potential applications in image-guided radiotherapy and personalized oncology.
Cancer Diagnosis
Effective cancer diagnosis is fundamental to patient management and precedes therapeutic decision-making. Accurate characterization involves both anatomical and molecular assessments of tumor burden and heterogeneity. Medical imaging plays a pivotal role, classified broadly into:
i. Nuclear imaging, which utilizes radiolabeled tracers (e.g., PET, SPECT),
ii. Non-nuclear imaging, which includes modalities such as MRI, CT, ultrasound, and optical imaging (Figure 3, Table 1).
While non-invasive imaging provides real-time whole-body evaluation, histopathological analysis of biopsied tissue remains essential for confirming malignancy and identifying molecular biomarkers. These data inform treatment strategies and aid in the selection of suitable imaging agents for disease monitoring, enabling a personalized and adaptive approach to cancer management [50] (Figure 3).
PET and SPECT Imaging
Positron Emission Tomography (PET) and Single Photon Emission Computed Tomography (SPECT) are advanced nuclear imaging modalities that enable non-invasive visualization and quantification of biochemical and molecular processes in vivo. These techniques are pivotal in oncology for assessing tumor metabolism, receptor expression, and treatment response, owing to their high sensitivity and deep tissue penetration.
PET imaging utilizes positron-emitting radionuclides (e.g., ^18F, ^68Ga, ^64Cu), while SPECT employs gamma-emitting isotopes (e.g., ^99mTc, ^123I, ^111In). Both methods rely on the development of radiolabeled molecular probes that exhibit high specificity and selectivity for a given biological target-such as cell surface receptors, enzymes, or transporters-implicated in tumorigenesis.
The process involves careful design and synthesis of targeted radiopharmaceuticals, which may be small molecules, peptides, antibodies, or nanoparticles. These agents bind selectively to the molecular targets expressed in cancer cells, allowing real-time, quantitative imaging of disease-associated biomarkers. PET offers superior spatial resolution and quantitative accuracy, whereas SPECT is more cost-effective and widely accessible.
Together, PET and SPECT have become essential tools in molecular oncology, contributing to early diagnosis, staging, patient stratification, and evaluation of therapeutic efficacy, while also playing a critical role in the development of personalized medicine [51] (Table 1).
A nanomolar dose of the radiolabeled imaging agent is administered intravenously, after which its biodistribution is dynamically monitored in vivo using PET or SPECT scanners. The emitted radiation is detected and quantitatively mapped to generate detailed spatial and temporal images of the tracer localization within the body Figure 4.
SPECT Imaging Mechanism
In Single Photon Emission Computed Tomography (SPECT), the administered radioisotope emits gamma (γ) photons that are detected by a gamma camera equipped with a 360° rotating array of photon detectors. This rotating detection system enables the acquisition of multiple planar projections around the patient, facilitating tomographic reconstruction of the spatial distribution of the radiotracer. The resultant three-dimensional images pinpoint the location and concentration of the radioisotope, thereby aiding in the accurate diagnosis of pathological conditions [50-53].
PET Imaging Mechanism
Positron Emission Tomography (PET) relies on the decay of positron-emitting radionuclides. Upon intravenous administration, the radiotracer emits positrons that travel a short distance-typically less than 2 mm-within the tissue before annihilating upon collision with electrons. This annihilation event produces two 511 keV photons emitted nearly simultaneously at approximately 180° to each other. These coincident photons are detected by a ring of detectors encircling the subject, allowing precise localization of the annihilation event and enabling high-resolution tomographic image reconstruction (Figure 5).
Radioisotopes Utilized in Nuclear Medicine
Radioisotopes are extensively employed in oncology for both diagnostic imaging and targeted radiotherapy, as well as in pharmaceutical development research. These isotopes can either be directly incorporated into specific molecular structures (e.g., ^18F in fluorodeoxyglucose (FDG), ^11C-labeled compounds) or coordinated within chelating ligands that stabilize radiometals under physiological conditions. The diversity of available radionuclides, spanning a wide range of half-lives and emission characteristics, allows for the tailored selection of isotopes based on the biological half-life of the targeting molecule, the imaging or therapeutic application, and radiation safety considerations [54].
Table 2 provides a comprehensive overview of commonly used radioisotopes in nuclear medicine, detailing their nuclear properties and clinical or research applications. This repertoire includes β^+, γ, and α emitters, each with unique advantages and limitations depending on the modality and therapeutic goal.
Magnetic Resonance Imaging (MRI)
MRI is a non-invasive imaging technique based on the principles of nuclear magnetic resonance (NMR), enabling detailed visualization of internal soft tissue structures through the detection of proton (primarily water) dynamics in a magnetic field. The technique employs a strong static magnetic field (typically 1.5–7 Tesla for clinical and research applications) to align hydrogen nuclei, generating a net magnetization along the longitudinal (z-) axis. Application of a radiofrequency (RF) pulse at the Larmor frequency perturbs this equilibrium, tipping the magnetization into the transverse (xy-) plane. Upon cessation of the RF pulse, the system undergoes relaxation, characterized by two distinct time constants: T1 (spin-lattice relaxation, recovery of longitudinal magnetization) and T2 (spin-spin relaxation, decay of transverse magnetization). By manipulating pulse sequence parameters (e.g., repetition time [TR], echo time [TE]), contrast between tissues can be enhanced based on differences in proton density, T1, or T2 relaxation times [53-57].
T1-weighted imaging emphasizes anatomical detail due to the rapid recovery of magnetization in fat-rich tissues, whereas T2-weighted imaging highlights pathological conditions (e.g., edema, tumors) where prolonged T2 relaxation yields hyperintense signals. Despite the inherent contrast provided by water protons, many clinical applications require exogenous contrast agents to amplify signal differences. Paramagnetic gadolinium (Gd³⁺) chelates are widely used to shorten T1, enhancing signal intensity in T1-weighted scans. Conversely, Superparamagnetic Iron Oxide Nanoparticles (SPIONs) induce local magnetic field inhomogeneities, reducing T2/T2* relaxation times and generating negative contrast in T2-weighted imaging [55-59].
MRI offers several advantages over other modalities, including:
i Absence of ionizing radiation
ii Superior soft-tissue contrast (exceeding CT resolution)
iii Multiplanar imaging capability (axial, sagittal, coronal views)
iv Functional and metabolic profiling (e.g., fMRI, diffusion-weighted imaging)
v Molecular imaging potential with targeted contrast agents However, limitations include relatively low sensitivity (μM–mM analyte concentrations), high operational costs, and safety concerns related to Gd-based agents (e.g., nephrogenic systemic fibrosis in renal-impaired patients). Ongoing advancements in high-field MRI (≥7T), hyperpolarized agents, and nanoparticle-based contrasts aim to address these challenges [33,58].
Optical Imaging
Optical imaging leverages non-ionizing radiation (visible to near-infrared [NIR] light) to probe cellular and molecular processes with high spatiotemporal resolution. Techniques such as fluorescence, bioluminescence, and absorption spectroscopy enable real-time visualization of biological events, often at subcellular scales. Key advantages include rapid acquisition, low cost, and high sensitivity (pM–nM detection limits), making it ideal for preclinical studies of nanomaterial–cell interactions [60]. Despite these benefits, optical imaging suffers from limited tissue penetration (≤1–2 cm) due to scattering and absorption by endogenous chromophores (e.g., hemoglobin, melanin). NIR fluorophores (650–900 nm) mitigate this by minimizing autofluorescence and improving depth penetration. Emerging technologies like photoacoustic imaging (combining optical excitation with ultrasonic detection) and Raman spectroscopy (vibrational fingerprinting) further enhance resolution and specificity [61]. Nanoparticle-based probes (e.g., quantum dots, gold nanorods) address photobleaching and low quantum yields of conventional dyes, enabling multiplexed and targeted imaging.
Multimodal Imaging
No single imaging modality provides comprehensive anatomical, functional, and molecular data. MRI and CT excel in spatial resolution but lack sensitivity; PET/SPECT offer nM-level sensitivity but poor resolution (1–6 mm); optical imaging is sensitive but depth-limited. Hybrid systems (e.g., PET/MRI, SPECT/CT) integrate complementary strengths, enabling simultaneous structural and metabolic profiling [60-64].
Challenges include:
i Hardware integration (e.g., MRI-compatible PET detectors)
ii Data co-registration algorithms
iii Multimodal contrast agents (e.g., Gd-labeled radiotracers, SPIONs with NIR dyes)
Nanoparticles (5–10 nm) are ideal platforms for multimodal probes due to their tunable surface chemistry, renal clearance, and capacity to co-load diverse imaging moieties (e.g., radionuclides, fluorophores). Their application in image-guided surgery (PET/optical) and theranostics (therapy + diagnostics) is an active research frontier [62-66].
