Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Endosulfatases: Promising Therapeutic Targets in Cancer and Inflammatory Diseases
*Corresponding author: prof. Dr. Nasser Thallaj Pharmaceutical chemistry and drug quality control Department, Faculty of Pharmacy, Al-Rachid privet University, Damascus, Syria. ORCID ID: 0000-0002-6279-768X.
Received: March 07, 2025; Published: May 21, 2025
DOI: 10.34297/AJBSR.2025.27.003523
Abstract
Endosulfatases, specifically HSulf1 and HSulf2, are key enzymes that regulate heparan sulfate (HS) chain structure by hydrolyzing 6-O-sulfate groups. This modulation of HS is crucial, as HS interacts with various proteins to influence critical biological processes. Dysregulation of HS sulfation is implicated in diseases like cancer and inflammation, highlighting endosulfatases as potential therapeutic targets. These enzymes function extracellularly at neutral pH, distinguishing them from other sulfatases and offering a pathway for selective therapeutic intervention. Current inhibition strategies focus on substrate mimetics, particularly sulfamate-containing compounds, which act as irreversible inhibitors. These inhibitors, however, present challenges for structural studies due to their mechanism of action.Emerging strategies explore inhibitors with functional groups for bioconjugation, leveraging the formylglycine group in the enzyme’s active site. Chemical, enzymatic, and genetic methods can introduce carbonyl groups into proteins, enabling the attachment of therapeutic agents. Reactions like oxime/hydrazone formation and cycloadditions offer versatile routes for inhibitor conjugation. The development of specific endosulfatase inhibitors holds promise for treating diseases with aberrant HS sulfation. Precisely modulating endosulfatase activity could disrupt pathological HS interactions, offering new therapeutic avenues. Future research should aim to enhance inhibitor potency and selectivity and explore targeted drug delivery to the extracellular matrix.
Keywords: Endosulfatases, Heparan sulfate, Inhibitors, Sulfatases, Cancer, Inflammation
Introduction
The sulfatase enzyme family plays a pivotal role in various biological processes, particularly in the hydrolysis of sulfate esters from a broad spectrum of substrates [1-5]. Among these enzymes, endosulfatases (HSulf1 and HSulf2) have emerged as critical players in the regulation of heparan sulfate chains, which are vital components of the extracellular matrix. These chains, classified as glycosaminoglycans, undergo extensive post-synthetic modifications that determine their biological functions, influencing cellular communication, development, and tissue repair [5-9]. Heparan sulfate (HS) exhibits a unique structural complexity characterized by a repeating disaccharide unit of uronic acid and glucosamine, which can be modified through sulfation and acetylation [9-13]. This structural heterogeneity allows HS to bind a diverse range of proteins, including growth factors, morphogens, and cytokines. Such interactions are crucial for various physiological processes, including cell signaling, adhesion, and migration [12-16]. However, dysregulation in HS sulfation patterns can lead to pathologies, including cancer, inflammation, and metabolic disorders. Endosulfatases, identified in the early 2000s, specifically target the 6-O-sulfate groups on glucosamine residues within HS chains [16-20]. Their unique action occurs extracellularly at neutral pH, contrasting with many other sulfatases that operate in lysosomal environments. This specificity is essential, as it allows endosulfatases to modulate the sulfation status of HS, thereby influencing ligand-receptor interactions and signaling pathways [21]. The identification of these enzymes has opened new avenues for therapeutic interventions, particularly in oncology, where their regulatory roles in tumor growth and progression have been increasingly recognized. [22]. Research has indicated that HSulf1 often functions as a tumor suppressor, while HSulf2 is associated with pro-oncogenic activities. The differential expression of these enzymes in various cancer types highlights their potential as biomarkers and therapeutic targets [23]. For instance, HSulf1 tends to be underexpressed in several malignancies, including breast and ovarian cancers, where restoring its expression has shown promise in enhancing the efficacy of chemotherapeutic agents. Conversely, HSulf2 is frequently overexpressed in aggressive tumors, correlating with poor patient prognosis [24]. Despite the significance of endosulfatases in disease mechanisms, their inhibition remains an underexplored area [25]. The development of specific inhibitors targeting these enzymes could represent a novel therapeutic strategy. Notably, compounds containing sulfamate groups have demonstrated potential as irreversible inhibitors of sulfatases by mimicking natural substrates [26]. These inhibitors can disrupt the catalytic activity of endosulfatases, providing a means to modulate HS sulfation and its associated biological functions [27].
In this article, we will delve into the structure, function, and regulatory roles of endosulfatases in heparan sulfate metabolism. We will further explore the implications of their activity in cancer and other diseases, highlighting the emerging therapeutic strategies aimed at inhibiting these enzymes. By understanding the in tricate balance between sulfation and desulfation mediated by endosulfatases, we can better appreciate their contributions to health and disease, paving the way for innovative approaches in targeted therapies [28].
The following sections will provide a comprehensive overview of the biochemical mechanisms underlying endosulfatase action, their interactions with heparan sulfate, and the potential of novel inhibitors in clinical applications [29]. This exploration is timely and critical as the quest for effective therapies against complex diseases continues, emphasizing the need for targeted approaches that can selectively influence enzyme activity and ultimately improve patient outcomes [30].
Endosulfatases
The Sulfatase Family
Sulfatases (EC 3.1.6-) are a class of esterases that catalyze the hydrolysis of sulfate esters from a wide variety of substrates. To date, approximately 4,600 sulfatases have been identified in both eukaryotic and prokaryotic organisms’ Schema 1. An online database was established in 2016 to catalog these enzymes and classify them based on sequence homology [31].
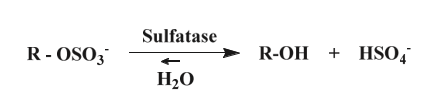
Scheme 1: Example of sulfatase-catalyzed hydrolysis of sulphate esters. The hydrolyzed substrate is then in the form of alcohol.
Among the approximately 4,600 known sulfatases, 17 human sulfatases have been identified to date. Six of these (ARSA, ARSB, GALNS, G6S, GDS, IDS) are found in lysosomes, are soluble, and function optimally at acidic pH. These enzymes play a crucial role in the degradation pathway of glycosaminoglycans and sulfolipids. Five additional sulfatases (ARSD, ARSF, ARSG, ARSI, ARSJ, ARSE) are located in the endoplasmic reticulum and Golgi apparatus, functioning at neutral pH. Four arylsulfatases (H, I, J, K) were identified in the 2000s, and their substrates remain largely unknown, except for ARSK, which was characterized in 2017 Table 1. Their expression is limited to embryonic tissues and cancer cells. Lastly, two sulfatases that hydrolyse 6-O-sulfates from heparan sulfate chains were identified in 2002, discovered either in the extracellular matrix or in the surrounding medium. These are classified as end sulfatases [32-36].
These 17 members of the sulfatase family exhibit high conservation, sharing sequence homology ranging from 20% to 60%. Notably, these enzymes possess the same catalytic mechanism and coordinate a divalent metal ion within their active site. A defining characteristic of this enzyme family is that all members demonstrate arylsulfatase activity Schema 2. Consequently, the synthetic substrate 4-methylumbelliferyl sulfate (4-MUS) is commonly utilized as a standard substrate for the in vitro measurement of these enzymes [36-39].
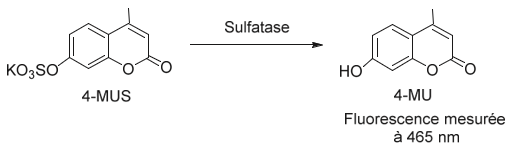
Scheme 2: Hydrolysis of the sulphate group of the synthetic substrate 4-methyl umbelliferyl sulphate (4-MUS). The resulting product is 4-methyl umbelliferol (4-MU) which fluoresces at 465 nm, which justifies its use in in vitro assays for the detection of arylsulfatase activity.
Human sulfatases are polypeptides consisting of 500 to 800 amino acids that share sequence homologies and exhibit similar tertiary structures. The sequence homology in the N-terminal region, approximately 150 amino acids long and encompassing the catalytic cycle along with several active residues, is more conserved than in other regions. This conservation supports the notion of a uniform catalytic mechanism across all sulfatases Schema 3. The alignment of sequences for the 17 human sulfatases is illustrated, highlighting three regions that contain highly conserved domains [40].
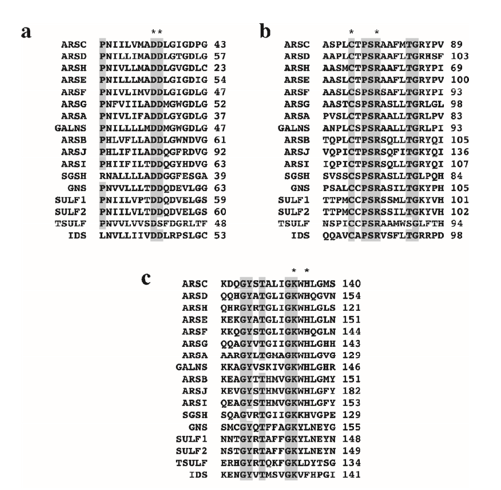
Scheme 3: Sequence alignment of three domains conserved among all human sulfatases. (a) and (c) represent domains of the N-terminal region of enzymes. (b) contains cysteine essential for the catalytic activity of sulfatases. Grey amino acids are retained in 90% of sulfatases. Asterisks mark amino acids known to have a function in the catalytic mechanism.
In domain (b), which represents the catalytic site of sulfatases, a conserved cysteine residue is noted. This amino acid is preserved in most human and non-human sulfatases across eukaryotic and prokaryotic organisms. However, some bacterial arylsulfatases, particularly those from Klebsiella pneumoniae and Pseudomonas aeruginosa, contain a serine residue in place of cysteine. Both residues are subsequently modified post-translationally to formyl glycine (FGly) [41-47].
Furthermore, the overlay of binding sites with the metal ion in six sulfatases (ARSA, ARSB, STS, IDS, GALNS, and SGSH) highlights the structural homology within the active sites of these enzymes, based on crystallographic data. The oxygen atoms from lysine (K347) and aspartate residues (D334, D45, D46) act as ligands for the divalent metal ion, while nitrogen atoms from histidine residues (H229, H138, H335) help stabilize FGly Figure 1. This metal cation is typically calcium, although magnesium may occasionally be present, and it plays a critical role in the catalytic mechanism for sulfate group hydrolysis by human sulfatases [48].
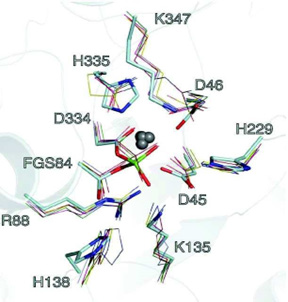
Figure 1: The superposition of the bivalent cation binding site for human sulfatases [ARSA (pink), ARSB (green), STS (yellow), IDS (blue), GALNS (dark blue) and SGSH (red)] shows their similarity. The metal ion is represented by gray spheres. Formylglycine is represented with a sulfate (FGS).
The identification of a cysteine residue common to most sulfatases, which undergoes post-translational modification to formylglycine (FGly), was made in 1995.Four years later, the same research group demonstrated that a sequence of 12 amino acids beginning with cysteine is essential for this modification in 10 sulfatases Figure 2. The pentapeptide CXPSR (where X can be T, S, C, or A) appears to be crucial for the formation of formylglycine and is highly conserved among eukaryotic and prokaryotic sulfatases. Additionally, a second “auxiliary” motif consisting of seven residues (X1X2X3LTGR) that aids in presenting cysteine to the modifying enzyme is also well conserved among human sulfatases Figure 3. The pentapeptide CXPSR and the auxiliary motif are consistently preserved in the sequences of endosulfatases (Sulf1 and Sulf2).
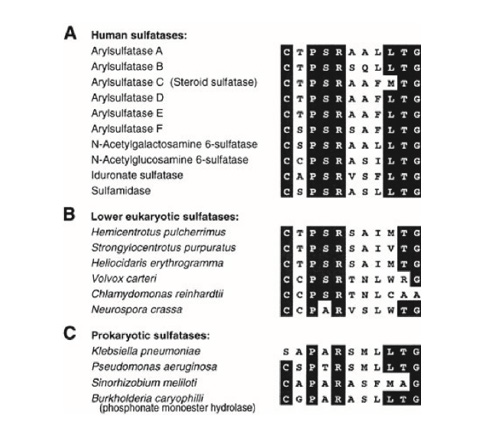
Figure 2: Sequence homology of the 12 demonstrated residues essential for the modification of cysteine to formylglycine (A) in 10 human sulfatases (B) in 6 lower eukaryotic sulfatases and (C) in 4 prokaryotic sulfatases. The sequence homology (in black, all conserved amino acids) CXPSR is noted in all human sulfatases and in 5 of the 6 lower eukaryotes. In prokaryotic sulfatases, the CXPXR motif is preserved, except for Klebsiella pneumoniae which has a serine residue instead of the cysteine residue. The XXXLTGR auxiliary motif is also very well conserved within human sulfatases.
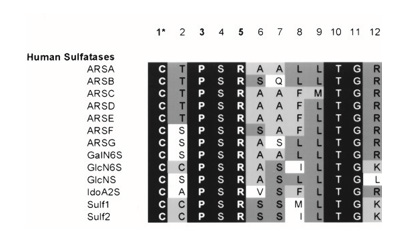
Figure 3: Sequence of the 12 amino acids of 13 human sulfatases. In black, the amino acids preserved in all sulfatases. From dark grey to white, the amino acids more or less conserved in these sequences.
The conserved sequence of 12 amino acids, C-X-P-S-R-X-X-X-L/ M-T-G-R/K/L, serves as a recognition motif for the enzyme that initiates the modification of cysteine to formylglycine. Mutations and truncations indicate that the highly conserved pentapeptide C-X-PX- R is the minimal motif required for this modification. Within the auxiliary motif, the four conserved residues LTGR are particularly important, as they are involved in the recognition of the sulfatase by the cysteine-modifying enzyme [49-55].
The conversion of cysteine to formylglycine occurs post-translationally through an enzyme known as Formylglycine-Generating Enzyme (FGE), a glycoprotein located in the endoplasmic reticulum. This oxidation mechanism, discovered in 2019, involves molecular oxygen and a copper(I) cofactor. The 3D structure of the enzyme reveals that two cysteine residues (Cys272 and Cys277) are responsible for binding with copper(I), which is also associated with the substrate (the cysteine of a sulfatase). This FGE-Cu-substrate complex reacts with O₂, leading to the conversion of copper(I) to copper (II), which activates the oxygen to form a reactive species Cu (II)- OO•. This reactive entity then deprotonates the carbon attached to the cysteine of the substrate, generating a radical that can form a double bond with sulfur Schema 4. Copper (II) is subsequently reduced back to copper(I) and dissociates rapidly from the sulfone, which is then hydrolyzed to formylglycine. Finally, FGE is reduced to return to its original form [55,56].
The conserved amino acids within the sulfatase family play a crucial role in the modification of cysteine to formylglycine (FGly) and are also essential for the hydrolysis of sulfate ester groups [56,67].
Crystallographic studies and mutational analyses have provided significant insights into the catalytic site of sulfatases. Many residues and structural features are highly conserved, indicating that most sulfatases likely share the same catalytic mechanism [58].
Within the key pentapeptide CXPSR, the arginine residue (R) has been identified as: 1) a critical determinant for the modification to formylglycine; its replacement with alanine nearly abolishes cysteine modification in vitro, 2) a stabilizing component of the active site for formylglycine, and 3) an essential structural element. Additionally, proline (P) is vital for cysteine modification to FGly, contributing to the helix that positions FGly within the active site [59,60].
Moreover, a second highly conserved sequence of 12 amino acids, G-Y/V-X-S/T-X-X-X-G-K-X-X-H, contains residues of lysine (K) and histidine (H), which are integral to the active site and play important roles in the catalytic mechanism by interacting with the sulfate group [61].
Based on the crystallographic structure of ARSA sulfatase in the presence of a sulfate group, the active site comprises 10 polar residues interconnected with a divalent metal ion. This metal ion may be magnesium or calcium, depending on the sulfatase; ARSA coordinates with Mg²⁺, while ARSB, ARSC, and PARS coordinate with Ca²⁺. A change in cation for ARSA leads to a significant reduction in substrate binding and catalytic activity, highlighting the importance of the metal ion in the hydrolysis of the sulfate group Figure 4. It appears to facilitate both substrate interaction and the reactivity necessary for nucleophilic attack [62-66].
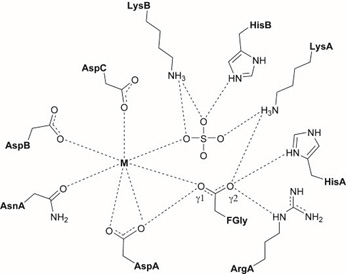
Figure 4: Restitution of the catalytic active site of sulfatases according to the crystallographic structures of ARSA, ARSB, ARSC and PARS. The interactions between the residues are represented by the dashed lines.
Additionally, it is notable that the catalytic pocket contains several positively charged residues, including LysA, LysB, and HisB, along with the cation, which are involved in interactions with the anionic sulfate group. These interactions likely help to neutralize the charge of the sulfate, reducing the electron density around the sulfur and rendering it more electrophilic. Furthermore, these residues assist in positioning the substrate in proximity to formylglycine, facilitating the nucleophilic attack [64-66].
Moreover, the residues HisA and LysA, each possessing a protonated nitrogen atom and carrying a positive charge, are involved in the protonation of the alkoxide formed during the hydrolysis of the sulfate group [66,67].
It is also observed that formylglycine exists in its hydrated form. In this state, oxygen 1 interacts with Asp1 and the metal ion, positioning it optimally for nucleophilic attack on the sulfur atom. Oxygen 2, which interacts with HisA, ArgA, and LysA, plays a role in the elimination of the sulfate group following hydrolysis Schema 5 [68]. The proposed role of each residue involved in the catalytic mechanism of sulfate group hydrolysis is summarized in Table 2.
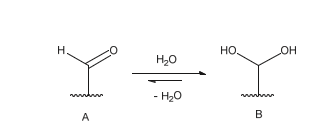
Scheme 5: Balance between formylglycine in its aldehyde form (A) and formylglycine in its hydrated form (B). According to the crystallographic structures obtained from the different sulfatases, this equilibrium seems to be in favor of formylglycine in hydrated form.
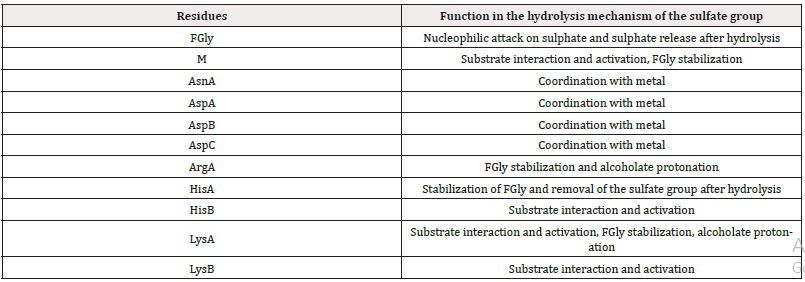
Table 2: Summary of the functions of the amino acids involved in the hydrolysis mechanism of the sulphate group.
In 1995, it was established that the presence of the FGly residue is essential for the catalysis of sulfate group hydrolysis. The resulting mechanism was elucidated with the advent of the first crystallographic structures of sulfatases containing sulfate groups. However, the mechanism remains a topic of discussion within the scientific community, and two hypotheses are currently proposed. The first mechanism was suggested by a research group in 1997 following the determination of the 3D structure of ARSB sulfatase, where formylglycine was present as a sulfate hemiacetal [69]. This mechanism describes a nucleophilic addition of the sulfate group to the free aldehyde of FGly, leading to the formation of an intermediate. Subsequently, the introduction of a water molecule would facilitate the hydrolysis of the substrate Schema 6. This intermediate is proposed to represent the resting state of the enzyme, although there exists an equilibrium toward the regenerated form of FGly (in its aldehyde form) [70].
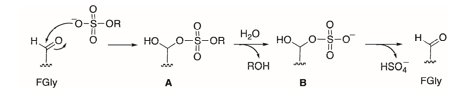
Scheme 6: Mechanism proposed by the Guss group of the hydrolysis of the sulfate group by sulfatases involving a nucleophilic addition step (A) and then a hydrolysis step (B), from the crystallographic structure of ARSB.
Based on the crystallographic structure of ARSA sulfatase, an alternative mechanism was proposed in 1998. This mechanism suggests that formylglycine exists in its hydrated form and posits an SN2-type nucleophilic attack by one of the two oxygen atoms on the sulfur of the sulfate group, resulting in the release of the substrate as an alcoholic product. The sulfate group would then be eliminated by the remaining alcohol, thereby regenerating formylglycine in its aldehyde form Schema 7. A water molecule would facilitate the reformation of hydrated formylglycine [71-77].
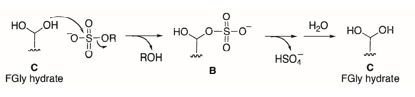
Scheme 7: Mechanism proposed – from the crystallographic structure of ARSA – by the Von Figura group for the hydrolysis of the sulfate group by sulfatases involving a nucleophilic addition step of type SN2 (B) then a hydrolysis step of the sulfate group and regeneration of FGly in its hydrated form (C).
This second mechanism for the hydrolysis of sulfate groups is strongly supported by the elucidation of the 3D structure of PARS sulfatase from the gram-negative bacterium Pseudomonas aeruginosa, achieved at atomic resolution in 2001. The structure of the catalytic site clearly indicates that formylglycine is in close proximity to the sulfate group but is not coordinated with it, a hypothesis initially proposed to explain the first mechanism. This sulfate group likely originates from the high concentrations of ammonium sulfate used during crystallographic studies Schema 8. The orientation of the sulfate group relative to formylglycine appears to be optimal for the nucleophilic attack by formylglycine [78-84]. Subsequently, a question arose regarding the nature of the bond cleavage between oxygen and sulfur. Is the hydrolysis associative (SN2-type mechanism) or dissociative (SN1-type mechanism)?.
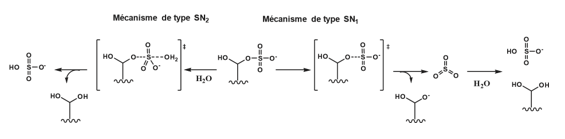
Scheme 8: Two different mechanisms for the hydrolysis of the sulfate group. Once the sulfate is bound to the oxygen of the formylglycine, it can either dissociate by an SN1 mechanism and lead to the free SO3 group which then reacts with water, or the water attacks the sulfur of the sulfate group and the latter is eliminated according to an SN2 mechanism.
Numerous studies have suggested an SN2 mechanism, as the SO₃ group resulting from an SN1 pathway is considered too high in energy Schema 9. However, theoretical and experimental investigations conducted in 2014 indicated that the hydrolysis of sulfate groups may actually follow an E2-type mechanism [82-86].
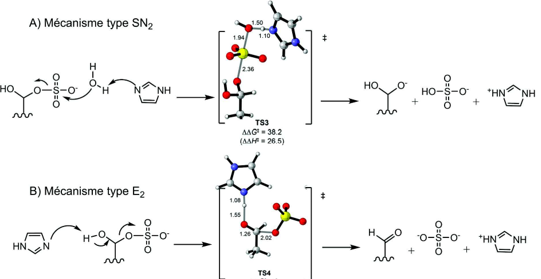
Scheme 9: Calculations of the transition states of the two hypothetical starting mechanisms of the sulphate group. Histidine, which is supposed to play a role in the hydrolysis of the sulfate group, is modeled here by an imidazole. In the transition states, nitrogen is shown in blue, carbons in grey, oxygens in red and sulphur in yellow. The calculation of the TS3 transition state of the SN2-type mechanism shows much higher values of activation energy G = 38.2 and enthalpy H = 26.5 than for the TS4 transition state of the E2-type mechanism (G = H = 0). These values strongly argue for a starting mechanism of the sulphate group of type E2.
Discovery of Human Endosulfatases (HSulfs)
Endosulfatases (Sulf1 and Sulf2) are enzymes identified in the 2000s among eukaryotes. In 2001, a gene from a quail embryo was first characterized, named QSulf1, which exhibited sequence homology with the N-terminal region of the GLNS sulfatase. This enzyme appears to be expressed on the cell surface and exhibits desulfation activity on heparan sulfate chains. Furthermore, QSulf1 contains a conserved cysteine at position 89, which undergoes post-translational modification to formylglycine, a modification essential for its function. These findings suggest that QSulf1 is an extracellular sulfatase specific to heparan sulfate [87].
In 2002, another gene, RSulf1FP1, was identified in rats as an ortholog of QSulf1. This sequence is longer than those of other sulfatases due to the presence of a hydrophilic insert within the gene. That same year, researchers cloned and expressed the human ortholog (HSulf1) and the mouse version (MSulf1), along with homologous proteins HSulf2 and MSulf2. They confirmed that QSulf1 and RSulfFP1 are indeed orthologs of HSulf1 and MSulf1, sharing 94% sequence homology. Sulf2, expressed from a separate gene, represents another member of the sulfatase family, closely related to Sulf1 in length and tertiary structure, despite having a lower sequence homology of 64%. Additionally, it was confirmed that these enzymes are expressed on the cell surface and possess sulfatase activity. They exhibit both arylsulfatase activity and highly specific endosulfatase activity towards the 6-O-sulfate groups of glucosamine residues in heparin [88].
The discovery of these new sulfatase family members in various eukaryotes continued with the identification of Sulf1 genes in chickens in 2004, Sulf1 and Sulf2 in Xenopus in 2008 and 2009, and in zebrafish, sea urchins, and Drosophila in subsequent years [89].
It is important to note that many human sulfatases are exosulfatases located in lysosomes, with optimal activity at acidic pH. In contrast, these newly identified human endosulfatases are unique as they function extracellularly at neutral pH and show high specificity for the heavily sulfated regions of heparan sulfate chains [90].
To date, 17 members of the sulfatase family have been identified. The catalytic site of these enzymes consists of a formylglycine residue and a divalent metal cation. The formylglycine arises from the post-translational modification of a cysteine, catalyzed by an enzyme known as Formylglycine-Generating Enzyme. This modification facilitates the hydrolysis of sulfate groups from numerous substrates. While all sulfatases share a highly conserved catalytic domain sequence that allows them to utilize the same desulfation mechanism, they differ in their localization and substrate specificity. Most sulfatases are intracellular and operate at acidic pH, whereas two newly discovered extracellular enzymes functioning at neutral pH exhibit endosulfatase activity on heparan sulfate chains [91].
Structure of Endosulfatases
HSulf1 and HSulf2 are enzymes with a significantly higher number of amino acids compared to other sulfatases, comprising 870 amino acids for HSulf1 and 871 for HSulf2, which is approximately 300 residues more than their family counterparts Figure 5. These 125-kDa polypeptides are cleaved by a furin-like protease to yield two subunits linked by a disulfide bond: a 75-kDa long chain containing the N-terminal portion and a 50-kDa short chain containing the C-terminal portion [92].
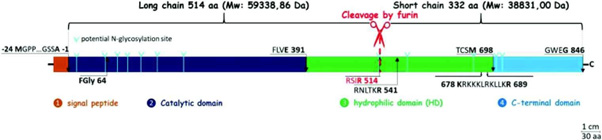
Figure 5: Schematic structure of HSulf2. The signal peptide of 24 aa is shown in orange, the catalytic domain of 391 aa in dark blue, the basic hydrophilic domain of 307 aa in green and finally the C-terminal part of 148 aa is shown in blue. The residue containing formylglycine is shown in position 64. RSIR 514 is the primary furin cut-off site, RNLTKR 541 is the secondary furin cut-off site. 678 KRKKKLRKLLKR 689 corresponds to the 12 aa conserved among vertebrates. Potential sites of N-glycosylation are represented by light blue arrows.
Overall, HSulfs consist of four domains ranging from the N- to the C-terminus:
i. Signal Peptide: Comprising 22 amino acids for HSulf1 and 24 for HSulf2, this domain directs the enzyme’s secretion into the extracellular space and is removed during this process.
ii. Catalytic Domain: This region, consisting of 392 amino acids for HSulf1 and 391 for HSulf2, is located in the N-terminal section and is essential for the enzyme’s arylsulfatase and endosulfatase activities. It exhibits a highly conserved sequence homology (42-44%) with the human sulfatase G6S.
iii. Hydrophilic Domain (HD): Unique to endosulfatases, this domain contains 327 amino acids for HSulf1 and 307 for HSulf2. It is crucial for endosulfatase activity but not for arylsulfatase activity. The HD domain is rich in charged amino acids, comprising 27% basic and 13% acidic residues. The N- and C-terminal sequences of this domain are highly conserved among vertebrate sulfatases, particularly a cluster of 12 basic residues.
iv. C-terminal Region: This region consists of 130 amino acids for HSulf1 and 148 for HSulf2 (Figure 5).
The previously mentioned mass of 125 kDa for endosulfatases is an experimental estimate obtained through Western blot analysis. Another study observed a mass of 132 kDa for the same enzymes, while the theoretical mass derived from the amino acid sequence is approximately 100 kDa (specifically 98,170 g/mol). A mass spectrometry analysis of HSulf2 conducted in 2019 provided clarification, revealing a mass of 133,115 ± 297 g/mol for this enzyme. This measured mass, significantly higher than the theoretical value, indicates that numerous post-translational modifications occur. Among these modifications are N-glycosylations, which were suggested by earlier findings; when HSulf1 and HSulf2 are treated with N-glycanase, their mass approaches around 100 kDa. HSulf1 features 10 potential N-glycosylation sites, while HSulf2 contains 12. The exact role of these N-glycosylations remains to be elucidated [93].
HSulf1 and HSulf2 are enzymes that exist as two chains linked by a disulfide bond, comprising four distinct domains. Notably, one domain is catalytic, enabling enzymatic activity through its formylglycine residue, while another hydrophilic domain is rich in basic residues [94].
Substrate of Endosulfatases: Heparan Sulfate Chains
Heparan sulfates (HS) are polysaccharides classified within the glycosaminoglycan (GAG) family, characterized by repeating disaccharide units composed of an uronic acid (either glucuronic acid [GlcA] or iduronic acid [IdoA]) and glucosamine (GlcN). Their biosynthesis occurs in the endoplasmic reticulum and Golgi apparatus through sequential glycosylation steps, initiating with the formation of a tetrasaccharide linker-comprising glucuronic acid, two galactose units, and xylose (GlcA-β1,3-Gal-β1,3-Gal-β1,4-Xyl)-which is covalently attached to a serine residue of a core protein. Each HS chain is bound to this core protein, and multiple HS chains may be associated with a single protein, Figure 6 collectively forming a heparan sulfate proteoglycan (HSPG) [95].