Nanoparticles in Multimodal Imaging
While nanoparticles are clinically established in oncology (e.g., liposomal doxorubicin), their use in imaging remains experimental. Ideal imaging nanoparticles exhibit rapid tumor uptake and clearance (unlike sustained-release drug carriers), necessitating precise control over size (5–10 nm for renal excretion), surface charge, and targeting ligands (e.g., peptides, antibodies). Core-shell designs (e.g., iron oxide cores with silica coatings) allow modular integration of MRI, PET, and optical probes, enabling early detection of micrometastases [60,65].
Monomolecular Multimodal Platform in cancer theranosis
The majority of imaging scans utilize small molecule-based tracers, typically under 2,000 kDa and measuring approximately 1 nm, such as 18F-FDG for PET, iodinated small molecules for CT, and chelated gadolinium for MRI. Recently, peptide-based imaging agents have gained traction due to their specificity and relative ease of synthesis compared to larger proteins. This trend has led to the approval of specific agents for targeted cancer imaging, such as NETSPOT™ (68Ga-DOTATATE) for neuroendocrine tumors Figure 5.
While protein imaging agents, including radiolabeled monoclonal antibodies, are less common, they provide precise molecular information and are an expanding area of research. Additionally, nanoparticles represent a promising class of imaging agents capable of facilitating both anatomic and molecular imaging. The current thesis centers on developing a platform that integrates these various modalities, focusing on Monomolecular Multimodal Platforms (MOMIPs) at Chematech and ICMUB.
MOMIPs aim to create systems (conjugates/nanoparticles) that are straightforward to develop and characterize while maintaining multimodality. For instance, the traditional approach of modifying an antibody for PET-optical probe development involves stepwise conjugation-first with the PET probe and subsequently with the optical probe. This method can be complex, complicating the optimization of conjugation processes and the precise characterization of the resulting conjugates [66,67].
In contrast, the MOMIP approach offers a streamlined, onestep conjugation process that allows for precise characterization of the bioconjugate. This simplification is especially beneficial in the development and functionalization of nanoparticles, which are inherently complex to characterize. The goal of this platform is to provide researchers and clinicians with integrated tools that encompass diverse imaging modalities, ultimately enhancing cancer and disease management. This thesis seeks to leverage this approach to create multimodal tools for cancer therapy, applicable in developing small molecule-based imaging ligands or ligands suitable for nanoparticle synthesis or antibody bioconjugation with significant theranostic potential. Figure 5 illustrates this approach in an infographic format, showcasing the adaptable and versatile nature of the multimodal platform based on product requirements and feasible chemistry [68].
Chelators in Multimodal Platforms
The increasing diversity of radioisotopes in nuclear medicine has driven the need for novel chelators that ensure stability in terms of both thermodynamic stability and kinetic inertness. Different radiometal ions exhibit unique physical and chemical properties, including preferences for ligand donor atoms (such as N, O, S), size, oxidation state, coordination number, and geometry. Therefore, selecting the appropriate chelator that matches the characteristics of the chosen radioisotope is critical for achieving optimal in vivo stability [69].
Chelators are generally classified into two categories: linear (acyclic) and macrocyclic. This thesis focuses on key radiometals relevant for theranostic applications, including Gadolinium (Gd) for MRI and radiosensitization, Copper-64 (64Cu) for PET, and Indium-111 (111In) for SPECT. The discussion will be limited to chelators effective for complexing these specific radiometals. A comprehensive review of the chelators used in radiochemistry [70] (Table 3).
DTPA (diethylenetriaminepentaacetic acid) is one of the oldest and most widely utilized acyclic chelators in radiochemistry, known for its ability to radiolabel a variety of radiometal ions at room temperature within a matter of minutes. However, the stability of DTPA complexes in vivo is a significant concern, as they are generally less stable than those formed with macrocyclic chelators. This limitation has led to a gradual decline in the use of DTPA, with many researchers now favouring chelators such as DOTA and its derivatives [71].
Despite these challenges, DTPA has found successful applications, notably in the FDA-approved SPECT agent OctreoScan™ (111In-DTPA-octreotide), a somatostatin-targeting peptide conjugate used for imaging neuroendocrine tumors. Additionally, DTPA (Gd-complexed) based contrast agents for MRI have been approved and marketed under various brand names, as detailed in Table 4.
Moreover, the first-generation gold nanoparticles developed by our research group utilized a bifunctional chelator (BFC), specifically thiolated DTDTPA. These nanoparticles are currently undergoing upgrades to incorporate advanced BFCs based on DOTA and NOTA, enhancing their stability and efficacy in biomedical applications [72].
DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) is a highly versatile and widely utilized chelator, particularly valuable in magnetic resonance imaging (MRI). Numerous commercially available products are based on gadolinium complexes of DOTA in various forms, as detailed in Table I-4. In contrast, DTPA (diethylenetriaminepentaacetic acid) based contrast agents exhibit relatively lower stability and are more prone to releasing Gd3+ ions in vivo, which raises concerns regarding potential nephrotoxicity and the risk of Nephrogenic Systemic Fibrosis (NSF). Consequently, DOTA-based complexes are increasingly favored [73,74].
DOTA is considered the gold standard for certain radioisotopes, forming stable complexes with isotopes such as 111In, 177Lu, 86/90Y, 225Ac, and 44/47Sc. However, DOTA does not form very stable complexes with positron emission tomography (PET) isotopes like 64Cu and 68Ga due to geometric incompatibilities. This limitation can be addressed by using NOTA (1,4,7-triazacyclononane-1,4-diacetic acid), a hexadentate N3O3 chelator that has proven effective for 67/68Ga and 64Cu. NOTA is now regarded as the “gold standard” for chelation of 64Cu2+ and Ga3+, offering favorable radiolabeling conditions (room temperature, 30–60 minutes) and excellent in vivo stability [75,76].
Influence of Chelators on Theranostic Properties and Stability
The selection of an appropriate chelator-metal combination is crucial for the successful development of theranostic agents. The in vivo kinetic inertness of the metal-chelate complex is a primary consideration when determining the optimal pairing. Although thermodynamic stability constants (KML = [ML]/[M] [L]) can provide initial comparisons, they do not always predict in vivo stability. Alternative assessments, such as acid dissociation and competitive radiolabeling experiments, have been proposed, but these methods may not accurately reflect physiological environments. Challenge studies, which involve incubating the metal- chelate complex with ions like Na+, K+, Ca2+, Mg2+, Cu2+, or Fe3+, can identify trans-chelation instabilities. Additionally, EDTA challenge assays can simulate the presence of endogenous chelators. Ultimately, in vitro stability assessments in serum and in vivo studies focusing on biodistribution and pharmacokinetics are essential for validating the chosen combination [77,78].
While stability and inertness with a given radiometal (e.g., NOTA vs. DOTA for 68Ga) are important, they may not always translate to optimal performance for specific applications, such as particular peptide vectors. For example, although NOTA forms a more stable complex with 68Ga than DOTA, the differences in charge and physical properties (e.g., neutral vs. charged complexes) may enable DOTA to provide superior in vivo characteristics with certain vectors. This highlights the complex interplay of variables to consider when developing radiometal-based radiopharmaceuticals [79,80].
Moreover, the choice of chelator can significantly modulate the binding affinity of peptide-based radiopharmaceuticals. Research by Maecke, et al. emphasizes that “the chelate makes a difference” in peptide imaging agents. Their findings indicate that the combination of chelator and metal can substantially influence binding affinity and tumor localization in animal models [81].
In the context of multimodal applications, selecting the appropriate bifunctional chelator is critical for successful conjugation to multimodal assemblies. This requires the use of bifunctional chelators designed to preserve the properties of the base chelator. One strategy for achieving bifunctionality involves appending a side arm containing two carboxylate groups to the macrocyclic ring. For instance, incorporating a glutaric arm during macrocycle synthesis can maintain the original coordination sphere while providing a site for conjugation, as seen with DOTAGA and NODAGA. Thus, the chelator remains a vital component in the development of multimodal theranostic agents [82].
Fluorescent Probes for NIR-Based Optical Imaging
NIR optical imaging offers several advantages over visible light imaging, primarily due to its reduced potential for damaging biological tissues. NIR light is less likely to cause harm compared to ionizing radiation, and most biomolecules exhibit low absorption coefficients in this range, resulting in minimal background auto-fluorescence. This increased signal-to-noise ratio enhances detection sensitivity. Additionally, NIR light penetrates tissue more effectively due to lower light scattering and reduced absorption by background materials. Although various biological windows have been proposed for NIR imaging, the range between 650 and 1450 nm is widely accepted as optimal for most studies, as illustrated in Figure 6.

Figure 6: Absorption spectrum of the oxygenated whole blood and the biological diagnostic window defined from 650–1450 nm.
An ideal fluorescent probe should meet several critical criteria to ensure optimal performance in biological applications:
i Good Photostability: The probe must retain its fluorescence over time under light exposure.
ii High Quantum Yields: Efficient conversion of absorbed light into emitted fluorescence is essential.
iii High Molar Extinction Coefficients: This property enhances the probe’s ability to absorb light.
iv Large Stokes Shifts: A significant energy difference between the absorption and emission maxima minimizes reabsorption effects.
v Chemical Stability and Solubility: The probe should maintain stability in physiological media and demonstrate adequate solubility.
vi Low Toxicity and Minimal Aggregation: Safety for biological systems is paramount, along with a reduced tendency to aggregate, which can hinder performance.
Near-infrared (NIR) imaging can be achieved using probes with varied physicochemical properties, which can be broadly categorized into four families: 1) Organic fluorophores, 2) Fluorescent proteins, 3) Quantum dots (QDs), and 4) Lanthanide (III)-based complexes and nanomaterials. This thesis particularly focuses on the utilization of organic fluorophores for developing multimodal platforms [83].
Organic fluorophores offer significant advantages, such as customizability and the potential for integration with other systems, enhancing multimodal applications. Within the realm of or ganic NIR-emitting probes, three primary classes are recognized: BODIPYs, squaraines, and cyanines [84].
BODIPY fluorescent probes, derived from borondipyrromethane, are distinguished by their high quantum yields and sharply defined absorption and emission spectra. They exhibit excellent photostability and chemical resilience under physiological conditions. However, practical applications are often limited due to drawbacks such as low water solubility, small Stokes shifts, and comparatively low molar extinction coefficients (around 104 M<- sup>-1·cm-1). Additionally, many BODIPYbased probes emit in the visible to deep red range, which poses challenges for NIR imaging applications Table 5.
Squaraines are zwitterionic fluorophores characterized by an oxocyclobutenolate core, which is substituted with heterocyclic or aromatic groups at opposing ends. This unique structure renders the photophysical properties of squaraine dyes highly sensitive to their surrounding environment, making them promising candidates for the development of responsive fluorescent probes. Squaraines exhibit high molar extinction coefficients, typically on the order of 10^5 M⁻¹·cm⁻¹, particularly in the near-infrared (NIR) region, along with moderate quantum yields and photostability. However, they face limitations such as relatively small Stokes shifts and solubility issues. Additionally, their susceptibility to nucleophilic attack and fluorescence quenching due to aggregation further constrain their applicability [85].
Cyanine fluorophores consist of two heteroaromatic rings linked by a polymethine bridge. The excitation and emission wavelengths of these probes are significantly affected by the length of the polymethine bridge; longer bridges result in increased absorption and emission wavelengths. This property can also be fine-tuned by enhancing aromaticity, as evidenced by the transition from Cy5 to Cy5.5, or from Cy7 to Cy7.5. Cyanine dyes generally demonstrate large molar extinction coefficients in the range of 10^5 M⁻¹·cm⁻¹ and exhibit high fluorescence quantum yields, including values up to 30% in the NIR spectrum [86].
In our research group, we have extensively utilized Cy5 for the development of antibody bioconjugatable probes (unpublished data), and we plan to extend this expertise to ligands for multifunctional gold nanoparticles. Selecting the appropriate dye is critical for theranostic development, as various properties of fluorescent dyes-such as lipophilicity, solubility, and polarity-can significantly influence ligand affinity for targets and the overall performance of multimodal platforms. A thorough understanding of these variables is essential during the developmental phase. Notably, Bunschoten, et al. conducted a systematic evaluation of how chemical modifications of fluorescent dyes affect integrin-targeted bimodal probes. Their work introduced a matrix-based scoring system that highlighted the substantial impact of minor alterations in the chemical design of fluorescent labels. This methodology not only led to the optimization of an αvβ3-integrin-targeted hybrid tracer but also established a systematic framework for optimizing similar tracers Figure 7.
Research involving IR-783, a commercially available near-infrared (NIR) fluorescent dye, has been relatively limited compared to the extensively utilized Cy5. However, IR-783’s multifaceted nature and its significant implications across a range of biological responses- spanning from imaging to photothermal therapy-have garnered attention. Notably, IR-783 exhibits unique physicochemical and biological properties, functioning effectively as both an imaging and targeting agent. Studies have demonstrated that IR-783 and its derivatives preferentially accumulate in tumor cells rather than normal cells, a phenomenon observed across various cancer cell lines, tumor xenografts, spontaneous tumors in transgenic models, and human tumor samples. This selective uptake is primarily mediated by factors such as tumor hypoxia and the activity of Organic Anion- Transporting Polypeptides (OATPs). These diverse attributes position IR-783 as a promising candidate for developing multimodal diagnostic and therapeutic platforms [87].
Targeting Ligands for Cancer Therapy
Cancer cells express a distinct array of receptors on their surfaces, often at significantly higher levels than their normal counterparts. This variation arises from the increased growth and metabolic demands of rapidly proliferating tumor cells, enabling differentiation between cancerous and healthy cells. Such differences present opportunities for selective targeting of cancer cells for diagnostic and therapeutic purposes, forming the basis for active targeting strategies. Typically, these cancer cell receptors can be engaged by ligands, which may include small molecules (less than 500 Da), peptides, or proteins/monoclonal antibodies [88].
Small Molecular and Peptidic Ligands
Small molecule ligands offer a versatile option for vectorizing various tracers, including small chemicals, macromolecules, and nanoparticles. The development of targeted tracers for theranostic applications based on these ligands has been a focal area of research in our group in Dijon. Numerous chelators and fluorescent dyes have been conjugated, either individually or in combination, with different targeting ligands, such as PSMA ligands, cRGDfk, folic acid, bombesin, and CXCR4 ligands, among others. A brief overview of the most widely utilized ligands is warranted in this context Figure 8.

Figure 8: Commonly used targeting ligands in targeted cancer therapeutics and diagnostics (Blue is the site of conjugation).
Folic Acid as a Targeting Ligand in Cancer Therapy
Folic acid (FA) is increasingly recognized for its role as a ligand in the selective targeting and delivery of macromolecular drugs to tumor cells via membrane-bound folate receptors. These glycosylphosphatidylinositol- linked membrane proteins bind ligands from the extracellular environment and facilitate their internalization through a non-destructive, recycling endosomal pathway. The overexpression of folate receptors (FRs) across a variety of cancer cells positions them as promising targets for ligand- and antibody-directed cancer theranostics. Notably, FR-α is frequently overexpressed in several tumor types, including ovarian, brain, kidney, breast, myeloid, and lung cancers, with receptor density often correlating with cancer stage and grade [89,90].
The capacity of folic acid to internalize ligands makes it particularly suitable for targeted delivery of anti-cancer therapeutics. The significance of FA in cancer research is underscored by the evaluation of five FA-based small molecule drug conjugates in clinical trials. The European Medicines Agency (EMA) approved ‘vintafolide’ and ‘etarfolide’ for use in women with platinum-resistant ovarian cancer; however, the sponsor, Endocyte, subsequently withdrew both applications due to challenges in conducting further clinical investigations. Vintafolide is a folate-conjugated form of vinblastine, while etarfolide serves as a companion diagnostic utilizing SPECT imaging to assess folate receptor expression in patients. Despite these setbacks, there remains significant interest in the development of folate-targeted therapeutics and diagnostics, with the potential for future approval of folate-based tools [90-93].
RGD Peptides in Tumor Angiogenesis
As tumors exceed a critical size (approximately 1 mm³), diffusion alone becomes insufficient to meet the oxygen and nutrient demands of tumor cells. To address this deficiency, tumors initiate angiogenesis, characterized by the formation of new blood vessels. This process is a hallmark of tumor growth and presents valuable targets for both imaging and therapeutic interventions. Key targets in angiogenesis include integrins, particularly αvβ3, and vascular endothelial growth factor (VEGF), both of which have been leveraged for cancer therapy and diagnostics. Cilengitide, an integrin inhibitor targeting αvβ3 and αvβ5, has been approved for anti-angiogenic therapy, while the monoclonal antibody bevacizumab (Avastin) blocks VEGF.
Integrin αvβ3 serves as a cell adhesion receptor that is predominantly expressed in tumor vasculature and some tumor cells. Its affinity for proteins and peptides containing the arginine-glycine- aspartate (RGD) sequence renders it an important tumor marker. RGD conjugates can act as imaging tracers due to their receptor- mediated binding, facilitating preferential accumulation in tumor vasculature. Both linear and cyclic RGD peptides are being utilized as targeting vectors for αvβ3-targeted imaging tracers; however, linear RGD peptides exhibit low binding affinity (IC50 > 100 nM), lack specificity, and are prone to rapid degradation by serum proteases. In contrast, cyclic RGD peptides demonstrate enhanced integrin binding affinity, particularly when cyclized with linkers such as disulfides, thioethers, or aromatic rings. This has led to an increased emphasis on peptides like c(RGDfk) for the development of RGD-targeted tracers and nanoparticles in cancer theranostics [94-97].
Prostate-Specific Membrane Antigen (PSMA) and its Implications in Prostate Cancer
The LNCaP human prostate cancer cell line was the first to demonstrate the presence of prostate-specific membrane antigen (PSMA), a type II integral membrane glycoprotein. Elevated expression of PSMA is commonly observed in prostate cancer tissues compared to benign prostatic tissues, while its expression is notably low in non-target tissues. This differential expression pattern makes PSMA a promising target for both diagnostic and therapeutic applications. Research has focused on developing monoclonal antibody-based ligands, which led to the approval of capromab pendetide (ProstaScint®) for Single-Photon Emission Computed Tomography (SPECT) imaging. Despite the advantages of monoclonal antibodies, their inherent limitations have prompted ongoing interest in the development of small-molecule ligands [98].