Subsequently, an additional glycosylation step introduces an N-acetylglucosamine (GlcNAc) residue via a β1,4 linkage. This is followed by a polymerization process involving alternating additions of glucuronic acid (GlcA) and N-acetylglucosamine, resulting in a heparan sulfate (HS) precursor with the repeating unit structure (GlcA-β1,4-GlcNAc-β1,4)_n. This nascent chain then undergoes a series of enzymatic modifications that refine its structure and functional properties Figure 7. These modifications include: N-deacetylation and N-sulfation of glucosamine residues, mediated by a family of N-deacetylase/N-sulfotransferase enzymes; epimerization of glucuronic acid to iduronic acid by C5-epimerase; 2-O-sulfation of iduronic acid by 2-O-sulfotransferase; and O-sulfation of glucosamine residues at the C-6 and C-3 positions, carried out by 6-O-sulfotransferases and 3-O-sulfotransferases, respectively [96,97] (Schema 10).
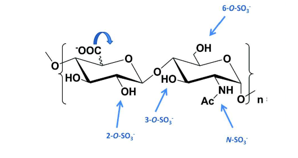
Figure 7: Disacharide repetitive unit of HS chains and the different modifications that may exist: on glucosamine, the amine can be free, acetylated or sulfated, alcohols in positions 3 and 6 can be sulfated or not schema10. Uronic acid can be glucuronic or iduronic and alcohol in position 2 can be sulfated or not.
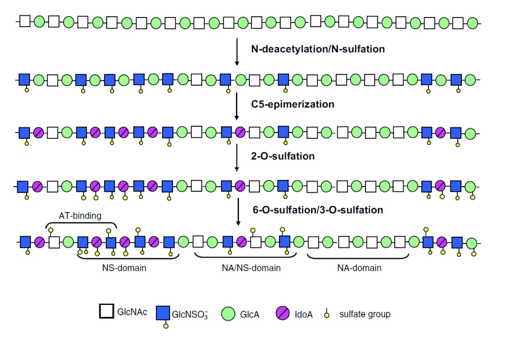
Scheme 10: Diagram of the biosynthesis of HS chains: once polymerization has taken place, each sugar can be modified by one or more enzymes. These modifications lead to the identification of several domains within the polysaccharide.
As a result of the selective activity of N-deacetylase/N-sulfotransferase enzymes, heparan sulfate chains acquire a unique and heterogeneous molecular structure composed of several distinct regions:
i. The NS domain, enriched in N-sulfated disaccharides, primarily features glucuronic acid residues epimerized to iduronic acid. This domain also exhibits variable O-sulfation at the C-6 (and occasionally C-3) positions of glucosamine and the C-2 position of iduronic acid. It is characterized by a high degree of sulfation.
ii. The NA domain consists predominantly of unmodified, N-acetylated disaccharides, showing little to no sulfation and displaying a relatively uniform structure.
iii. The NA/NS domain represents a transitional region between the NA and NS domains and is the most structurally heterogeneous part of the chain.
Further post-synthetic regulation of 6-O-sulfate groups occurs through the action of endosulfatases, contributing to additional structural diversity [98].
These extensive modifications result in heparan sulfate chains that vary significantly in size, ranging from approximately 50 to 250 disaccharide units (corresponding to 20–100 kDa), as well as in the spacing, size, and composition of NA and NS domains, the degree of epimerization, and patterns of sulfation within both defined and transitional regions [99].
Structural modeling of an NS domain decasaccharide-featuring 2-O-sulfation and epimerization of glucuronic acid, along with N- and 6-O-sulfation of glucosamine residues-reveals a helical conformation with clusters of three sulfate groups projecting outward on either side of the oligosaccharide Figure 8. This spatial arrangement underscores the significance of these sulfated motifs in ligand recognition and interaction [100].
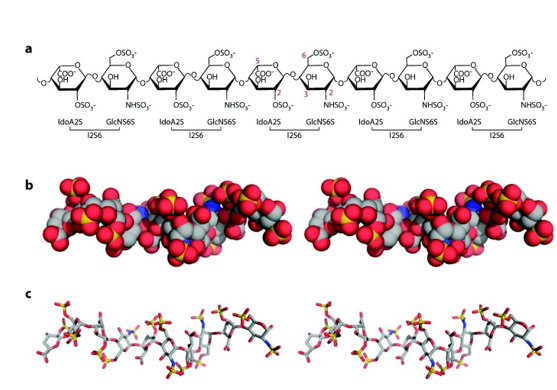
Figure 8: Structure of Heparan Sulfate. (a) Chemical structure of a decasaccharide. The carbons noted in red represent the sites of modification. (b) View of the decasaccharide in full representation, based on the “Protein Data Bank” identifying 1E0O (crystallography structure of the ternary complex of fibroblast growth factor 1 with its receptor and heparin). Carbons are shown in grey, oxygens in red, nitrogen in blue and sulphates in yellow. (c) View of the decasaccharide in stick representation. Note the helical nature of the chain and the alternating clusters of three sulfates on each side of the sugar backbone.
Due to the presence of highly sulfated domains, heparan sulfate (HS) chains of proteoglycans are capable of binding a wide variety of proteins through ionic interactions. Negatively charged sulfate and carboxylate groups on HS preferentially associate with positively charged amino acid residues such as lysine and arginine. Additionally, polar residues including asparagine, glutamine, and histidine can contribute to these interactions through hydrogen bonding. These target proteins, commonly referred to as heparan sulfate-binding proteins (HSBPs), are highly diverse and include: chemokines and cytokines (~60); growth factors and morphogens involved in development and tissue repair (~50); blood coagulation factors such as serine proteases and their inhibitors (~25); extracellular matrix proteins like collagen, fibronectin, and vitronectin (~25); and cell adhesion molecules (~10). Overall, HS chains are implicated in the binding of more than 400 proteins that play central roles in cellular communication [101].
Furthermore, HS chains are frequently exploited by pathogens during infection, as many microorganisms have developed or acquired HSBPs to facilitate their interaction with host cells [102].
The primary biological function of HS lies in its ability to bind and spatially present proteins within tissues. By interacting with extracellular proteins and subsequently releasing them, HS contributes to the formation of protein concentration gradients. These interactions also protect proteins from proteolytic degradation, thereby extending their extracellular half-life.
Morphogens, which govern the spatial and temporal organization of cells and tissues during embryonic development, operate via such concentration gradients. A cell’s positional identity-its knowledge of location within a developing tissue-is defined by its exposure to a specific morphogen concentration Schema 11. This spatial information is provided by a signalling center known as the organizer, which emits morphogenic signals that diffuse through the tissue, creating gradients. Cells interpret their distance from the organizer based on the morphogen concentration they receive, thereby adopting fates appropriate to their position [103].
Heparan sulfate chains contribute to the regulation of these morphogen gradients through several mechanisms:
i. (A)Core proteins of HSPGs (such as syndecan, glypican, and perlecan) can bind morphogens, potentially inhibiting their signaling activity.
ii. (B)Morphogens may bind directly to HS chains, which restricts their diffusion and helps establish localized concentration gradients.
iii. (C)Some morphogens are lipid-modified and transported via lipoprotein complexes; HS can modulate their distribution by interacting with these carriers.
iv. (D)The number of HSPGs on the cell surface is regulated through proteolytic cleavage, a process known as shedding.
v. (E)HS chains may be cleaved by extracellular heparanase, leading to the release of bound morphogens into the extracellular space.
vi. (F)HSPGs can mediate the internalization of morphogens via endocytosis.
vii. (G)Once internalized, morphogens can be degraded within lysosomes.
viii. (H)Alternatively, they may undergo transcytosis, allowing transport from the apical to the basal surface of the cell. Through these multifaceted mechanisms, HS chains play a central role in fine-tuning morphogen activity and distribution during tissue development [104] (Schema 11).
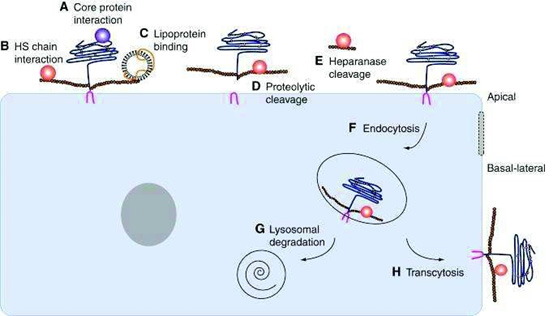
Scheme 11: Regulation of HSPGSs on the surface of cells. HSPGs can interact with morphogens through (A) their protein core (B) through their HS chain (C) or through lipoproteins. The proteolytic cut of the HPSGs (D) and the extracellular heparanases that cut the HS (E) chains thus release the morphogens. HSPGs can be transported inside the cell by endocytosis (F) and then either degraded in lysosomes (G) or transported to the basal pole of the cell by transcytosis (H).
Fibroblast Growth Factors (FGFs) comprise a family of 23 proteins identified in humans, classified within the broader group of morphogens. They are key regulators of numerous biological processes, including cell proliferation, survival, migration, and differentiation [105].
Among these, FGF2 has been shown to utilize Heparan Sulfate (HS) chains within the extracellular matrix for its transport. In the pericellular matrix, Heparan Sulfate Proteoglycans (HSPGs) form a structured network containing multiple binding sites. Rather than the HS chains or their core proteins moving freely-given their interactions with various ligands-it is the FGF2 molecules themselves that travel by sequentially binding to and dissociating from these sites. This mode of movement involves repeated cycles of transient interaction between FGF2 and HS, facilitated by the electrostatic nature of the binding, which supports rapid association and dissociation kinetics [106].
The residence time of FGF2 in its unbound state within the matrix is minimal compared to the duration it remains associated with HS, with dissociation times as short as 42 milliseconds-too brief to be directly measured with conventional methods. FGF2 contains three distinct HS-binding sites with varying affinities: one high-affinity site (dissociation constant Kd ~10⁻⁸ to 10⁻⁶ M), a medium- affinity site (in the millimolar range), and a third low-affinity site. This arrangement enhances the likelihood of re-association with HS immediately following dissociation Figure 9. Moreover, these multiple binding sites enable FGF2 to engage with adjacent HS chains while still bound to the original one, effectively allowing it to move progressively along the polysaccharide network [107].
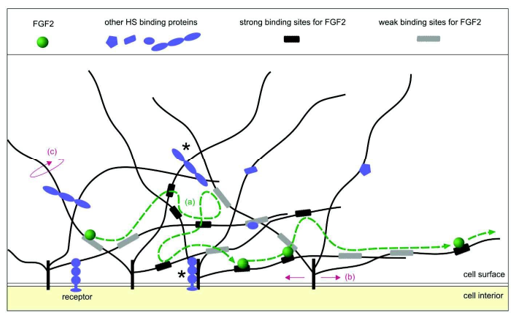
Figure 9: Schematic representation of FGF2 transport in the extracellular matrix by HSPGs: in the congested matrix, proteins and proteoglycans interact with each other. In this diagram, only HSPG (in black and grey), FGF2 (in green) and other ligands of HS (in blue) are shown. These ligands can be growth factors, cytokines, chemokines, enzymes, template proteins, and cell surface receptors. The main proteins of the proteoglycans are represented in black, inserted into the membrane, the HS chains are the black lines which have a size ranging from 40 to 160 nm. The high-affinity sites of HS for FGF2 are represented by black rectangles and those of low affinity by gray rectangles. In this network of HSPGs, FGF2 moves from one link site to another. (a) The dashed green line represents the path taken by FGF2 and the green circles its successive positions. This displacement of FGF2 is independent of the proper motions of HSPGs. (b) Movement of HS chains (c) to which FGF2 is attached and which can allow the displacement of FGF2 in the extracellular matrix. Ligands bound to HS chains and the main proteins of HSPGs and which may impede their movement are indicated by an asterisk.
These examples highlight the critical role of binding affinity between Heparan Sulfate (HS) chains and their ligands in shaping morphogen concentration gradients and guiding their diffusion within the extracellular matrix. This affinity can be modulated by the removal of 6-O-sulfate groups, a reaction catalyzed by endosulfatase enzymes [108].
Mechanism and Function of Endosulfatases
Endosulfatases are specialized enzymes that catalyze the hydrolysis of 6-O-sulfate groups on heparan sulfate chains. Although relatively recent in their discovery, these enzymes remain partially understood due to the complexity of their catalytic mechanisms, which are still under active investigation [109].
Since their identification in 2001, endosulfatases have been recognized for their specific interaction with HS chains. However, they do not exhibit uniform affinity across all HS domains. Their substrate recognition is influenced by a binding region rich in positively charged basic residues, enabling electrostatic interactions with negatively charged HS sequences.
Endosulfatases preferentially target the highly sulfated NS domain of HS, which includes disaccharide motifs that are triply sulfated-comprising N-sulfated glucosamine, 6-O-sulfate on glucosamine, and 2-O-sulfate on iduronic acid Figure 10. This high-affinity substrate allows the enzymes to act selectively, altering HS structure and thereby modulating protein interactions and signaling pathways within the extracellular environment [110].
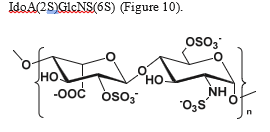
Figure 10: Disaccharide motif from the NS domain of HS chains comprising the following modifications: sulfation of amine and alcohol at position 6 of glucosamine, epimerization of glucuronic acid to iduronic acid, and sulfation of alcohol at position 2 of iduronic acid.
The high affinity of endosulfatases for the trisulfated disaccharide motif has been demonstrated through experiments involving human endosulfatases HSulf1 and HSulf2. When incubated with heparin-a highly sulfated glycosaminoglycan rich in trisulfated disaccharide units-under neutral pH conditions, these enzymes catalyze the hydrolysis of approximately 80% of the 6-O-sulfate groups. In contrast, when substrates lacking 2-O-sulfation on the iduronic acid residue are used, no enzymatic activity is observed [111].
This substrate specificity has been further validated through analytical studies where heparan sulfate was treated with endosulfatases, enzymatically digested into disaccharides, and the remaining 6-O-sulfate content was quantified. The results revealed that 65% of the trisulfated disaccharides [IdoA(2S)-GlcNS(6S)] underwent 6-O-desulfation, whereas only 20% of disulfated disaccharides [IdoA-GlcNS(6S)] were modified in the same way. Notably, the amount of monosulfated disaccharides [IdoA-GlcNAc(6S)] remained unchanged following treatment. These findings highlight the critical role of the trisulfated disaccharide motif [IdoA(2S)- GlcNS(6S)]-and specifically the presence of the N-sulfate group on glucosamine-in enabling HSulf activity [112].
Further insights into enzyme function were provided by studies investigating the role of the hydrophilic domain (HD) in HSulf1. In one such study, the wild-type enzyme and three mutants were engineered:
i. Sulf1 HD, in which the entire hydrophilic domain (residues 417–735) was deleted;
ii. Sulf1 HDB, lacking the HD except for the basic cluster near the C-terminal end (residues 417–720);
iii. Sulf1 HDC, with the HD removed except for the outer N-terminal and C-terminal segments (residues 459–618).
These mutants were used to assess how structural features of the hydrophilic domain contribute to catalytic efficiency and substrate interaction Figure 11.
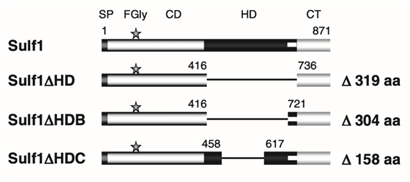
Figure 11: Schematic representation of the structure of HSulf1 and its mutants: Sulf1 comprises four domains: the signal peptide, the catalytic domain including the formylglycine residue, the HD hydrophilic domain and the C-terminal domain. The Sulf1 HD mutant lost its entire HS domain, the Sulf1 HDB mutant retained the basic cluster at the C-terminal and the Sulf1 HDC mutant retained the two outer regions at the N-terminal and C-terminal.