Current investigations have identified various chemical classes of PSMA ligands, including phosphorous-based (phosphonates, phosphates, and phosphoramidates), thiol-based, carbamate-based, and urea-based compounds. While phosphorous-based ligands exhibit nanomolar inhibitory potency, their high polarity and suboptimal pharmacokinetic profiles hinder clinical application. Thiol- based and carbamate ligands present alternative options due to improved membrane permeability and oral bioavailability; however, they face challenges related to metabolic stability and selectivity. To address these issues, novel urea-based PSMA ligands have been synthesized, with particular focus on those incorporating the Gluurea- Lys binding motif. This motif has yielded promising lead candidates that combine effective radiolabeling, chelation, and linker strategies. Given the advantageous properties of Glu-urea-Lys and our group’s extensive experience in developing related imaging agents, it is positioned as an ideal targeting ligand for multimodal theranostics in prostate cancer [99].
Monoclonal Antibodies in Cancer Treatment
Monoclonal antibodies (mAbs) have significantly advanced immunotherapy, transforming cancer treatment paradigms. By specifically targeting receptors on cancer cells, mAbs initiate processes such as antibody-dependent cellular cytotoxicity (ADCC), leading to tumor cell apoptosis. In addition to serving as standalone therapies, mAbs can act as delivery vehicles for potent anticancer agents (e.g., maytansinoids), radioisotopes (e.g., ^177Lu for brachytherapy), and imaging tracers. The effective use of mAbs for targeted delivery necessitates the application of chemical biology techniques for the conjugation of therapeutic agents. Our research group in Dijon is particularly focused on the application of monoclonal antibodies for imaging purposes. The selection of appropriate targets is critical, as mAbs can bind to various receptors on cancer cells, including HER-2, EGFR, CD20, and CD30, among others [100]. A summary of approved mAbs and their associated tumor antigens is provided in Table 6.
How does the targeting using small ligand differ from the antibody-based targeting?
The use of targeting moieties underpins the principles of active targeting in theranostics. A key consideration for researchers is whether to select small molecule ligands or antibodies based on the specific disease and its target. This decision is influenced by the unique characteristics of each option. Monoclonal antibodies typically exhibit high specificity for receptors expressed on cancer cells, making them a strong candidate for targeting. However, the repertoire of small molecule ligands that are specific to certain receptors is limited. One significant distinction between antibodies and small molecules lies in their pharmacokinetic properties. Monoclonal antibodies have a longer circulation half-life, ranging from 3 to 4 days, compared to hours for small molecules. This is primarily due to the rapid excretion of small molecules from the body, which is not the case for larger, macromolecular antibodies, except in instances of high protein binding. The ability of a ligand to penetrate cancerous tissue effectively favors small molecules, as their smaller size allows for quicker diffusion. In the development of targeted nanoparticles, it is crucial to consider how conjugation with either antibodies or chemical ligands impacts nanoparticle properties and pharmacokinetics [101-105]. The limitations of organic solvents in nanoparticle synthesis can further restrict the choice of antibodies for use. Additionally, antibodies can be more costly to produce compared to small molecule ligands.
Thus, while small chemical ligands may be more suitable for imaging and theranostic applications, emerging antibody formats that are less bulky may help mitigate the challenges faced with conventional antibodies. In some cases, employing antibodies or their fragments may be necessary when corresponding small molecule ligands are unavailable or underdeveloped [106-109].
Conjugation Site and Chemistry
Effective conjugation chemistry serves as the foundation for attaching multimodal functionality to biological macromolecules (antibodies, antibody fragments) or nanoparticles. As illustrated in Figure 9, current bioconjugation approaches primarily target nucleophilic sites on these substrates. Native lysine residues provide abundant amine groups for modification, while engineered or reduced cysteine residues offer selective thiol handles. Similarly, nanoparticle surfaces can be functionalized with amine groups (e.g., AGuIX) or thiolated using reagents like 2-iminothiolane (Traut’s reagent). Successful bioconjugation requires optimization of reaction stoichiometry and maintenance of mild aqueous conditions to preserve biomolecular integrity while achieving efficient coupling [110].
Gold nanoparticle functionalization predominantly exploits the robust thiol-gold interaction (40-50 kcal/mol binding energy), which forms stable Au-S bonds regardless of the thiol compound’s aliphatic or aromatic nature [78]. This reliable chemistry enables precise surface engineering with multifunctional thiolated ligands. Amide bond formation remains a cornerstone of bioconjugation, typically achieved through N-hydroxysuccinimide (NHS) ester chemistry. These activated esters, either pre-formed or generated in situ using coupling agents like TSTU or carbodiimide/NHS, react selectively with primary amines at pH 7.2-9 to form stable amide linkages. The commercial availability of NHS-activated fluorescent dyes, chelators, and linker molecules has made this approach particularly versatile. Alternative amine-targeting strategies employ isocyanate or isothiocyanate groups. While isocyanates are primarily limited to organic phase polymer chemistry due to their hydrolytic instability, isothiocyanates demonstrate superior aqueous stability.
These groups react efficiently with amines at pH ≥9, though such alkaline conditions may compromise some sensitive biomolecules. Similarly, hydrazine/aminooxy groups enable selective conjugation through oxime ligation or hydrazone formation with carbonyl compounds [79].
Thiol-based conjugation offers exceptional versatility due to the enhanced nucleophilicity of the thiolate anion (pKa ~8.5) compared to corresponding alcohols. This property enables diverse reactions including:
i Nucleophilic additions to α-halocarbonyls
ii Michael additions to maleimides or vinyl derivatives
iii Displacement reactions with perfluoroaromatics
iv Cycloadditions with 2-cyanobenzothiazoles or phenyloxadiazole sulfones [80]
These thiol-specific reactions provide powerful tools for site-selective bioconjugation while maintaining excellent reaction kinetics under physiological conditions Figure 9.
Maleimide-thiol chemistry remains the gold standard for bioconjugation since its initial development, representing the most widely employed Michael addition reaction in biomolecular modification. This reaction demonstrates optimal efficiency under mildly acidic to neutral conditions (pH 6.5-7.5), as alkaline environments (pH >8) promote competing hydrolysis reactions that generate unreactive maleamic acid derivatives while also risking unwanted amine conjugation [110-113]. Our research leverages both halo- substitution and maleimide-based Michael addition chemistry to develop multifunctional theranostic platforms [114-118].
Click chemistry has revolutionized bioconjugation strategies across diverse fields including pharmaceutical development, polymer science, and nanomedicine. Its exceptional efficiency makes it particularly valuable for time-sensitive applications such as protein modification and radiochemistry, where reaction speed must accommodate biomolecular stability and radionuclide half-life constraints. Three principals click reaction classes dominate current applications:
i Copper-catalyzed azide-alkyne cycloaddition (CuAAC) - forms stable 1,2,3-triazole linkages with minimal steric perturbation
ii Strain-promoted azide-alkyne cycloaddition (SPAAC) - avoids copper catalysts but introduces hydrophobic benzocyclooctatriazole moieties
iii Inverse electron-demand Diels-Alder (iEDDA) - offers ultra- fast kinetics (k > 800 M⁻¹s⁻¹) via tetrazine/trans-cyclooctene coupling [117-121]
While iEDDA represents the fastest bioorthogonal reaction known, its application is constrained by the substantial [6.4.0] bicyclic adduct formed during conjugation. Solubility challenges with click reagents (alkynes, DBCO, tetrazines) in aqueous systems can be mitigated through PEGylation strategies. The commercial availability of diverse click chemistry toolkits continues to expand their utility in bioconjugation [122-126].
Surface functionalization via silanization chemistry enables precise modification of silica-based nanomaterials. APTES (3-aminopropyltriethoxysilane) serves as the benchmark reagent for generating amine-terminated surfaces through complex condensation reactions forming Si-O-Si networks [90, 91]. This approach has been successfully adapted for engineering iron oxide and silica nanoparticles, including the functionalization of AGuIX nanoparticles with chelating moieties. Our work extends this methodology to create multifunctional theranostic agents through controlled surface modification [126-129].
Key advantages of these conjugation strategies include:
i High reaction specificity under physiological conditions
ii Compatibility with sensitive biomolecules
iii Tunable surface chemistries for multifunctionalization
iv Scalability for clinical translation
These advanced bioconjugation techniques provide the foundation for developing next-generation theranostic platforms with precisely controlled surface functionalities and multimodal capabilities [130-132].
The multimodal platform has been utilized to synthesize customized chemical entities applicable across a wide range of systems, as illustrated in Figure 10. This approach effectively employs precursors for the functionalization of AGuIX nanoparticles, highlighting their physicochemical and radiochemical properties. Investigations using the TSA tumor model further emphasize the relevance of these platforms in theranostics. The study encompasses ligands tailored for PET, PET-MRI, and PET-optical imaging, as well as PSMA-targeted ligands for SPECT imaging. Given that gold is a high atomic number (Z) element, the nanoparticles developed with these ligands are particularly suitable for radiosensitizing applications, thereby creating advanced theranostic tools [131-134].

Figure 10: Application of the monomolecular multimodal platform towards development of theranostics.
The focus is specifically on tools beneficial for prostate cancer management, particularly the development of small molecule-based PSMA-targeted bimodal (PET-optical) imaging agents. The research not only includes the synthesis of these probes but also evaluates their suitability regarding photophysical, binding, and radiochemical characteristics. This analysis is supported by systematic characterization and testing in relevant animal models. These objectives align well with the expertise and research priorities at Chematech/ ICMUB, as well as the overarching aims of the ARGENT program, which seeks to enhance processes and technologies for managing breast, prostate, and brain cancers [132-135].
Conclusion
The development of multifunctional platforms for cancer theranostics represents a significant leap forward in precision medicine, integrating diagnostics and therapy into a unified approach. This research underscores the potential of nanoparticles, particularly gold (Au) and gadolinium (Gd)-based systems, as versatile tools for imaging and radiosensitization. By leveraging the enhanced permeability and retention (EPR) effect and active targeting strategies, these platforms enhance tumor specificity while minimizing off-target toxicity-a critical advancement over conventional therapies. Key findings highlight the importance of multimodal imaging, where PET, SPECT, MRI, and optical techniques synergize to provide comprehensive disease characterization. The combination of high-sensitivity nuclear imaging (PET/SPECT) with high-resolution MRI addresses the limitations of individual modalities, enabling more accurate tumor localization and treatment monitoring. Additionally, the integration of Near-Infrared (NIR) fluorescent probes, such as cyanine dyes, offers real-time intraoperative guidance, further refining surgical and therapeutic interventions. The selection of targeting ligands-ranging from small molecules (e.g., folic acid, RGD peptides) to monoclonal antibodies-plays a pivotal role in enhancing tumor accumulation. Small-molecule ligands, with their rapid pharmacokinetics and deep tissue penetration, are particularly advantageous for imaging applications, while antibody- based strategies provide high specificity for therapeutic delivery. Chelators like DOTA and NOTA ensure the stable complexation of radiometals, optimizing both diagnostic and therapeutic efficacy.
Conjugation chemistry remains a cornerstone of theranostic platform development. Innovations in bioconjugation, including maleimide-thiol coupling and click chemistry, enable precise functionalization of nanoparticles and biomolecules, ensuring reproducible and scalable synthesis. Furthermore, surface modifications, such as silanization, enhance nanoparticle biocompatibility and targeting efficiency. Despite these advancements, challenges persist, including variability in EPR effects among patients, potential long-term toxicity of inorganic nanoparticles, and the need for standardized protocols for clinical translation. Future research should focus on optimizing nanoparticle pharmacokinetics, improving tumor penetration, and developing next-generation ligands with higher affinity and selectivity. Additionally, theranostic platforms must undergo rigorous preclinical and clinical validation to ensure safety and efficacy across diverse cancer types.
This work demonstrates the transformative potential of multifunctional theranostic platforms in oncology. By combining advanced imaging, targeted therapy, and innovative nanomaterials, these systems pave the way for personalized cancer treatment with improved precision and reduced side effects. Continued interdisciplinary collaboration among chemists, biologists, and clinicians will be essential to overcome existing challenges and accelerate the translation of these technologies into clinical practice. Ultimately, theranostics holds promise not only for cancer but also for other complex diseases, heralding a new era of integrated diagnostic and therapeutic solutions.
Acknowledgement
None.
Conflict of Interest
None.
References
- Thallaj N (2021) Synthesis of a New Ligand Tris (2-pyridylmethyl) amine functionalized by a methoxy group and study of Dichloroferrous complexes, its reactivity to dioxygen both in the presence and absence of substrate. International journal of applied chemistry and biological sciences 2(4): 65-77.
- L Labban, N Thallaj, Z Malek (2020) International Journal of Medical Studies 5(12): 23-36.
- L Labban, M Kudsi, Z Malek, N Thallaj (2020) Pain Relieving Properties of Ginger (Z. officinale) and Echinacea (E.angustifolia) Extracts Supplementation among Female Patients with Osteoarthritis. A Randomized Study. Advances in Medical, Dental and Health Sciences 3(3): 45-48.
- L Labban, N Thallaj, M Al Masri (2020) Journal of Advanced Research in Food Science and Nutrition 3(1): 34-41.
- Thallaj N, agha M I H, nattouf AH, katib CH, karaali A et al. (2020) Evaluation of Antimicrobial Activities and Bioactive Compounds of Different Extracts Related to Syrian Traditional Products of Damask Rose (Rosa damascena). open access library journal 7(5): 1-21.
- L labban, N Thallaj, A labban (2020) Archives of Medicine 12(2): 1-5.
- L labban, N Thallaj (2020) International Journal of Herbal Medicine 8(2): 33-37.
- L Labban, N Thallaj, Z Malek (2019) The implications of E-cigarettes or vapingon the nutritional status. Journal of Medical Research and Health Sciences 2(11): 784-787.
- L labban, N Thallaj (2019) The Effect of Magnesium Supplementation on Hba1c Level and Lipid Profile Among Type 2 Diabetics. Acta Scientific Nutritional Health 3(10): 7-12.
- Malek ZS, Labban L (2020) The International Journal of Neuroscience 1-7.
- Malek ZS, Labban L (2019) A Comparative Study of Tryptophan Hydroxylase's Circadian Rhythm in the Functional Parts of Dorsal Raphe Nuclei in the Mesencephalon European Journal of Pharmaceutical and Medical Research 6(11): 527-532.
- Malek ZS (2018) Journal of AlBaath University 40(4): 39-62.
- Malek ZS (2018) Analytical Study for the Determination of Norfloxacin in Pure and Pharmaceutical Formulations using Alizarin by Visible Spectrophotometric Method. Tishreen University Journal for Research and Scientific Studies 40(2).
- ZS Malek, LM Labban (2021) Photoperiod regulates the daily profiles of tryptophan hydroxylase-2 gene expression the raphe nuclei of rats. International Journal of Neuroscience 131(12): 1155-1161.
- ZS Malek, LM Labban (2020) Photoperiod regulates the daily profiles of Tryptophan Hydroxylase-2 gene expression the raphe nuclei of rats. Journal of current research in physiology and pharmacology 4(1): 1-5.
- LM Labban, MM Alshishkli, A Alkhalaf, Z Malek (2017) J Adv Res Dent Oral Health 2(3&4): 1-4.
- L Labban, ZS Malek (2018) Open Access Library Journal 5(07): 1-11.
- L Labban, ZS Malek (2019) Ann Food Nutr Res J 1: 1
- Labban L, N Thallaj (2019) Acta Scient Nutr Health 3: 7-12.
- N Thallaj (2022) Tishreen university journal 44(1): 59-77.
- N Thallaj (2022) Tishreen university journal 44(2): 87-105.
- A Abbood, N Thallaj (2023) Arab Journal of Pharmaceutical Sciences 7(1).
- N Thallaj (2023) Tishreen University Journal-Medical Sciences Series 44(6): 21-29.
- Machkour A, Thallaj NK, Benhamou L, Lachkar M, Mandon D (2006) The coordination chemistry of FeCl3 and FeCl2 to bis[2-(2,3-dihydroxyphenyl)-6-pyridylmethyl] (2-pyridylmethyl) amine: access to a diiron (III) compound with an unusual pentagonal-bipyramidal/square-pyramidal environment. Chemistry 12(25): 6660-
- Thallaj N, Machkour A, Mandon D, Welter R (2005) Square pyramidal geometry around the metal and tridentate coordination mode of the tripod in the [6-(3′-cyanophenyl)-2-pyridylmethyl] bis(2-pyridylmethyl) amine FeCl2 complex: a solid-state effect New J Chem 29: 1555 - 1558.
- Thallaj NK, Rotthaus O, Benhamou L, Humbert N, Elhabiri M (2008) Reactivity of molecular dioxygen towards a series of isostructural dichloroiron(III) complexes with tripodal tetraamine ligands: general access to mu-oxodiiron(III) complexes and effect of alpha-fluorination on the reaction kinetics. Chemistry-A European Journal 14(22): 6742-6753.
- Wane A, Thallaj NK, Mandon D (2009) Biomimetic Interaction between FeII and O2: Effect of the Second Coordination Sphere on O2 Binding to FeII Complexes: Evidence of Coordination at the Metal Centre by a Dissociative Mechanism in the Formation of μ-Oxo Diferric Complexes. Chemistry 15(40): 10593-10602.
- Thallaj NK, Orain PY, Thibon A, Sandroni M, Welter R (2014) Steric congestion at, and proximity to, a ferrous center leads to hydration of α-nitrile substituents forming coordinated carboxamides. Inorg Chem53(15): 7824-7836.