To assess the arylsulfatase activity of the four HSulf1 mutants, each was tested using the pseudosubstrate 4-methylumbelliferyl sulfate (4-MUS). While wild-type Sulf1 and the Sulf1 HDC mutant exhibited similar activity levels, Sulf1 HDB showed approximately 50% activity, and Sulf1 HD retained only 30%. These results indicate that the complete removal of the hydrophilic domain (HD) does not abolish enzymatic activity but significantly reduces it, suggesting that the HD contributes to optimal substrate presentation at the catalytic site.
When tested with native heparan sulfate (HS) chains, both Sulf1 and Sulf1 HDC maintained comparable activity. In contrast, Sulf1 HDB exhibited a 50% reduction in activity, and Sulf1 HD showed no detectable activity. Additionally, none of the mutants displayed activity against chondroitin sulfate (which contains 6-O-sulfation) or dermatan sulfate (which contains 4-O-sulfation), underscoring the enzyme’s specificity for HS.
These findings demonstrate that complete deletion of the HD domain eliminates the enzyme’s ability to act on its natural substrate, whereas partial retention of the domain, as in Sulf1 HDC, preserves both catalytic function and substrate specificity. The flanking N-terminal and C-terminal regions of the HD appear crucial for substrate recognition and effective endosulfatase activity.
Further evidence of the HD’s role in substrate binding comes from Surface Plasmon Resonance (SPR) analysis measuring the affinity of the HD domain of HSulf1 for heparin. Given the domain’s length, it is likely to contain multiple binding sites. Assuming two binding sites-one high-affinity and one moderate-affinity-the dissociation constants were determined to be KD1 = 0.6 nM and KD2 = 17 nM. These values highlight the HD’s contribution to the enzyme’s strong binding affinity for HS. Additional studies confirmed that the HD interacts strongly with HS chains, but this interaction is lost when the 6-O-sulfate groups are removed, emphasizing the importance of this sulfation pattern for binding.
In a separate development, the successful recombinant expression and purification of HSulf2 was achieved, overcoming previous limitations in yield and purity that hindered in vitro studies. This advancement enabled detailed functional analyses of HSulf2 domains. Two HS-binding motifs-V179KEK and L404KKK-were identified within the catalytic region. Single and double alanine-substitution mutants of these motifs were generated to evaluate their functional significance. While individual mutations did not eliminate enzymatic activity, they reduced it moderately. However, the double mutant showed a marked decrease in activity toward heparin, suggesting that although these motifs are not essential for catalysis, they enhance enzymatic efficiency.
Interestingly, these two motifs are conserved exclusively among Sulf isoforms and orthologs, despite the catalytic domain’s sequence conservation across the sulfatase family, suggesting a specific role in substrate recognition. Structural modeling of HSulf2 lacking the HD domain in complex with a heparin oligosaccharide [GlcNS(6S)-IdoA(2S)-GlcNS(6S)]₅ revealed that the VKEK and LKKK motifs align spatially with the active site residue, formylglycine. The distance between these two motifs corresponds to the length of an octasaccharide, indicating that these binding sites may facilitate interaction with extended HS sequences, beyond the disaccharide unit, contributing to substrate recognition and efficient endosulfatase activity Figure 12.
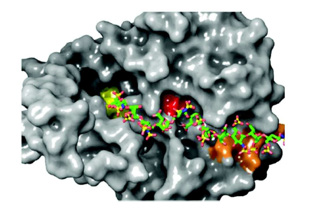
Figure 12: Modeling of HSulf2 without its HD domain in complex with a heparin oligosaccharide GlcNS(6S)-[Idoa(2S)-GlcNS(6S)]5. The location of Formylglycine (C88) is shown in red, the VKEK and LKKK peptides are shown in yellow and orange.
The hydrolysis of 6-O-sulfate groups on heparan sulfate (HS) chains by endosulfatases is not a random process but follows a specific and directional mechanism. Studies have demonstrated that HSulf2 preferentially targets the non-reducing end of HS chains. Additional investigations confirmed that both the terminal non-reducing end and internal NS domains of HS serve as favorable initiation sites for HSulf2 activity.
Based on these observations, it is proposed that HSulf2 operates through a processive and directional mode of action. The Hydrophilic Domain (HD) facilitates specific recognition and binding to the highly sulfated NS domains of HS. Upon binding, the catalytic domain of the enzyme is positioned near the non-reducing end of the HS chain, where it begins the stepwise removal of 6-O-sulfate groups. As desulfation proceeds, the enzyme translocates along the polysaccharide until it reaches a region outside the NS domain. At this point, the enzyme’s affinity for the substrate decreases significantly, resulting in dissociation of the enzyme-substrate complex Figure 13.
The previously identified VKEK and LKKK peptide motifs are thought to contribute to substrate recognition by aligning the HS chain for optimal access to the catalytic site. Although the absence of these motifs does not abolish enzymatic activity, it reduces the efficiency of catalysis, suggesting their role in enhancing substrate positioning and processivity (Figure 13).
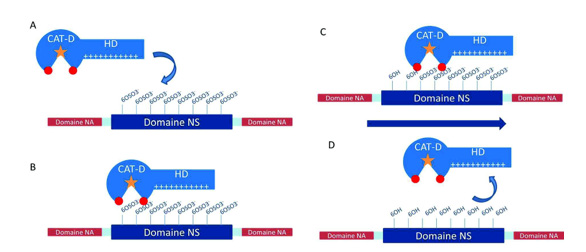
Figure 13: Schematic representation of the processive and oriented mechanism of desulphation of HS chains by HSulfs. The formylglycine residue is represented by the orange star, the VKEK and LKKK peptides in red. A) The hydrophilic (HD) domain of the enzyme recognizes the NS domain of the substrate and binds to it through the 6-Osulfate groups. B) The CAT-D catalytic domain is positioned at the non-reducing end of the NS domain. C) Once desulphation has been carried out, the enzyme advances on the substrate to position another sulphate group at the catalytic site. D) Once the reducing side of the NS domain is reached, the absence of the 6-O-sulfate groups induces a loss of affinity between the enzyme and the substrate and the complex dissociates.
By modulating the sulfation pattern of Heparan Sulfate (HS) chains, endosulfatases regulate the binding affinity of HS for various ligands and thereby influence a wide range of biological processes. This regulation is context-dependent and varies according to the specific signaling pathway involved.
For instance, desulfation of HS chains can facilitate access of certain ligands-such as Wnt, a glycoprotein involved in embryonic development and oncogenesis, or bone morphogenetic proteins (BMPs)-to their respective receptors, thereby promoting signaling. In contrast, in pathways involving Fibroblast Growth Factors (FGFs), endosulfatase activity can disrupt the HS-mediated stabilization of the FGF-receptor complex, effectively terminating the signaling response. Additionally, endosulfatases can influence the spatial distribution of chemokines by altering their gradients, which may affect immune cell migration and tissue patterning Figure 14.
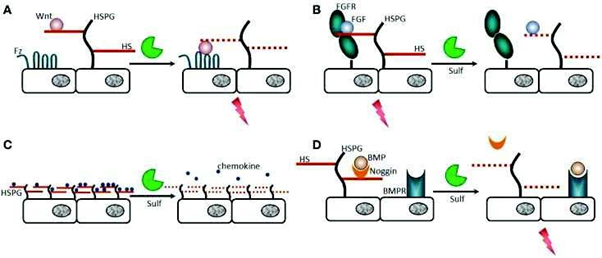
Figure 14: Regulation of the affinity of HS with their substrates by endosulfatases. A) The ligand Wnt is a glycoprotein involved in embryonic development and cancer. It binds to HS chains with high affinity (HS shown in solid red line) which prevents interaction with its receptor. Desulfation of HS by Sulfs decreases HS/ligand affinity (HS shown in red dotted line). The ligand is then free to interact with its receptor, which triggers a biological response, such as angiogenesis or cell migration. B) FGF1 and FGF2 are involved in angiogenesis, wound healing and cancers. FGF2 and its receptor (FGFR) form a complex that requires the 6-O-sulfate groups. Desulphation of HS no longer allows the formation of this complex and inhibits the signal. C) chemokines, proteins involved in the immune system, bind to HS in order to form gradients. The loss of sulfate groups alters these gradients, which can have impacts on the development of cancers such as leukemia. D) The BMP (Bone Morphogenic Protein) ligand is involved in the morphogenesis of bones and cartilage. It is sequestered by a “Noggin” protein that binds to HS. When Noggin loses its affinity for HS, it also loses its affinity for the BMP ligand which can then go to its BMPR receptor and induce a signal.
Differences Between HSulf1 and HSulf2
HSulf1 and HSulf2 are two distinct enzymes within the sulfatase family. They share the same catalytic mechanism and both exhibit desulfation activity on the 6-O-sulfates of Heparan Sulfate (HS) chains, but their amino acid sequences show only 64% homology. This raises the question of the functional significance of the remaining 36% sequence divergence. In vitro experiments have demonstrated that HSulf1 and HSulf2 follow the same desulfation process, albeit at different rates. For instance, when an octasaccharide mimicking an HS S-domain fragment is treated with HSulf1, complete 6-O-desulfation occurs within 48 hours. However, treatment with HSulf2 results in only partial desulfation. Despite these differences, the enzymes exhibit redundant, overlapping, and even antagonistic functions in vivo, depending on the biological context. In knockout (KO) mouse models, single KO of either HSulf1 or HSulf2 does not result in lethality or severe anomalies, while the double KO leads to increased mortality and numerous phenotypic abnormalities, suggesting functional compensation between the two enzymes. The 6-O-sulfate content on HS chains in double KO mice is significantly higher than in single KO mice, indicating a cooperative role between HSulf1 and HSulf2. Furthermore, expression of HSulf1 can compensate for the loss of HSulf2, but not vice versa. Notably, several studies have reported differences in HSulf1 and HSulf2 expression in various cancer types. While HSulf1 appears to act as an anti-oncogenic factor, HSulf2 is typically associated with a pro-oncogenic role.
The biosynthesis of heparan sulfate chains is a highly regulated process involving numerous enzymes and occurs primarily in the Golgi apparatus and endoplasmic reticulum. These HS chains consist of multiple domains, including the highly sulfated NS domain, which contains repetitive trisulfated disaccharide units such as IdoA(2S)-GlcNS(6S). The sulfation pattern of HS chains can be post-synthetically regulated by endosulfatases. HSulf1 and HSulf2 act as endosulfatases that catalyze the hydrolysis of 6-O-sulfate groups on glucosamine residues within HS chains. The desulfation process follows a processive and directional mechanism, facilitated by the hydrophilic domain (HD) of the enzymes, which preferentially recognizes the trisaccharide IdoA(2S)-GlcNS(6S) present in the NS domains of HS. The basic HD domain binds electrostatically to the negatively charged sulfate groups, positioning the catalytic domain at the non-reducing end of the NS domain. The enzyme then “slides” along the substrate, removing 6-O-sulfates. Upon reaching the reducing end of the NS domain, the absence of sulfate groups weakens the enzyme-substrate affinity, leading to dissociation of the complex.
The role of endosulfatases is highly complex and context-dependent, influenced by the specific enzyme (HSulf1 or HSulf2), the ligand, and the biological setting. For example, desulfation of HS may enable certain ligands to bind their receptors and inhibit biological responses to other ligands. HSulf1 and HSulf2 can cooperate, exhibiting both redundant and antagonistic functions: HSulf1 tends to be anti-oncogenic, while HSulf2 generally displays pro-oncogenic activity.
Endosulfatases and Associated Pathologies
The hydrolysis of 6-O-sulfates by HSulfs reduces the overall sulfate content of HS chains by approximately 5 to 7%. Although this alteration in sulfation may seem minimal, it plays a crucial role in maintaining essential physiological processes such as embryogenesis and tissue regeneration. However, it is also implicated in various pathological conditions, including cancer, amyloidosis, chronic renal fibrosis, inflammation, and diabetes. Since the discovery of these enzymes in 2001, their involvement in pathological processes has been extensively studied, with emerging evidence linking dysregulated HSulf expression to disease progression.
HSulf1 and HSulf2 in Cancer
Following the identification of HSulfs, their involvement in human cancers was soon recognized. Studies have shown that HSulf1 is underexpressed in several cancer types, including ovarian, breast, pancreatic, renal, and hepatocellular carcinomas. Re-expression of HSulf1 has been shown to reduce cancer cell proliferation in vitro and increase their sensitivity to apoptosis-inducing drugs. Furthermore, HSulf1 has been repeatedly described as possessing tumor-suppressive activity in certain cancers, such as breast, pancreatic, head and neck cancers, and myeloma. However, this anti-oncogenic role of HSulf1 is contested by studies reporting its overexpression in leukemias, gastric cancers, brain tumors, and invasive breast carcinomas. In contrast, HSulf2 expression is upregulated in many cancers, and the enzyme is associated with pro-oncogenic activity in hepatocellular carcinoma, pancreatic, breast, and lung cancers. Overexpression of HSulf2 is linked to poor prognosis in patients with myeloma and hepatocellular carcinoma. Additionally, studies have shown that knockdown of HSulf2 in mouse models reduces cancer cell growth and tumor size.
HSulf2 as a Biomarker for Cirrhosis
In 2010, it was shown that the mRNA of HSulf2 can be detected in patients with healthy livers, with expression levels increasing by 2.8-fold in patients with non-cancerous liver fibrosis.Given that liver fibrosis is the first stage of liver degeneration leading to cirrhosis, in 2015, researchers measured HSulf2 mRNA levels in cirrhotic patients and found a similar 2.8-fold increase in expression compared to healthy controls. Since liver secretions enter the bloodstream, the question arose as to whether the concentration of HSulf2 would also be elevated in the blood of these patients. To address this, an ELISA test was developed using monoclonal antibodies specific to HSulf2, enabling measurement of HSulf2 levels in both healthy individuals (728 ± 400 pg/mL) and cirrhotic patients (1460 ± 1160 pg/mL). These findings suggest that HSulf2 may serve as a non-invasive biomarker for liver fibrosis, detectable via blood tests, which would provide a more patient-friendly and cost-effective alternative to liver biopsy.
The overexpression of HSulf2 in many cancers, particularly those with poor prognosis, makes it an attractive therapeutic target. Its extracellular localization also positions it as a potential biomarker for cirrhosis and other diseases where the enzyme is overexpressed. Additionally, its extracellular location makes it accessible to inhibitors whose structures can be designed to bypass the cellular membrane.
Inhibition of Endosulfatases: Which Molecules to Choose?
The involvement of endosulfatases in various diseases has led researchers to explore molecules that could inhibit these enzymes.
Multi-target Molecules
Several molecules, already tested or marketed for their anticancer activity, have prompted some research groups to investigate their potential as inhibitors of HSulfs.
Indirect Inhibitors
It has been shown that no HSulf1 activity is detected in certain cancer cells, and this repression is due to hypermethylation of the DNA in the HSulf1 promoter region. Treatment of these cells with a demethylating agent (5-aza-2-deoxycytidine) reactivates HSulf1 expression. Furthermore, HSulf1 expression has been linked to improved responses to chemotherapy, both in vitro and in vivo, with a higher number of patients in remission correlating with elevated HSulf1 levels.
HSulf2 is overexpressed in breast cancer, and studies have shown that proteasome inhibitors (MG132, Lactacystin, and Bortezomib) abolish HSulf2 expression in several cancer cell lines. In addition, treatment of MCF10DCIS cells (a breast cancer cell line with high HSulf2 expression) with Bortezomib leads to reduced tumor size, massive apoptosis, and decreased HSulf2 expression in vivo. However, the mechanisms underlying the reduction of HSulf2 expression by proteasome inhibitors remain unclear.