- NK Thallaj, J Przybilla, R Welter, D Mandon (2008) A Ferrous Center as Reaction Site for Hydration of a Nitrile Group into a Carboxamide in Mild Conditions. J Am Chem Soc 130: 2414-2415.
- NK Thallaj, D Mandon, KA White (2007) The Design of Metal Chelates with a Biologically Related Redox-Active Part: Conjugation of Riboflavin to Bis(2-pyridylmethyl) amine Ligand and Preparation of a Ferric Complex. Eur J of Inorg Chem 2007: 44-47.
- Thallaj N (2021) Synthesis of a New Ligand Tris (2-pyridylmethyl) amine functionalized by a methoxy group and study of Dichloroferrous complexes, its reactivity to dioxygen both in the presence and absence of substrate. International journal of applied chemistry and biological sciences 2(4): 65-77.
- Thallaj N (2023) Review of a Few Selected Examples of Intermolecular Dioxygenases Involving Molecular Oxygen and Non-Heme Iron Proteins. Int J Adv Parmacutical Sci Res (IJAPSR) 3: 1-18.
- L Labban, M Kudsi, Z Malek, N Thallaj (2020) Pain Relieving Properties of Ginger (Z. officinale) and Echinacea (E. angustifolia) Extracts Supplementation among Female Patients with Osteoarthritis. A Randomized Study. Advances in Medical, Dental and Health Sciences 3(3): 45-48.
- L Labban, N Thallaj, M Al Masri (2020) The Nutritional Value of Traditional Syrian Sweets and Their Calorie Density. Journal of Advanced Research in Food Science and Nutrition 3(1): 34-41.
- L labban, N Thallaj, A labban (2020) Assessing the Level of Awareness and Knowledge of COVID 19 Pandemic among Syrians. archives of medicine 12(2): 1-5.
- L Labban, N Thallaj, Z Malek (2019) The implications of E-cigarettes or vaping on the nutritional status. Journal of Medical Research and Health Sciences 2(11): 784-787.
- Malek ZS, Sage D, Pevet P, Raison S (2007) Daily Rhythm of Tryptophan Hydroxylase-2 Messenger Ribonucleic Acid within Raphe Neurons Is Induced by Corticoid Daily Surge and Modulated by Enhanced Locomotor Activity. Endocrinology 148 (11): 5165-5173.
- Malek ZS, Dardente H, Pevet P, Raison S (2005) Tissue-specific expression of tryptophan hydroxylase mRNAs in the rat midbrain: anatomical evidence and daily profiles. European Journal of Neuroscience 2005 22(4): 895-901.
- A Abbood, SA Malik, D aldiab, HH Ali, N Thallaj (2025) Investigation of the charge variant profile of non-cleavable conjugated antibodies. Research J Pharm and Tech 18(1): 185-190.
- Malek S, Pevet P, Raison S (2004) Neuroscience 125(3): 749-758.
- Malek ZS, Labban L (2020) The International Journal of Neuroscience :1-7.
- ZS Malek, LM Labban (2020) Journal of current research in physiology and pharmacology 4(1): 1-5.
- LM Labban, MM Alshishkli, A Alkhalaf, Z Malek (2017) J Adv Res Dent Oral Health 2(3&4): 1-4.
- L Labban, ZS Malek, Open Access Library Journal, 2018, 5 (07), 1-11.
- Y alhomush, Z malek, A Abboud, N Thallaj (2022) Research Journal of Pharmacy and Technology 15: 10.
- A Abbood, Z Malek, N Thallaj (2022) Research Journal of Pharmacy and Technology 15(11): 4935-4939.
- Thallaj N, agha MIH, nattouf AH, katib CH, karaali A, Moustapha A, et al. (2020) open access library journal 7(5): 1-21.
- N Thallaj (2021) Indian journal of advanced chemistry 1(2): 20-26.
- N Thallaj (2022) Indian journal of advanced chemistry 2(2): 1-11.
- N Thallaj (2022) Indian journal of advanced chemistry 2(1): 5-9.
- N Thallaj (2022) Indian journal of advanced chemistry 2(1): 10-14.
- N Thallaj, Xi'an ShiyouDaxueXuebao (ZiranKexue Ban) (2022) Journal of Xi'an Shiyou University, Natural Sciences Edition 65(06): 289-301.
- N Thallaj, Xi'an ShiyouDaxueXuebao (ZiranKexue Ban) (2022) Journal of Xi'an Shiyou University, Natural Sciences Edition 65(06): 313-328.
- Z MALEK, A ABBOOD, N THALLAJ, Xi'an ShiyouDaxueXuebao (ZiranKexue Ban) (2022) Journal of Xi'an Shiyou University, Natural Sciences Edition 65(06): 302-312.
- N Thallaj, Xi'an Shiyou Daxue Xuebao (Ziran Kexue Ban) (2022) Journal of Xi'an Shiyou University, Natural Sciences Edition 65(7): 169-184.
- Z MALEK, Xi'an Shiyou Daxue Xuebao (Ziran Kexue Ban) (2022) Journal of Xi'an Shiyou University, Natural Sciences Edition 65(7): 143-152.
- Thallaj, Xi'an Shiyou Daxue Xuebao (Ziran Kexue Ban) (2022) Journal of Xi'an Shiyou University, Natural Sciences Edition 65(7): 110-142.
- N Thallaj (2023) Tishreen University Journal-Medical Sciences Series 44(6): 21-29.
- N Thallaj (2022) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 2(3): 1-28.
- N Thallaj (2022) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 2(4): 1-15.
- N Thallaj (2023) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 3(2): 1-18.
- N Thallaj (2022) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 2(6): 1-12.
- N Thallaj (2023) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 3(3): 1-10.
- N Thallaj (2024) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(1): 32-52.
- N Thallaj (2024) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(5): 29-49.
- N Thallaj (2024) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(4): 7-21.
- N Thallaj. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 2024.4, 6,7-27.
- N Thallaj (2024) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR)4(6): 33-48.
- Besherb S, Alallan L, Hassan Agha MA, AIshamas I, Thallaj N, et al. (2024) Influence of soil salinity on the chemical composition of essential oil of Rosmarinus Officinalis in Syria, Research J. Pharm. and Tech 17(5).
- Thallaj N (2024) Advancements in Pharmaceutical Science: Synthesis and Application of Molecular Cages Integrating N-Heterocyclic Carbenes for Enhanced Stability and Functionality. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 5(1): 6-19.
- Ayat Abbood, Hassan Hadi Ali, Samir Azzat Malik, Dima AlDiab, Nasser Thallaj, et al. (2025) Investigation of the Charge Variant Profile of Non-cleavable Conjugated Antibodies. Research Journal of Pharmacy and Technology 18(1): 185-190.
- Thallaj N (2025) Analyzing Charge Variant Profiles of Monoclonal Antibodies Conjugated to Cytotoxic Agents. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(3): 20-26.
- Thallaj N (2025) Biomimetic Synthesis and Phytochemical Analysis of Lodopyridone: Insights into 4-Pyridone Derivatives and Thiopeptide Antibiotic. Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(3): 9-19.
- Mousa Al Khleif, Nour Alhuda Alzoubi, Ghassan Shannan, Zeina S Malek, Nasser Thallaj (2025) Exploring Circadian Rhythms:A Comprehensive Study on Sleep Patterns and Disorders in Syrian Society. Am J Biomed Sci & Res 26(2).
- Ranim Abdul Rahman, Louai Alallan, Ahmed Khalid Aldhalmi, Nasser Thallaj (2025) Separation, Determination, and Potential Application of Active Compounds in Porosopis cineraria: A Comprehensive Analysis. Research Journal of Pharmacy and Technology 18(4):1604-1610.
- Dalia Aboufakher, Rita Zeinaldin, Racha Khatib, Rawa Khreit, Mohamed Sami Joha, et al. (2025) Prevalence and AntibioticResistance Patterns of Multidrug-Resistant Gram-Negative Bacilli in Hospitalized Patients in Sweida, Syria. Am J Biomed Sci & Res. 2025 26(3): 309-315
- Besher S, Alallan L, Hasan Agha MI, Alshamaa I, Thallaj N (2024) Influence of Soil Salinity on the Chemical Composition of Essential Oil of Rosmarinus officinalis in Syria. Research Journal of Pharmacy and Technology 17(5): 2282- 2288.
- Khatib O, Alshimale T, Alsaadi A, Thallaj N (2024) The Global Impact of HIV: A Comprehensive Review. IJAPSR 4(3): 6-19.
- Samer alkhoury, Rasha Kateeb, Rawa Akasha and Nasser Thallaj (2025) Analysis of Crocin Content in Saffron (Crocuss ativus L) Cultivated in Syria Using Liquid Chromatography-Mass Spectrometry. Am J Biomed Sci & Res. 2025 26(3).
- Zanboua R, Abbood A (2024) Survey of Knowledge About the Interaction Between Food and Drugs Among the Syrian Population. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(4): 22-28.