Direct Inhibitors
HSulf2 is overexpressed in most human hepatocellular carcinomas (HCC) and promotes tumor growth by enhancing Wnt and FGF2 signaling pathways. Patients with high HSulf2 expression have higher relapse rates and poorer prognosis. The molecule 2,4-Disulfophenyl-N-tert-butylnitrone (OKN-007), which has been tested for ischemic stroke treatment, has shown antitumor activity in HCC models Schema 12.
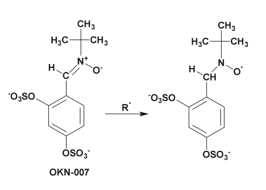
Scheme 12: The OKN-007 molecule contains a nitrone known to react with free radicals and has been studied for its anti-cancer activities in many cancers.
Studies have shown that treatment of Hepatocellular Carcinoma (HCC) cancer cells expressing HSulf2 with OKN-007 inhibits cell proliferation and migration while promoting apoptosis. However, when OKN-007 is used on cancer cells with suppressed HSulf2 expression, the effects on proliferation, migration, and apoptosis are significantly reduced. This suggests that the anti-tumor efficacy of OKN-007 is, at least in part, dependent on the expression of HSulf2. This indicates that OKN-007 likely inhibits the enzymatic activity of HSulf2, an oncogenic protein that promotes HCC progression.
The exact mechanism of HSulf2 inhibition by OKN-007 has not been discussed, but it is hypothesized that the sulfate groups of the molecule may interact with the catalytic site, with the radical present possibly reacting with specific amino acids within this site.
Additionally, PI-88 is a mixture of phosphorylated mannosides consisting of 90% pentasaccharides and tetrasaccharides, with a sulfate content ranging from 2.9 to 3.1 sulfates per monosaccharide Figure 15.
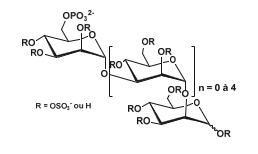
Figure 15: Structure of PI-88: mixture of phosphate oligosaccharides ranging from disaccharide to hexasaccharide with a strong predominance of pentasaccharides and tetrasaccharides (90% of its composition). The oligosaccharides are partially sulfated with 2.9 to 3.1 sulfate groups per monosaccharide unit.
This mixture of heparanase inhibitors, an enzyme responsible for degrading heparan sulfate chains, has reached Phase II clinical trials for its inhibitory action on angiogenesis and its anti-metastatic activity during solid tumor growth. Additionally, a research group tested its inhibitory activity on Endosulfatases 1 and 2, finding that PI-88 inhibits both HSulf1 and HSulf2 with IC50 values ranging from 0.6 to 3.6 μg/mL, comparable to those observed for heparanase (2 μg/mL). Since HSulf2 is proangiogenic and is overexpressed in many cancers, it is plausible that the anti-tumor effects of PI-88 may, in part, be attributed to its inhibition of HSulf2 [111].
32Following the discovery of endosulfatases in the early 2000s, many research groups investigated the biological role of these enzymes and found that HSulf2 is overexpressed in various cancers. As a therapeutic target, existing inhibitors of other enzymes were tested on HSulf2, and some were found to be effective. However, a major issue is that these inhibitors are not specific to endosulfatases.
Sulfatase Inhibitors
Identification of the Sulfamate Group as an Inhibitor of Steroid Sulfatase
Steroid sulfatase (also known as estrone sulfatase, STS) is the enzyme responsible for hydrolyzing the sulfate ester group of estrone sulfate to release estrone, an estrogen hormone secreted by the ovary in premenopausal women and by adipose tissue in both men and postmenopausal women Schema 13.
Estrone is found at significantly higher concentrations in the blood of postmenopausal women with breast cancer compared to healthy women. As early as 1993, several research groups sought to synthesize inhibitors of steroid sulfatase, the major enzyme responsible for the elevated levels of this hormone. One group successfully synthesized the first steroid sulfatase inhibitor: a modified estrone molecule where the sulfate group was replaced with a thiophosphonate group, resulting in estrone 3-methylthiophosphonate (E1-3-MTP) (Figure 16). A year later, the same group developed additional estrone sulfate derivatives, including estrone 3-sulfamate.
The sulfamate functional group had previously been employed in the development of compounds with antibacterial, antiviral, and antitumor properties, but its potential as a sulfatase inhibitor had not been explored. Inhibition assays revealed that estrone 3-sulfamate is a significantly more potent inhibitor of steroid sulfatase than estrone 3-methylthiophosphonate, demonstrating time- and concentration-dependent inactivation of the enzyme. Furthermore, the inhibition is irreversible; after a 2-hour incubation at 37 °C followed by overnight dialysis, no enzymatic activity could be recovered.A related compound, estrone 3-N, N-dimethylsulfamate (Figure 16), was also synthesized and found to act as a reversible inhibitor of the same enzyme. This study was the first to demonstrate the sulfamate group’s capacity to irreversibly inhibit a sulfatase in a time- and dose-dependent manner. However, in vivo administration of estrone 3-sulfamate resulted in adverse effects, including increased uterine weight in ovariectomized rats, leading to its exclusion as an antitumor drug candidate. As a result, research shifted toward non-steroidal sulfatase inhibitors for oncological applications [112].
While estrone 3-sulfamate was discontinued as an anticancer agent, estradiol 3-sulfamate (E2MATE) progressed to phase II clinical trials as an estradiol prodrug for hormone replacement therapy and remains a promising candidate for treating hormone-dependent disorders such as endometriosis (Figure 17).
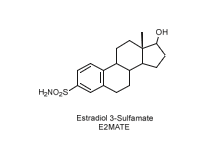
Figure 17: Estradiol 3-sulfamate, a potential candidate as a prodrug in hormone-dependent disease therapies.
In the search for potent steroid sulfatase (STS) inhibitors, efforts were directed toward the development of a non-steroidal compound with oral bioavailability, compliance with Lipinski’s rule of five, and a structural framework resembling that of estrone. A coumarin derivative, 3,4-dimethylcoumarin-7-O-sulfamate (commonly referred to as 667 COUMATE), was identified as a promising candidate. This molecule demonstrated strong inhibitory activity against STS in MCF-7 cells, with an IC₅₀ value of 30 nM (Figure 18).
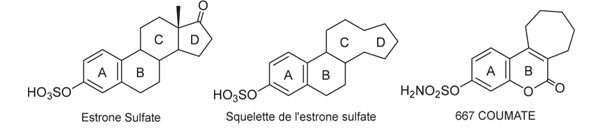
Figure 18: Representation of estrone sulfate, the strome sulfate backbone for the search for new STS inhibitors and the coumarin-derived 667 COUMATE having very good inhibitory activity on STS.
Potential of Sulfamate-Based Compounds for Inhibition of Human Endosulfatases
In 2010, a study reported the inhibitory effects of glucosamine- based monosaccharide derivatives on human endosulfatases. These compounds featured a sulfamate group at the 6-position of the glucosamine residue. Four variants were evaluated: two N-sulfated glucosamines bearing either a primary sulfamate group (1a) or a dimethylated sulfamate group (1b), and two N-acetylated glucosamines with the same respective substitutions (2a and 2b) (Figure 19).
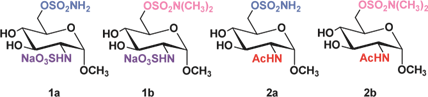
Figure 19: The four differently substituted glucosamine used for the inhibition studies against HSulf1 and 2.
The two N-sulfated glucosamine derivatives, 1a and 1b, demonstrated inhibitory activity against human endosulfatases, with IC₅₀ values of 95 μM and 180 μM for HSulf1, and 130 μM and 240 μM for HSulf2, respectively. The free sulfamate compound 1a exhibited approximately twice the potency of its N, N-dimethylated counterpart 1b. This difference is attributed to increased steric hindrance from the methyl groups in 1b and, more importantly, to the irreversible nature of inhibition by the free sulfamate, compared to the reversible inhibition observed with the dimethylated variant. Notably, the results from derivatives 2a and 2b highlight the critical role of the N-sulfate group on glucosamine. These N-acetylated analogs showed significantly reduced inhibitory activity-approximately 20- to 50-fold lower-relative to their N-sulfated counterparts. While type 1 compounds displayed IC₅₀ values below 250 μM, type 2 compounds inhibited only about 50% of HSulf activity at 5 mM inhibitor concentration [113].
In early 2020, research expanded to include the synthesis of heparan sulfate fragments containing sulfamate groups on one or more glucosamine residues. Initially, oligosaccharides ranging from disaccharides to hexasaccharides were synthesized. Since direct quantification of endosulfatase activity remains technically challenging, surrogate assays measuring either arylsulfatase activity or the release of free sulfate were employed. Using this approach, compounds 3 to 6 were tested, and it was observed that they effectively inhibited the arylsulfatase activity of HSulf1 (Figure 20).
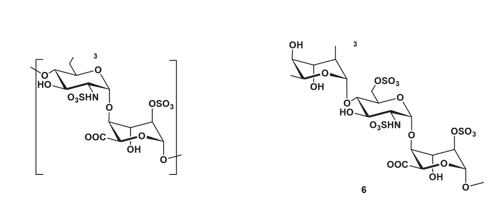
Figure 20: Synthesized HS fragments. Molecules 3 to 5 are of the type [GlcNS(6S)-IdoA(2S)]: with a glucosamine at the non-reducing end while 6 is of the IdoA(2S)-GlcNS(6S) type: with an iduronic acid at the non-reducing end.
The results demonstrated that disaccharide 3 does not compete with 4-methylumbelliferyl sulfate (4-MUS) and is unable to block its access to the catalytic site of HSulf1. In contrast, tetrasaccharide 4, hexasaccharide 5, and 3 kDa heparin were all capable of competing with 4-MUS, indicating their potential to interact with the enzyme’s active site.
To identify the minimal saccharide length required for effective inhibition, trisaccharide 6 was evaluated and showed a marked reduction in 4-MUS hydrolysis. Among the tested fragments, trisaccharide 6 demonstrated the most potent inhibition. The IC₅₀ values for tetrasaccharide 4 and trisaccharide 6 were determined to be 87.8 μM and 3.9 μM, respectively.
This inhibitory effect was further supported by sulfate quantification assays, where greater sulfate release was observed when trisaccharide 6 was used as a substrate. This enhanced activity may be related to structural differences, as trisaccharide 6 terminates with an iduronic acid residue at the reducing end, while tetrasaccharide 4 ends with a glucosamine unit [114].
Subsequently, three additional fragments were synthesized, each containing one or two sulfamate groups at the 6-position of glucosamine-the site targeted by endosulfatase-mediated hydrolysis- to evaluate their inhibitory potential against HSulf1 (Figure 21).
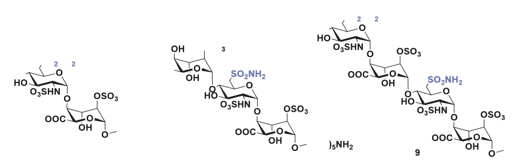
Figure 21: The 3 molecules synthesized to test their inhibitory activity against HSulf1. Molecules 7 and 9 are of the GlcNS-IdoA(2S) type. Disaccharide 7 having a sulfamate at position 6 at the non-reducing end and tetrasaccharide 9 having two sulfamates at position 6 of the two glucosamines. Trisaccharide 8 is of the IdoA(2S)-GlcNS type with a sulfamate at position 6 on glucosamine.
Inhibition assays revealed that disaccharide 7 exhibited the weakest inhibitory effect, reducing HSulf1 activity by only 20% at an inhibitor concentration of 0.7 mM. In contrast, trisaccharide 8 and tetra saccharide 9 showed significantly stronger inhibition, with IC₅₀ values of 0.53 μM and 29.6 μM, respectively. Among the three, disaccharide 7 was clearly the least effective. Trisaccharide 8, which features an IdoA-GlcNS motif, demonstrated the highest inhibitory activity, outperforming tetrasaccharide 9, which contains a GlcNS-IdoA sequence and bears two sulfamate groups [115].
Kinetic analysis of trisaccharide 8 provided a Ki value of 0.36 μM, and surface plasmon resonance (SPR) measurements determined its dissociation constant (KD) with the hydrophilic domain (HD) of HSulf1 to be 12 nM, indicating strong binding affinity.
These findings establish that a trisaccharide is the minimal structural requirement for effective interaction with the catalytic site of HSulf1, competing with the pseudo-substrate 4-MUS. Substituting the 6-O-sulfate of the glucosamine residue in compound 6 with a sulfamate group, as in compound 8, results in a potent inhibitor with favorable inhibition constants (IC₅₀ = 0.53 μM, Ki = 0.36 μM, KD = 12 nM). Notably, the higher inhibitory efficacy of trisaccharide 8, which has a non-reducing end iduronic acid, compared to tetrasaccharide 9, which features a non-reducing end glucosamine, underscores the importance of terminal sugar identity [116].
This study represents the first reported example of heparan sulfate fragments containing sulfamate groups acting as inhibitors of HSulf1 and supports the strategy of targeting HSulf2 using similar chemical designs. The promising inhibition profiles highlight the potential for developing novel and selective endosulfatase inhibitors [117].
In a related effort, a set of HSulf2 inhibitors incorporating either a classical sulfamate or a novel trichloroethylsulfamate moiety- tested here for the first time-was synthesized. To enhance specificity for HSulf2, the compounds were designed through molecular modeling by aligning their structures with heparin to replicate the spatial distribution of negative charges. This guided the synthesis of biphenyl and biphenyl ether derivatives bearing sulfamate and/ or trichloroethylsulfamate functionalities (Figure 22).
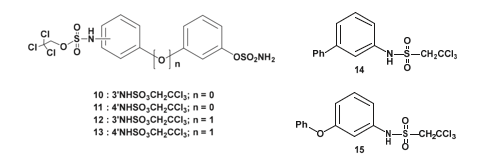
Figure 22: Molecules synthesized for HSulf2 inhibition. synthesized and tested the inhibitory activity of about thirty molecules, but only the molecules with the best inhibitions are represented here.
The inhibitory activity of the synthesized molecules was evaluated against HSulf2, as well as the arylsulfatases ARSA and ARSB, to assess their selectivity toward endosulfatases. All compounds shown in Figure 22 exhibited greater than 95% inhibition of HSulf2 at a concentration of 1 mM.
However, they also demonstrated notable inhibitory effects on ARSA and ARSB, in many cases exceeding their inhibition of HSulf2. For instance, compound 10 showed an IC₅₀ of 167 μM for HSulf2, but lower values of 55 μM and 130 μM for ARSA and ARSB, respectively. Similarly, compounds 11 through 15 exhibited IC₅₀ values exceeding 250 μM for HSulf2 while maintaining IC₅₀ values below 150 μM for ARSA and ARSB [118].
These findings indicate that the synthesized inhibitors lack selectivity for HSulf2 and display relatively weak binding affinity for this enzyme. The spatial arrangement of negatively charged substituents alone appears insufficient to ensure effective interaction with the Hydrophilic Domain (HD) of HSulf2. Nevertheless, the study successfully demonstrated that the trichloroethylsulfamate moiety can function as a novel sulfatase inhibitor. The precise mechanism by which this functional group exerts its inhibitory effect remains to be elucidated.
Mechanism of Sulfatase Inhibition by the Sulfamate Group
The inhibitory mechanism of sulfatases by sulfamate-based compounds has been thoroughly investigated, particularly through studies on the arylsulfatase from Pseudomonas aeruginosa. Initial analyses revealed that irreversible enzyme inactivation begins with the cleavage of the RO–SO₂NH₂ bond within the sulfamate group. This discovery enabled determination of the inhibition stoichiometry, with enzyme assays using four different coumarin derivatives indicating that irreversible inhibition requires approximately three to six inhibitor molecules per enzyme molecule [119]. Further investigations showed that the sulfamate hydrogen is relatively acidic, with pKa values ranging from 7 to 8, suggesting that the deprotonation step is likely facilitated by a basic residue within the enzyme’s active site. These results support an E1cb-type elimination mechanism: deprotonation of the sulfamate nitrogen triggers cleavage of the oxygen–sulfur bond, leading to formation of a sulfamoyl intermediate (SO₂NH). Energy calculations suggest that this species is sufficiently stable to exist transiently. The resulting iminosulfur dioxide intermediate may then react covalently with catalytic residues such as formylglycine, resulting in enzyme inactivation (Figure 23). This multistep mechanism is consistent with the observed inhibitor stoichiometry and the requirement for the presence of an N–H bond within the sulfamate group [120].
The E1cb pathway also explains why N, N-dimethylated sulfamate analogs, which lack a proton on the nitrogen, fail to cause irreversible inhibition-as illustrated by compounds 1a and 1b (Schema 14). Without this proton, the initial deprotonation step cannot occur, thereby preventing the cascade of reactions needed for irreversible enzyme inactivation [121] (Figure 23).
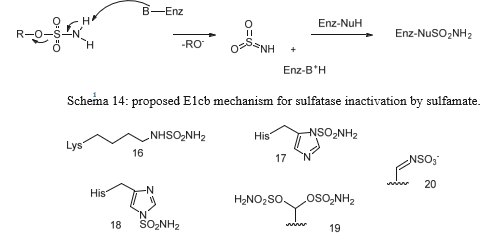
Figure 23: Some hypothesized products resulting from the reaction of the iminosulfan dioxide intermediate SO2NH with the residues present in the catalytic site that would lead to the inactivation of the enzyme. Product 20 is the one hypothesized to lead to the irreversible transformation of formylglycine.
Compound 20 was identified as the species responsible for sulfatase inactivation. It results from the covalent reaction between the SO₂NH intermediate and the formylglycine residue located within the enzyme’s active site (Schema 15).
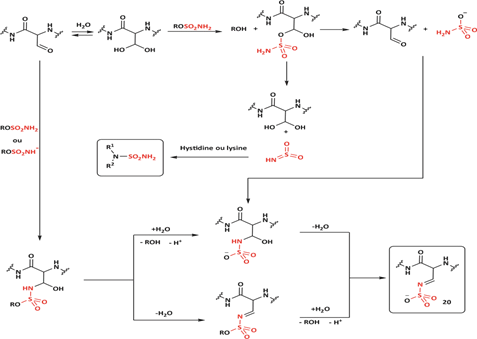
Scheme 16: Diagram representing the compounds that can be formed during the inhibition of the catalytic site of sulfatases by a sulfamate.
Schema 16 illustrates the potential products that may be generated during the inhibition of endosulfatases by a compound containing a sulfamate functional group [113].
The discovery of the sulfamate group as an inhibitor of sulfatases dates back to 1994, when researchers were investigating inhibitors of steroid sulfatase involved in hormone-dependent breast cancers. Subsequently, sulfamates were also used to synthesize inhibitors of endosulfatases, enzymes implicated in various cancers. The identification of its mechanism of inhibition revealed that sulfamates act as irreversible inhibitors through the cleavage of the oxygen-sulfate bond. The resulting SO₂NH intermediate can then interact with various residues in the enzyme’s active site, leading to enzyme inhibition. This mechanism explains the inhibition stoichiometry, which ranges between 3 and 6 [122].
While the complete mechanism of sulfatase inhibition by sulfamates remains incompletely understood, these molecules do not form covalent bonds with the substrate but rather cause destruction of the catalytic site. The inhibition of the catalytic site via a covalent bond with formylglycine holds great potential for further enzyme studies.
Other Functional Groups Reacting with Aldehydes
The presence of an aldehyde group within sulfatases offers a significant advantage for bioconjugation, as this electrophilic functional group exhibits reactivity orthogonal to the amino acids and functional groups within proteins. It can thus react with a wide variety of functionalized molecules to enhance protein properties.
For example, conjugation of Polyethylene Glycol (PEG) to a protein or drug can extend its half-life, coupling with a fluorescent probe can enable in vivo imaging or protein tracking, and the formation of Antibody-Drug Conjugates (ADCs) is a growing area in cancer therapy. The presence of this functional group is so advantageous that numerous research groups have sought to incorporate it into various proteins through chemical, genetic, or enzymatic methods [123].
Aldehyde Incorporation into Proteins
Aldehydes can be generated through the oxidation of threonine or serine residues at the N-terminal of a protein using sodium periodate (NaIO₄). This reaction, known as the “Malaprade reaction,” is analogous to the oxidation of vicinal diols (Schema 17).
The resulting electron-deficient aldehyde is termed -oxo aldehyde or glyoxylamide (Scheme 18). This oxidation reaction is advantageous as it is 100 to 1000 times faster than the oxidation of vicinal diols, although it can also oxidize methionine and cysteine residues within the protein.
Scheme 18: Formation of -oxo aldehyde by reaction of an N-terminal serine or threonine with sodium periodate.
Another method for selectively oxidizing the N-terminal residue has been developed, enabling its application to proteins containing alanine, glycine, aspartate, glutamate, or glutamine at the N-terminal position. This oxidation process is a biomimetic transamination that utilizes pyridoxal 5-phosphate (the active form of vitamin B6, which is involved in amino acid metabolism). The aldehyde group of pyridoxal 5-phosphate condenses with the amine of the amino acid to form an imine. Following a rearrangement and hydrolysis, the -oxo aldehyde is generated (Schema 19). This reaction is a transamination of the amino acid with pyridoxal 5-phosphate [124].
Scheme 19: Mechanism of formation of -oxo aldehyde by biomimetic transamination of the N-terminal residue using pyridoxal-5-phosphate.
In 2006, Poulter’s group developed a method based on farnesyltransferase (PFTase) for functionalizing proteins. PFTase is an enzyme that adds an isoprenoid lipid, such as farnesyl, to the thiol groups of cysteines in proteins (farnesylation). This enzyme is specific for a cysteine located within a C-terminal tetrapeptide and utilizes Farnesyl Pyrophosphate (FPP) for this modification. PFTase can also use FPP derivatives to incorporate various functional groups into the protein. For instance, Poulter’s group initially employed FPP derivatives to introduce groups, such as azides or alkynes, that enable Huisgen cycloaddition (Schema 20).
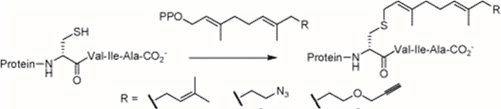
Scheme 20: Functionalization of the protein with PPF derivatives comprising groups allowing the Huisgen reaction such as azide or alkyne. The tetrapeptide essential for the recognition of PFTase is generally: cysteine-valine-isoleucine-alanine.
Subsequent research groups developed additional derivatives of FPP containing aldehyde groups to enable the functionalization of target proteins (Figure 24).
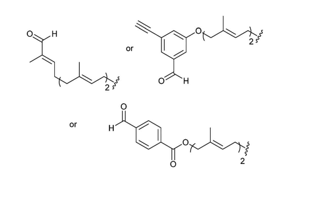
Figure 24: Examples of different derivatives of FPP allowing the introduction of an aldehyde function on the target protein.
Tubulin tyrosine ligase (TTL) is an enzyme that post-translationally modifies α-tubulin by adding a tyrosine residue to its C-terminal end. Recent work by a research group employed this enzyme to introduce tyrosine analogs containing an aldehyde group, such as 3-formyl-L-tyrosine (3ForTyr) (Schema 21). The incorporation of this derivative was optimized, achieving a conversion rate of up to 99% [122].
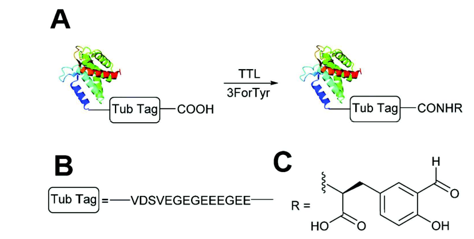
Scheme 21: A) Tubulin tyrosine ligase recognizes the “Tub Tag” present at the C-terminal end of tubulin and adds a tyrosine residue to it. B) The “Tub Tag” is a peptide of 14 amino acids allowing the recognition of TLL. C) The tyrosine derivative used to introduce an aldehyde function on the protein: 3-formyl tyrosine.
Starting in 2007, a research group utilized the enzyme formylglycine- generating enzyme (FGE), which modifies cysteine residues to form formylglycine in sulfatases, to incorporate aldehydes into proteins. The minimal pentapeptide required for FGE activity (CXPXR) was introduced at the N- or C-terminal of the protein sequence, and recombinant proteins were expressed in cells containing FGE to facilitate the aldehyde incorporation. This method demonstrated a conversion rate exceeding 85% of cysteine to formylglycine [119-124].
A second enzyme, AtsB, allows the formation of formylglycine from cysteine or serine. AtsB is a member of the “Radical S-Adenosyl Methionine” (radical-SAM) protein family and is almost exclusively found in prokaryotes. This enzyme catalyzes the conversion of deoxyadenosine and cysteine to formylglycine (Schema 22).
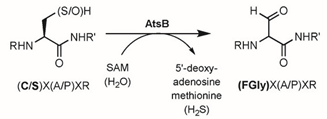
Scheme 22: Formation of FGly from a cysteine or serine by the enzyme AtsB. The pentapeptide required for the recognition of the enzyme is (C/S)X(A/P)XR.
A research group utilized an anaerobic enzyme to incorporate aldehydes into proteins using serine residues. By employing two different recognition peptides, they were able to convert two cysteines into aldehydes on the same protein: AtsB converted the cysteine in the peptide CTAGR to formylglycine, while FGE converted the cysteine in the peptide CTPSR to formylglycine [125-128].
Modifications of Enzymes Containing an Aldehyde Group
There are numerous methods for modifying substrates that contain an aldehyde group, developed based on the desired type of bond, the reactivity of the aldehyde, and modification conditions such as pH or the addition of co-solvents. The most commonly developed methods involve the formation of oximes or hydrazones. This section presents four distinct methods employed for the functionalization of aldehydes (Schema 23).
Reactions involving hydrazine or oxime groups with aldehyde- containing proteins are among the most widely used and optimized methods. The formation of oximes and hydrazones occurs through the condensation of an oxime/hydrazide with an aldehyde/ ketone under acidic catalysis (pH 3–7). This reaction releases a water molecule and is reversible, as the generated water can hydrolyze the C=N bond formed (Schema 24).
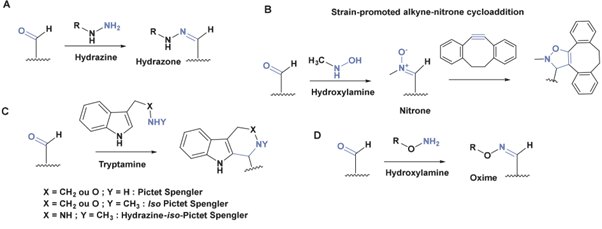
Scheme 23: Representation of four methods used for the functionalization of molecules with an aldehyde function. A) Functionalization by formation of a hydrazone from a hydrazine. B) Functionalization of the aldehyde by reaction with an oxyamine to form an intermediate nitrone that reacts in a cycloaddition [3+2] with a cyclic alkyne. C) Functionalization of an aldehyde with tryptamine derivatives by Pictet-Spengler reactions. D) Functionalization of the aldehyde by reaction with an oxyamine to form an oxime.
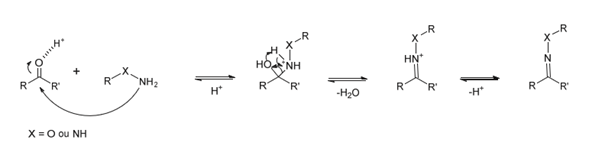
Scheme 24: Mechanism of oxime/hydrazone formation from oxyamine/hydrazide and aldehyde/ketone, catalyzed by acid.
The hydrolysis of the C=N double bond begins with the protonation of the nitrogen. The atom X directly attached to the nitrogen influences the stability of the conjugate: the more electronegative X is, the lower the basicity of the nitrogen. Therefore, an oxime is more stable than a hydrazone, which in turn is more stable than an imine (electronegativity values: p(O) = 3.5; p(N) = 3.0; p(C) = 2.6). The stability of the double bond is also reduced under acidic conditions and at high temperatures [124-128].
The Pictet-Spengler reaction is a cyclization process between an aldehyde and a tryptamine, proceeding through an iminium intermediate, which results in the formation of a C-C bond between the two reactants Schema 25. The product formed is stable. This reaction has been carried out on tryptamines containing a free amine, an oxime group, a methylated amine (iso-Pictet-Spengler), and methylated hydrazides (iso-Pictet-Spengler). The reaction can be performed in a pH range of 4 to 7 [126-130]. The Pictet-Spengler reaction has been utilized to conjugate PEG, biotin, and fluorescent probes to α-oxo aldehyde-myoglobins, maltose-binding proteins, and FGly antibodies. The Huisgen reaction between azides and alkynes is well-established for protein functionalization. However, it is copper-catalyzed, which limits its applicability in living systems due to the toxicity of copper to both bacterial and mammalian cells. To address this, a new [3+2] cycloaddition method was developed that does not require a catalyst. By using cyclic alkynes with significant ring strain, the reaction is accelerated and can proceed in physiological conditions. Further advancements by Van Delft’s group involved reacting nitrones with cyclic alkynes, which provided a higher reaction rate and enabled the use of aldehydes Schema 26. The formation of the cyclo-adduct proceeds in three steps starting from the protein’s N-terminal residue: first, the residue is oxidized to an aldehyde, which then reacts with an oxyamine to form a nitrone, which subsequently reacts with the alkyne [122-125].
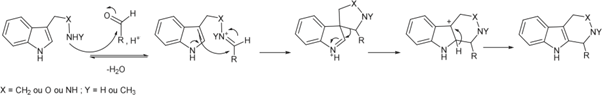
Scheme 25: Mechanism of the Pictet Spengler reaction between an aldehyde and a tryptamine leading to the formation of a C-C ring and bond.
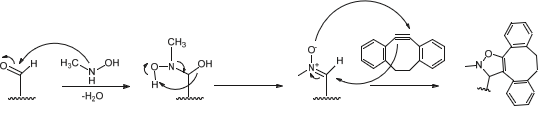
Scheme 26: Mechanism for the functionalization of substrate by cycloaddition [3+2]. The aldehyde formed from the N-terminal residue of the protein reacts with oxyamine to form nitrone which in turn reacts with the cyclic alkyne.
This functionalization method has been employed to add a PEG group to interleukin 8. The discovery of endosulfatases in the early 2000s has led to limited knowledge of these enzymes. However, many studies have highlighted the involvement of HSulfs in diseases such as cancer, making them important therapeutic targets. Despite this, there has been little research focused on the development of specific inhibitors for endosulfatases. Currently, the most promising strategy is the synthesis of inhibitors that mimic their natural substrate, heparan sulfate. Researchers have synthesized mimetics containing a sulfamate group, which irreversibly inhibit endosulfatases with a Ki in the micromolar range [125-127].