- Qattan M, Dashash M, S Malek Z (2024) Enhancing Academic Success: A mixed Study on the Influencing Factors among Pharmacy Students in Syrian Universities. F1000Res 13: 868.
- Thallaj N (2022) Design and Synthesis Ligands Tetradents Substituted with Halogenes in α- Position and Conjugation with Riboflavin (Bioconjugates): Conjugate ligands Type TPA’s with Flavonoids as un–Electron Mediator. Biomedicine and Chemical Sciences, 1(2): 47-56.
- Thallaj N, Alrasho JF, Sofi FK (2024) Advancements in Antiviral Therapeutics: A Comprehensive Review of Hepatitis C Virus and Novel Flavone Leads. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 5(1): 28-40.
- Thallaj N (2025) Analyzing Charge Variant Profiles of Monoclonal Antibodies Conjugated to Cytotoxic Agents. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 5(3): 20-26.
- Thallaj N (2025) Biomimetic Synthesis and Phytochemical Analysis of Lodopyridone: Insights into 4-Pyridone Derivatives and Thiopeptide Antibiotics. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 5(3): 9-19.
- Ghassan Shannan, Zeina S (2025) Malek and Nasser Thallaj. Pactamycin: A Comprehensive Review of Its Biological Activity, Biosynthesis, And Synthetic Strategies in the Fight Against Antibiotic Resistance. European Journal of Biomedical and Pharmaceutical Sciences (EJBPS) 12(4): 334-353.
- Ghassan Shannan, Zeina S (2025) Malek and Nasser Thallaj. A Review of Antibiotic-Induced Drug Allergies: Mechanisms, Prevalence, And Future Perspectives. European Journal of Biomedical and Pharmaceutical Sciences (EJBPS) 12(4): 387-399.
- Alma Soudan, Joudy Abokassem, Shaam Ammar and Nasser Thallaj (2025) Advancements in the Total Synthesis of Cyclotripeptidic Natural Products: Exploring the Therapeutic Potential Of Marine-Derived Compounds. European Journal of Biomedical and Pharmaceutical Sciences (EJBPS) 12(4): 354-377.
- Labban L, Thallaj N, Labban A (2020) Assessing the level of awareness and knowledge of COVID 19 pandemic among syrians. Arch Med 12(2): 8.
- Labban L, Kudsi M, Malek Z, Thallaj N (2020) Advances in Medical. Dental and Health Sciences 3(3): 45-48.
- Thallaj N (2021) Efficiency in transporting molecular oxygen to iron (II) complexes with ligands type tri (2-pyridylmethyl) amine substitution aromatic in (α) position by a mechanism that mimics biological oxidation. Journal homepage: ijrpr. com ISSN, 2582, 7421.
- Labban L (2020) International Journ. IJMS 5(12): 23-36.
- Thallaj N (2022) Tishreen University Journal-Medical Sciences Series 44(1): 59-77.
- Thallaj N (2020) Evaluation of antimicrobial activities and bioactive compounds of different extracts related to syrian traditional products of damask rose (Rosa damascena). Open Access Library Journal 7(05): 1.
- Thallaj N (2023) Characterization of charge heterogeneity of antibody-drug conjugate by anion-exchange chromatofocusing. Tishreen University Journal-Medical Sciences Series 44(6): 21-29.
- Labban L, M Kudsi, Z Malek, N Thallaj (2020) Advances in Medical, Dental and Health Sciences 3(3): 45-48.
- Thallaj N (2023) A Brief Overview of the General Characteristics and Reactivity Towards Dioxygen of the Ferrous Tris (2-Pyridylmethyl Amine) Series Complexes is Presented. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 3(3): 1-18.
- Abbood A, Thallaj N (2023) Comparison between chromatofocusing and icIEF charge variant profiles of unconjugated monoclonal antibodies and their drug conjugates. Arab Journal of Pharmaceutical Sciences 7(1).
- Thallaj N (2024) Detecting Antioxidant Behavior for Phenolic Content of Some Beauty Care Creams in Syrian Market. Indian Journal of Advanced Chemistry 2(1): 10-14.
- Thallaj N (2024) Advancements in Pharmaceutical Science: Synthesis and Application of Molecular Cages Integrating N-Heterocyclic Carbenes for Enhanced Stability and Functionality. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 5(1): 6-19
- Thallaj N (2023) Tishreen University Journal-Medical Sciences Series 44(6): 21-29.
- Thallaj N (2024) Conductive Nanocomposites Based on Graphene and Natural Polymers. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(6): 7-27.
- Thallaj N (2022) Design and Synthesis Ligands Tetradents Substituted with Halogenes in α-Position and Conjugation with Riboflavin (Bioconjugates): Conjugate ligands Type TPA’s with Flavonoids as un Electron Mediator. Biomedicine and Chemical Sciences 1(2): 47-56.
- Dayoub L, Alkuddsi Y, Thallaj N (2023) Investigating the interaction between some of Bipolaris sorokiniana’s toxins and the Gα Subunit of the Wheat G-Protein using bioinformatics tools. University of Thi-Qar Journal of agricultural research 12(1): 181-200.
- Thallaj N, Abbood A (2023) Tishreen University Journal-Medical Sciences Series 45: 47-57.
- Thallaj N (2022) HPLC method validation for determination of pentoxifylline in pharmaceutical dosage forms. Indian J Adv Chem 2(1): 5-9.
- Thallaj N (2022) Ligands tris (2-pyridylmethyl) amine with nitrile group in α-substituted and the corresponding FeCl2 complexes.
- Thallaj N (2022) Review of Calixarene-Derivatives in Transition Metal Chemistry. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 2(3): 1-30.
- Thallaj N (2022) Ligands tris (2-pyridylmethyl) amine with nitrile group in α-complexes 2 substituted and the corresponding FeCl. Journal of Biological Pharmaceutical And Chemical Research 9(1): 25-47
- Thallaj N (2022) Journal of Biological Pharmaceutical And Chemical Research 9 (3): 40-47.
- NK Thallaj (2018) Damascus University Journal for Basic Sciences. Conjunction of riboflavin to TPAs ligands with two halogens atoms in α-position substitution 34: 1.
- NK Thallaj (2017) Designing and synthesis of DPAs ligands to induce structural modification of Riboflavin. Journal of AlBaath University 39(8): 33-72.
- THALLAJ, Nasser Ferrous complexes with aromatic α-substituted tris (2-pyridylmethyl) amine ligands: effect of the substituents and biomimetic reactivity.
- Thallaj N (2024) Advancements in Peptide Vectors for Cancer Therapy and Tumor Imaging: A Comprehensive Review. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(5): 29-49.
- Thallaj N, ali Hamad DH, Batieh NA, Saker GA (2023) A Review of Colon Cancer Treatment using Photoactive Nanoparticles. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 3(4): 1-32.
- Labban, Louay (2020) International Journ. IJMS 5(12): 23-36.
- Labban L, Malek Z (2020) Photoperiod regulates the daily profiles of tryptophan hydroxylase-2 gene expression the raphe nuclei of rats. International Journal of Current Research in Physiology and Pharmacology 1-5.
- Labban, Louay, Nasser Thallaj, and Zeina Malek (2019) The implications of E-cigarettes or vaping on the nutritional status. Jour Med Resh and Health Sci 2(11): 784-787.
- MALEK Z (2018) The effect of regular exercise on the expression of tryptophan hydroxylase-2 gene within Raphe complex: functional relationship with adrenal hormones and glucose blood levels. in Journal of AlBaath University.
- MALEK Z (2018) The effect of Glucocorticoids rhythm on serotonin release within the hypothalamic suprachiasmatic nuclei; the locus of the Biological Clock: functional relationship with blood glucose. Tishreen University Journal for Research and Scientific Studies 40: 1-17.
- Labban L (2018) The Association between Visceral Fat, Dietary Patterns, and Comorbidities. Open Access Library Journal 5(07): 1.
- Labban LM, Alshishkli MM, Alkhalaf A, Malek Z (2017) The effects of dental amalgam toxicity on health and nutritional status. J. Adv. Res. Dent. Oral Health 2: 1-4.
- Shannan G, Malek ZS, Thallaj N (2025) Advancements in Protein Synthesis: Triazole Multi-Ligation and Cycloadditions for Pharmaceutical Applications. Am J Biomed Sci & Res 26(5): 685-700.
- Channan G (2018) Communicable diseases in the Mediterranean region. EJIFCC 29(3): 164-170.
- Shannan G (2011) TUBERCULOSIS IN THE AFCB COUNTRIES; RE-EMERGING DISEASE. In CLINICAL CHEMISTRY AND LABORATORY MEDICINE. GENTHINER STRASSE 13, D-10785 BERLIN, GERMANY: WALTER DE GRUYTER & CO 49: S41-S41.
- Channan GM (1977) Studies of vitamin D hydroxylation in chronic renal failure.
- Aldakhoul M, Sleman S, Alragheb N, Alfarwan M, Alallan L, et al. (2024) Phytochemical Composition and Health Benefits of Pumpkin. Research Journal of Pharmacy and Technology 17(10): 4915-4921.
- Khabbazeh Sally, Saly Alhaddad, Yazan Kiwan, Louai Alallan and Nasser Thallaj, et al. (2024) The Benefits of Jujube: Extracting Phenols and Saponins for Medicinal and Nutritional Uses. Azerbaijan Pharmaceutical and Pharmacotherapy Journal 23(4): 1-7.
- Nasser Thallaj, Dalia Aboufakher, Rita Zeinaldin, Rawa Khreit, Racha Khatib (2025) Prevalence and Antibiotic Resistance Patterns of Multidrug-Resistant Gram-Negative Bacilli in Hospitalized Patients in Sweida, Syria. FJHMS 1(1): 70-78.
- Thallaj Dr N (2022) Review of Calixarene-Derivatives in Transition Metal Chemistry. International Journal of Advanced Pharmaceutical Sciences and Research 2(3): 1-28.
- Nasser Thallaj, Dalia Aboufakher, Rita Zeinaldin, Rawa Khreit, Racha KhatibPrevalence (2025) Antibiotic Resistance Patterns of Multidrug-Resistant Gram-Negative Bacilli in Hospitalized Patients in Sweida, Syria. FJHMS 1(1): 70-78.
- Ghassan Shannan, Zeina S Malek, Nasser Thallaj (2025) Advances in Supported Synthesis of Oligosaccharides Using Thioglycoside Donors. Am J Biomed Sci & Res 27(1).
- Ghassan Shannan, Zeina S Malek, Nasser Thallaj (2025) Bioinspired Iron (III) Complexes: Catalysts for Sustainable Oxygen Atom Transfer Reactions. Am J Biomed Sci & Res 2025 27(1).
- Ghassan Shannan, Zeina S Malek and Nasser Thallaj (2025) Endosulfatases: Promising Therapeutic Targets in Cancer and Inflammatory Diseases. Am J Biomed Sci & Res 27(1).
- Samer alkhoury, Rasha Kateeb, Rawa Akasha and Nasser Thallaj (2025) Analysis of Crocin Content in Saffron (Crocus sativus L) Cultivated in Syria Using Liquid Chromatography-Mass Spectrometry. Am J Biomed Sci & Res 26(3).
- Dalia Aboufakher, Rita Zeinaldin, Racha Khatib, Rawa Khreit, Nasser Thallaj et.al. (2025) Prevalence and Antibiotic Resistance Patterns of Multidrug-Resistant Gram-Negative Bacilli in Hospitalized Patients in Sweida, Syria. Am J Biomed Sci & Res 26(3).




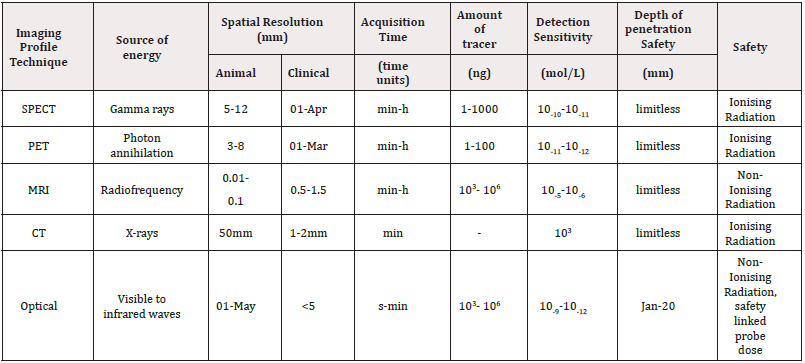


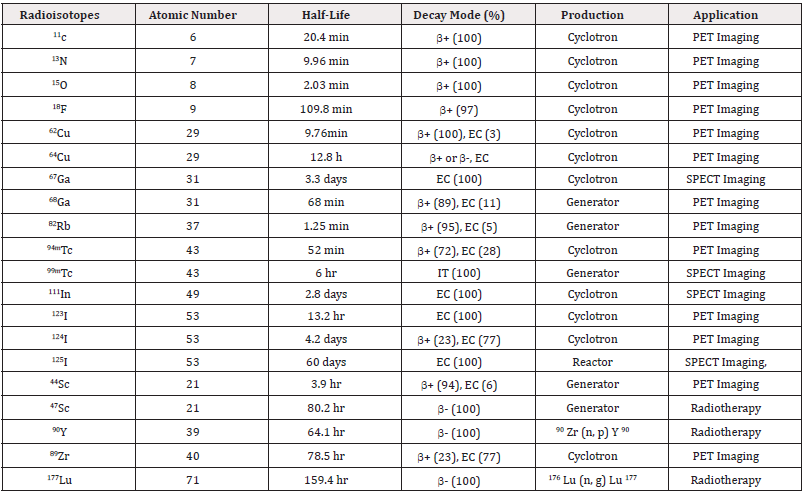
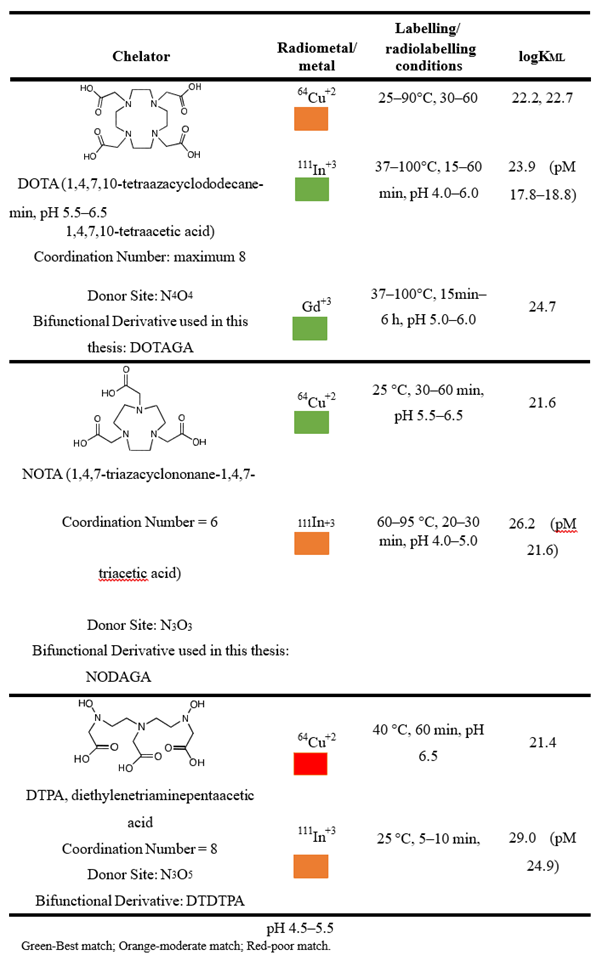
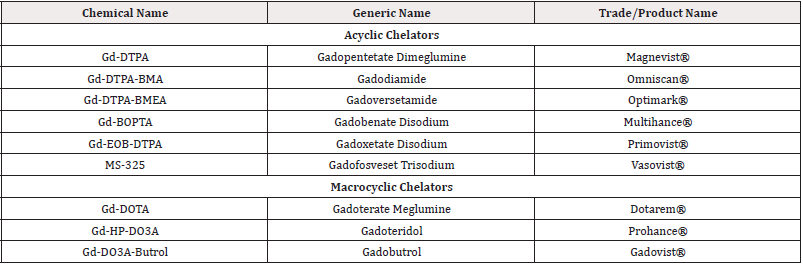
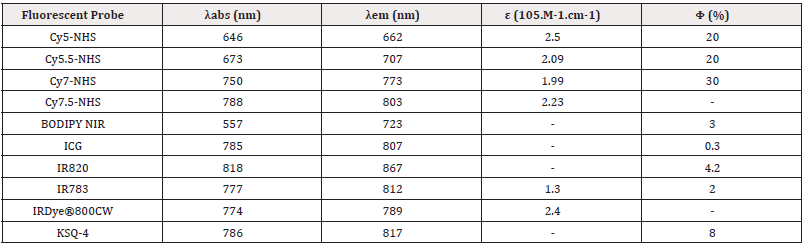

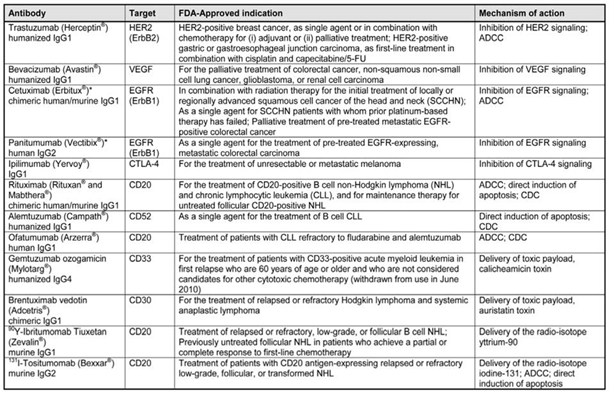



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.