The sulfamate group, discovered in 1994 as an inhibitor of sulfatases during the search for steroid sulfatase inhibitors, mimics the sulfate group. It is also hydrolyzed by sulfatases via an E1cb mechanism, resulting in a sulfonylamine that reacts with residues in the enzyme’s catalytic site and formylglycine, leading to enzyme inactivation. This inhibition mechanism has a stoichiometry of 3 to 6. The hydrolysis of the sulfamate group and the degradation of the catalytic site prevent co-crystallization of the enzyme with its substrate [128-130].
The synthesis of inhibitors that include a functional group enabling bioconjugation presents a promising alternative. Endosulfatases are advantageous in that they possess a formylglycine group. Various groups have developed chemical, enzymatic, or genetic methods to introduce aldehydes into proteins that do not naturally contain them, facilitating their functionalization. Once the carbonyl group is introduced, several reactions can be employed to attach molecules of interest to the target protein, such as the formation of oximes or hydrazones, the Pictet-Spengler reaction, or [3+2] cycloaddition via a nitrone intermediate.
Conclusion
The endosulfatase enzyme family, comprising HSulf1 and HSulf2, stands as a crucial modulator of Heparan Sulfate (HS) within the extracellular matrix. Their specific action in hydrolyzing 6-O-sulfate groups on glucosamine residues plays a pivotal role in regulating the intricate structural landscape of HS chains. This structural heterogeneity of HS is fundamental to its ability to interact with a diverse array of proteins, thereby influencing a wide spectrum of biological processes, including cellular communication, development, and tissue repair. The dysregulation of HS sulfation patterns, however, has been implicated in the pathogenesis of various diseases, notably cancer, inflammation, and metabolic disorders, underscoring the therapeutic relevance of endosulfatases. The unique extracellular activity of endosulfatases at neutral pH distinguishes them from other sulfatases that function within the lysosomal environment. This specificity highlights their potential as therapeutic targets, offering a more selective approach to modulating HS-protein interactions without broadly affecting lysosomal sulfatase activity. Understanding the precise mechanisms by which endosulfatases influence these interactions is paramount for developing targeted therapeutic interventions.
The current landscape of endosulfatase inhibition primarily focuses on the development of substrate mimetics. Among these, sulfamate-containing compounds have shown promise, acting as irreversible inhibitors with micromolar affinities. The sulfamate group, mimicking the sulfate moiety of the natural substrate, undergoes hydrolysis by the enzyme via an E1cb mechanism. This process leads to the formation of a sulfonylamine intermediate that reacts with the catalytic site, involving the formylglycine residue, ultimately resulting in enzyme inactivation. The mechanism, characterized by a 3 to 6 stoichiometry, unfortunately complicates structural studies through co-crystallization due to the degradation of the enzyme’s active site. To circumvent these challenges, a promising alternative strategy lies in the synthesis of inhibitors equipped with functional groups that enable bioconjugation. The presence of the formylglycine group within the active site of endosulfatases presents a unique opportunity for targeted modification. Leveraging chemical, enzymatic, or genetic methodologies to introduce carbonyl groups into proteins, even those lacking them naturally, opens avenues for attaching molecules of interest to the target enzyme. Subsequent reactions, such as oxime or hydrazone formation, the Pictet-Spengler reaction, or [3+2] cycloadditions utilizing nitrone intermediates, offer versatile approaches for conjugating inhibitors or other therapeutic agents to endosulfatases.
The development of specific and effective inhibitors for HSulf1 and HSulf2 holds significant promise for the treatment of diseases characterized by aberrant HS sulfation. By precisely modulating endosulfatase activity, it may be possible to disrupt pathological interactions involving HS and various signaling molecules, thereby offering novel therapeutic avenues for cancer and inflammatory disorders. Future research should focus on refining existing inhibitor designs to enhance potency and selectivity, as well as exploring innovative strategies for targeted drug delivery to the extracellular matrix where these enzymes exert their activity. The potential to harness the unique catalytic mechanism and active site features of endosulfatases for therapeutic gain represents a compelling direction in drug discovery.
Acknowledgement
None.
Conflict of Interest
None.
References
- Thallaj N (2021) Synthesis of a New Ligand Tris (2-pyridylmethyl) amine functionalized by a methoxy group and study of Dichloroferrous complexes, its reactivity to dioxygen both in the presence and absence of substrate. International journal of applied chemistry and biological sciences 2(4): 65-77.
- L Labban, N Thallaj, Z Malek (2020) International Journal of Medical Studies 5(12): 23-36.
- L Labban, M Kudsi, Z Malek, N Thallaj (2020) Pain Relieving Properties of Ginger (Z officinale) and Echinacea (E.angustifolia) Extracts Supplementation among Female Patients with Osteoarthritis. A Randomized Study. Advances in Medical, Dental and Health Sciences 3(3): 45-48.
- L Labban, N Thallaj, M Al Masri (2020) Journal of Advanced Research in Food Science and Nutrition 3(1): 34-41.
- Thallaj N, agha M I H, nattouf AH, katib CH, karaali A et al. (2020) Evaluation of Antimicrobial Activities and Bioactive Compounds of Different Extracts Related to Syrian Traditional Products of Damask Rose (Rosa damascena). open access library journal 7(5): 1-21.
- L labban, N Thallaj, A labban (2020) Archives of Medicine 12(2): 1-5.
- L labban, N Thallaj (2020) International Journal of Herbal Medicine 8(2): 33-37.
- L Labban, N Thallaj, Z Malek (2019) The implications of E-cigarettes or "vaping"on the nutritional status. Journal of Medical Research and Health Sciences 2(11): 784-787.
- L labban, N Thallaj (2019) The Effect of Magnesium Supplementation on Hba1c Level and Lipid Profile Among Type 2 Diabetics. Acta Scientific Nutritional Health 3(10): 7-12.
- Malek ZS, Labban L (2020) The International Journal of Neuroscience 1-7.
- Malek ZS, Labban L (2019) A Comparative Study of Tryptophan Hydroxylase's Circadian Rhythm in the Functional Parts of Dorsal Raphe Nuclei in the Mesencephalon European Journal of Pharmaceutical and Medical Research 6(11): 527-532.
- Malek ZS (2018) Journal of AlBaath University 40(4): 39-62.
- Malek ZS (2018) Analytical Study for the Determination of Norfloxacin in Pure and Pharmaceutical Formulations using Alizarin by Visible Spectrophotometric Method. Tishreen University Journal for Research and Scientific Studies 40(2).
- ZS Malek, LM Labban (2021) Photoperiod regulates the daily profiles of tryptophan hydroxylase-2 gene expression the raphe nuclei of rats. International Journal of Neuroscience 131(12): 1155-1161.
- ZS Malek, LM Labban (2020) Photoperiod regulates the daily profiles of Tryptophan Hydroxylase-2 gene expression the raphe nuclei of rats. Journal of current research in physiology and pharmacology 4(1): 1-5.
- LM Labban, MM Alshishkli, A Alkhalaf, Z Malek (2017) J Adv Res Dent Oral Health 2(3&4): 1-4.
- L Labban, ZS Malek (2018) Open Access Library Journal 5(07): 1-11.
- L Labban, ZS Malek (2019) Ann Food Nutr Res J 1: 1
- Labban L, N Thallaj (2019) Acta Scient Nutr Health 3: 7-12.
- N Thallaj (2022) Tishreen university journal 44(1): 59-77.
- N Thallaj (2022) Tishreen university journal 44(2): 87-105.
- A Abbood, N Thallaj (2023) Arab Journal of Pharmaceutical Sciences 7(1).
- N Thallaj (2023) Tishreen University Journal-Medical Sciences Series 44(6): 21-29.
- Machkour A, Thallaj NK, Benhamou L, Lachkar M, Mandon D (2006) The coordination chemistry of FeCl3 and FeCl2 to bis[2-(2,3-dihydroxyphenyl)-6-pyridylmethyl] (2-pyridylmethyl) amine: access to a diiron (III) compound with an unusual pentagonal-bipyramidal/square-pyramidal environment. Chemistry 12(25): 6660-
- Thallaj N, Machkour A, Mandon D, Welter R (2005) Square pyramidal geometry around the metal and tridentate coordination mode of the tripod in the [6-(3′-cyanophenyl)-2-pyridylmethyl] bis(2-pyridylmethyl) amine FeCl2 complex: a solid-state effect New J Chem 29: 1555 - 1558.
- Thallaj NK, Rotthaus O, Benhamou L, Humbert N, Elhabiri M (2008) Reactivity of molecular dioxygen towards a series of isostructural dichloroiron(III) complexes with tripodal tetraamine ligands: general access to mu-oxodiiron(III) complexes and effect of alpha-fluorination on the reaction kinetics. Chemistry-A European Journal 14(22): 6742-6753.
- Wane A, Thallaj NK, Mandon D (2009) Biomimetic Interaction between FeII and O2: Effect of the Second Coordination Sphere on O2 Binding to FeII Complexes: Evidence of Coordination at the Metal Centre by a Dissociative Mechanism in the Formation of μ-Oxo Diferric Complexes. Chemistry 15(40): 10593-10602.
- Thallaj NK, Orain PY, Thibon A, Sandroni M, Welter R (2014) Steric congestion at, and proximity to, a ferrous center leads to hydration of α-nitrile substituents forming coordinated carboxamides. Inorg Chem53(15): 7824-7836.
- NK Thallaj, J Przybilla, R Welter, D Mandon (2008) A Ferrous Center as Reaction Site for Hydration of a Nitrile Group into a Carboxamide in Mild Conditions. J Am Chem Soc 130: 2414-2415.
- NK Thallaj, D Mandon, KA White (2007) The Design of Metal Chelates with a Biologically Related Redox-Active Part: Conjugation of Riboflavin to Bis(2-pyridylmethyl) amine Ligand and Preparation of a Ferric Complex. Eur J of Inorg Chem 2007: 44-47.
- Thallaj N (2021) Synthesis of a New Ligand Tris (2-pyridylmethyl) amine functionalized by a methoxy group and study of Dichloroferrous complexes, its reactivity to dioxygen both in the presence and absence of substrate. International journal of applied chemistry and biological sciences 2(4): 65-77.
- Thallaj N (2023) Review of a Few Selected Examples of Intermolecular Dioxygenases Involving Molecular Oxygen and Non-Heme Iron Proteins. Int J Adv Parmacutical Sci Res (IJAPSR) 3: 1-18.
- L Labban, M Kudsi, Z Malek, N Thallaj (2020) Pain Relieving Properties of Ginger (Z. officinale) and Echinacea (E. angustifolia) Extracts Supplementation among Female Patients with Osteoarthritis. A Randomized Study. Advances in Medical, Dental and Health Sciences 3(3): 45-48.
- L Labban, N Thallaj, M Al Masri (2020) The Nutritional Value of Traditional Syrian Sweets and Their Calorie Density. Journal of Advanced Research in Food Science and Nutrition 3(1): 34-41.
- L labban, N Thallaj, A labban (2020) Assessing the Level of Awareness and Knowledge of COVID 19 Pandemic among Syrians. archives of medicine 12(2): 1-5.
- L Labban, N Thallaj, Z Malek (2019) The implications of E-cigarettes or "vaping “on the nutritional status. Journal of Medical Research and Health Sciences 2(11): 784-787.
- Malek ZS, Sage D, Pevet P, Raison S (2007) Daily Rhythm of Tryptophan Hydroxylase-2 Messenger Ribonucleic Acid within Raphe Neurons Is Induced by Corticoid Daily Surge and Modulated by Enhanced Locomotor Activity. Endocrinology 148 (11): 5165-5173.
- Malek ZS, Dardente H, Pevet P, Raison S (2005) Tissue-specific expression of tryptophan hydroxylase mRNAs in the rat midbrain: anatomical evidence and daily profiles. European Journal of Neuroscience 2005 22(4): 895-901.
- A Abbood, SA Malik, D aldiab, HH Ali, N Thallaj (2025) Investigation of the charge variant profile of non-cleavable conjugated antibodies. Research J Pharm and Tech 18(1): 185-190.
- Malek S, Pevet P, Raison S (2004) Neuroscience 125(3): 749-758.
- Malek ZS, Labban L (2020) The International Journal of Neuroscience :1-7.
- ZS Malek, LM Labban (2020) Journal of current research in physiology and pharmacology 4(1): 1-5.
- LM Labban, MM Alshishkli, A Alkhalaf, Z Malek (2017) J Adv Res Dent Oral Health 2(3&4): 1-4.
- L Labban, ZS Malek, Open Access Library Journal, 2018, 5 (07), 1-11.
- Y alhomush, Z malek, A Abboud, N Thallaj (2022) Research Journal of Pharmacy and Technology 15: 10.
- A Abbood, Z Malek, N Thallaj (2022) Research Journal of Pharmacy and Technology 15(11): 4935-4939.
- Thallaj N, agha MIH, nattouf AH, katib CH, karaali A, Moustapha A, et al. (2020) open access library journal 7(5): 1-21.
- N Thallaj (2021) Indian journal of advanced chemistry 1(2): 20-26.
- N Thallaj (2022) Indian journal of advanced chemistry 2(2): 1-11.
- N Thallaj (2022) Indian journal of advanced chemistry 2(1): 5-9.
- N Thallaj (2022) Indian journal of advanced chemistry 2(1): 10-14.
- N Thallaj, Xi'an ShiyouDaxueXuebao (ZiranKexue Ban) (2022) Journal of Xi'an Shiyou University, Natural Sciences Edition 65(06): 289-301.
- N Thallaj, Xi'an ShiyouDaxueXuebao (ZiranKexue Ban) (2022) Journal of Xi'an Shiyou University, Natural Sciences Edition 65(06): 313-328.
- Z MALEK, A ABBOOD, N THALLAJ, Xi'an ShiyouDaxueXuebao (ZiranKexue Ban) (2022) Journal of Xi'an Shiyou University, Natural Sciences Edition 65(06): 302-312.
- N Thallaj, Xi'an Shiyou Daxue Xuebao (Ziran Kexue Ban) (2022) Journal of Xi'an Shiyou University, Natural Sciences Edition 65(7): 169-184.
- Z MALEK, Xi'an Shiyou Daxue Xuebao (Ziran Kexue Ban) (2022) Journal of Xi'an Shiyou University, Natural Sciences Edition 65(7): 143-152.
- Thallaj, Xi'an Shiyou Daxue Xuebao (Ziran Kexue Ban) (2022) Journal of Xi'an Shiyou University, Natural Sciences Edition 65(7): 110-142.
- N Thallaj (2023) Tishreen University Journal-Medical Sciences Series 44(6): 21-29.
- N Thallaj (2022) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 2(3): 1-28.
- N Thallaj (2022) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 2(4): 1-15.
- N Thallaj (2023) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 3(2): 1-18.
- N Thallaj (2022) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 2(6): 1-12.
- N Thallaj (2023) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 3(3): 1-10.
- N Thallaj (2024) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(1): 32-52.
- N Thallaj (2024) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(5): 29-49.
- N Thallaj (2024) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(4): 7-21.
- N Thallaj. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 2024.4, 6,7-27.
- N Thallaj (2024) International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR)4(6): 33-48.
- Besherb S, Alallan L, Hassan Agha MA, AIshamas I, Thallaj N, et al. (2024) Influence of soil salinity on the chemical composition of essential oil of Rosmarinus Officinalis in Syria, Research J. Pharm. and Tech 17(5).
- Thallaj N (2024) Advancements in Pharmaceutical Science: Synthesis and Application of Molecular Cages Integrating N-Heterocyclic Carbenes for Enhanced Stability and Functionality. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 5(1): 6-19.
- Ayat Abbood, Hassan Hadi Ali, Samir Azzat Malik, Dima AlDiab, Nasser Thallaj, et al. (2025) Investigation of the Charge Variant Profile of Non-cleavable Conjugated Antibodies. Research Journal of Pharmacy and Technology 18(1): 185-190.
- Thallaj N (2025) Analyzing Charge Variant Profiles of Monoclonal Antibodies Conjugated to Cytotoxic Agents. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(3): 20-26.
- Thallaj N (2025) Biomimetic Synthesis and Phytochemical Analysis of Lodopyridone: Insights into 4-Pyridone Derivatives and Thiopeptide Antibiotic. Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(3): 9-19.
- Mousa Al Khleif, Nour Alhuda Alzoubi, Ghassan Shannan, Zeina S Malek, Nasser Thallaj (2025) Exploring Circadian Rhythms:A Comprehensive Study on Sleep Patterns and Disorders in Syrian Society. Am J Biomed Sci & Res 26(2).
- Ranim Abdul Rahman, Louai Alallan, Ahmed Khalid Aldhalmi, Nasser Thallaj (2025) Separation, Determination, and Potential Application of Active Compounds in Porosopis cineraria: A Comprehensive Analysis. Research Journal of Pharmacy and Technology 18(4):1604-1610.
- Dalia Aboufakher, Rita Zeinaldin, Racha Khatib, Rawa Khreit, Mohamed Sami Joha, et al. (2025) Prevalence and AntibioticResistance Patterns of Multidrug-Resistant Gram-Negative Bacilli in Hospitalized Patients in Sweida, Syria. Am J Biomed Sci & Res. 2025 26(3): 309-315
- Besher S, Alallan L, Hasan Agha MI, Alshamaa I, Thallaj N (2024) Influence of Soil Salinity on the Chemical Composition of Essential Oil of Rosmarinus officinalis in Syria. Research Journal of Pharmacy and Technology 17(5): 2282- 2288.
- Khatib O, Alshimale T, Alsaadi A, Thallaj N (2024) The Global Impact of HIV: A Comprehensive Review. IJAPSR 4(3): 6-19.
- Samer alkhoury, Rasha Kateeb, Rawa Akasha and Nasser Thallaj (2025) Analysis of Crocin Content in Saffron (Crocuss ativus L) Cultivated in Syria Using Liquid Chromatography-Mass Spectrometry. Am J Biomed Sci & Res. 2025 26(3).
- Zanboua R, Abbood A (2024) Survey of Knowledge About the Interaction Between Food and Drugs Among the Syrian Population. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(4): 22-28.
- Qattan M, Dashash M, S Malek Z (2024) Enhancing Academic Success: A mixed Study on the Influencing Factors among Pharmacy Students in Syrian Universities. F1000Res 13: 868.
- Thallaj N (2022) Design and Synthesis Ligands Tetradents Substituted with Halogenes in α- Position and Conjugation with Riboflavin (Bioconjugates): Conjugate ligands Type TPA’s with Flavonoids as un–Electron Mediator. Biomedicine and Chemical Sciences, 1(2): 47-56.
- Thallaj N, Alrasho JF, Sofi FK (2024) Advancements in Antiviral Therapeutics: A Comprehensive Review of Hepatitis C Virus and Novel Flavone Leads. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 5(1): 28-40.
- Thallaj N (2025) Analyzing Charge Variant Profiles of Monoclonal Antibodies Conjugated to Cytotoxic Agents. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 5(3): 20-26.
- Thallaj N (2025) Biomimetic Synthesis and Phytochemical Analysis of Lodopyridone: Insights into 4-Pyridone Derivatives and Thiopeptide Antibiotics. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 5(3): 9-19.
- Ghassan Shannan, Zeina S (2025) Malek and Nasser Thallaj. Pactamycin: A Comprehensive Review of Its Biological Activity, Biosynthesis, And Synthetic Strategies in the Fight Against Antibiotic Resistance. European Journal of Biomedical and Pharmaceutical Sciences (EJBPS) 12(4): 334-353.
- Ghassan Shannan, Zeina S (2025) Malek and Nasser Thallaj. A Review of Antibiotic-Induced Drug Allergies: Mechanisms, Prevalence, And Future Perspectives. European Journal of Biomedical and Pharmaceutical Sciences (EJBPS) 12(4): 387-399.
- Alma Soudan, Joudy Abokassem, Shaam Ammar and Nasser Thallaj (2025) Advancements in the Total Synthesis of Cyclotripeptidic Natural Products: Exploring the Therapeutic Potential of Marine-Derived Compounds. European Journal of Biomedical and Pharmaceutical Sciences (EJBPS) 12(4): 354-377.
- Labban L, Thallaj N, Labban A (2020) Assessing the level of awareness and knowledge of COVID 19 pandemic among syrians. Arch Med 12(2): 8.
- Labban L, Kudsi M, Malek Z, Thallaj N (2020) Advances in Medical. Dental and Health Sciences 3(3): 45-48.
- Thallaj N (2021) Efficiency in transporting molecular oxygen to iron (II) complexes with ligands type tri (2-pyridylmethyl) amine substitution aromatic in (α) position by a mechanism that mimics biological oxidation.
- Labban L (2020) International Journal. IJMS 5(12): 23-36.
- Thallaj N (2022) Tishreen University Journal-Medical Sciences Series 44(1): 59-77.
- Thallaj N (2020) Evaluation of antimicrobial activities and bioactive compounds of different extracts related to syrian traditional products of damask rose (Rosa damascena). Open Access Library Journal 7(05): 1.
- Thallaj N (2023) Characterization of charge heterogeneity of antibody-drug conjugate by anion-exchange chromatofocusing. Tishreen University Journal-Medical Sciences Series 44(6): 21-29.
- Labban L, M Kudsi, Z Malek, N Thallaj (2020) Advances in Medical, Dental and Health Sciences 3(3): 45-48.
- Thallaj N (2023) A Brief Overview of the General Characteristics and Reactivity Towards Dioxygen of the Ferrous Tris (2-Pyridylmethyl Amine) Series Complexes is Presented. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 3(3): 1-18.
- Abbood A, Thallaj N (2023) Comparison between chromatofocusing and icIEF charge variant profiles of unconjugated monoclonal antibodies and their drug conjugates. Arab Journal of Pharmaceutical Sciences 7(1).
- Thallaj N (2024) Detecting Antioxidant Behavior for Phenolic Content of Some Beauty Care Creams in Syrian Market. Indian Journal of Advanced Chemistry 2(1): 10-14.
- Thallaj N (2024) Advancements in Pharmaceutical Science: Synthesis and Application of Molecular Cages Integrating N-Heterocyclic Carbenes for Enhanced Stability and Functionality. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 5(1): 6-19
- Thallaj N (2023) Tishreen University Journal-Medical Sciences Series 44(6): 21-29.
- Thallaj N (2024) Conductive Nanocomposites Based on Graphene and Natural Polymers. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(6): 7-27.
- Thallaj N (2022) Design and Synthesis Ligands Tetradents Substituted with Halogenes in α-Position and Conjugation with Riboflavin (Bioconjugates): Conjugate ligands Type TPA’s with Flavonoids as un Electron Mediator. Biomedicine and Chemical Sciences 1(2): 47-56.
- Dayoub L, Alkuddsi Y, Thallaj N (2023) Investigating the interaction between some of Bipolaris sorokiniana’s toxins and the Gα Subunit of the Wheat G-Protein using bioinformatics tools. University of Thi-Qar Journal of agricultural research 12(1): 181-200.
- Thallaj N, Abbood A (2023) Tishreen University Journal-Medical Sciences Series 45: 47-57.
- Thallaj N (2022) HPLC method validation for determination of pentoxifylline in pharmaceutical dosage forms. Indian J Adv Chem 2(1): 5-9.
- Thallaj N (2022) Ligands tris (2-pyridylmethyl) amine with nitrile group in α-substituted and the corresponding FeCl2 complexes.
- Thallaj N (2022) Review of Calixarene-Derivatives in Transition Metal Chemistry. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 2(3): 1-30.
- Thallaj N (2022) Ligands tris (2-pyridylmethyl) amine with nitrile group in α-complexes 2 substituted and the corresponding FeCl. Journal of Biological Pharmaceutical and Chemical Research 9(1): 25-47
- Thallaj N (2022) Journal of Biological Pharmaceutical and Chemical Research 9 (3): 40-47.
- NK Thallaj (2018) Damascus University Journal for Basic Sciences. Conjunction of riboflavin to TPAs ligands with two halogens atoms in α-position substitution 34: 1.
- NK Thallaj (2017) Designing and synthesis of DPAs ligands to induce structural modification of Riboflavin. Journal of AlBaath University 39(8): 33-72.
- THALLAJ, Nasser Ferrous complexes with aromatic α-substituted tris (2-pyridylmethyl) amine ligands: effect of the substituents and biomimetic reactivity.
- Thallaj N (2024) Advancements in Peptide Vectors for Cancer Therapy and Tumor Imaging: A Comprehensive Review. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 4(5): 29-49.
- Thallaj N, ali Hamad DH, Batieh NA, Saker GA (2023) A Review of Colon Cancer Treatment using Photoactive Nanoparticles. International Journal of Advanced Pharmaceutical Sciences and Research (IJAPSR) 3(4): 1-32.
- Labban, Louay (2020) International Journ. IJMS 5(12): 23-36.
- Labban L, Malek Z (2020) Photoperiod regulates the daily profiles of tryptophan hydroxylase-2 gene expression the raphe nuclei of rats. International Journal of Current Research in Physiology and Pharmacology 1-5.
- Labban, Louay, Nasser Thallaj, and Zeina Malek (2019) The implications of E-cigarettes or" vaping" on the nutritional status. Jour Med Resh and Health Sci 2(11): 784-787.
- MALEK Z (2018) The effect of regular exercise on the expression of tryptophan hydroxylase-2 gene within Raphe complex: functional relationship with adrenal hormones and glucose blood levels. in Journal of AlBaath University.
- MALEK Z (2018) The effect of Glucocorticoids rhythm on serotonin release within the hypothalamic suprachiasmatic nuclei; the locus of the Biological Clock: functional relationship with blood glucose. Tishreen University Journal for Research and Scientific Studies 40: 1-17.
- Labban L (2018) The Association between Visceral Fat, Dietary Patterns, and Comorbidities. Open Access Library Journal 5(07): 1.
- Labban LM, Alshishkli MM, Alkhalaf A, Malek Z (2017) The effects of dental amalgam toxicity on health and nutritional status. J. Adv. Res. Dent. Oral Health 2: 1-4.
- Shannan G, Malek ZS, Thallaj N (2025) Advancements in Protein Synthesis: Triazole Multi-Ligation and Cycloadditions for Pharmaceutical Applications. Am J Biomed Sci & Res 26(5): 685-700.
- Channan G (2018) Communicable diseases in the Mediterranean region. EJIFCC 29(3): 164-170.
- Shannan G (2011) TUBERCULOSIS IN THE AFCB COUNTRIES; RE-EMERGING DISEASE. In CLINICAL CHEMISTRY AND LABORATORY MEDICINE. GENTHINER STRASSE 13, D-10785 BERLIN, GERMANY: WALTER DE GRUYTER & CO 49: S41-S41.
- Channan GM (1977) Studies of vitamin D hydroxylation in chronic renal failure.
- Aldakhoul M, Sleman S, Alragheb N, Alfarwan M, Alallan L, et al. (2024) Phytochemical Composition and Health Benefits of Pumpkin. Research Journal of Pharmacy and Technology 17(10): 4915-4921.
- Khabbazeh Sally, Saly Alhaddad, Yazan Kiwan, Louai Alallan and Nasser Thallaj, et al. (2024) The Benefits of Jujube: Extracting Phenols and Saponins for Medicinal and Nutritional Uses. Azerbaijan Pharmaceutical and Pharmacotherapy Journal 23(4): 1-7.
- Nasser Thallaj, Dalia Aboufakher, Rita Zeinaldin, Rawa Khreit, Racha Khatib (2025) Prevalence and Antibiotic Resistance Patterns of Multidrug-Resistant Gram-Negative Bacilli in Hospitalized Patients in Sweida, Syria. FJHMS 1(1): 70-78.
- Thallaj Dr N (2022) Review of Calixarene-Derivatives in Transition Metal Chemistry. International Journal of Advanced Pharmaceutical Sciences and Research 2(3): 1-28.

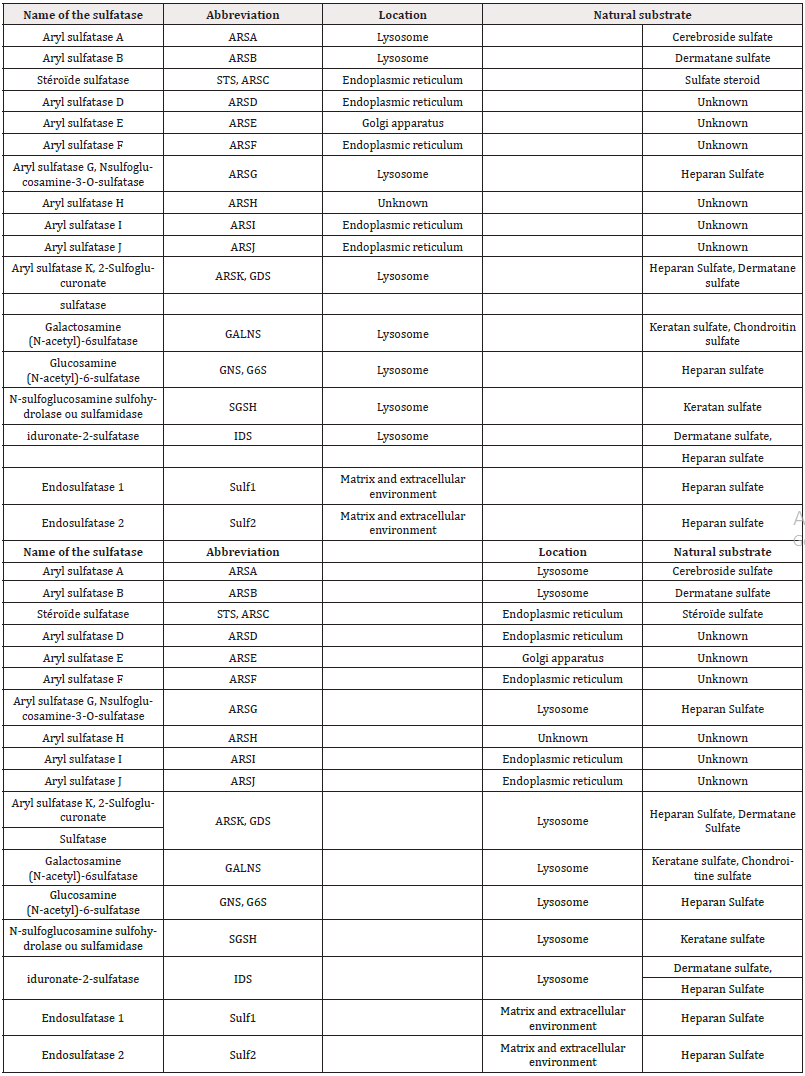
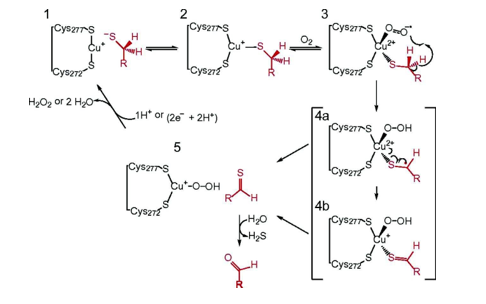
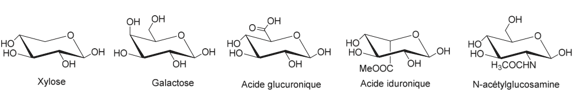
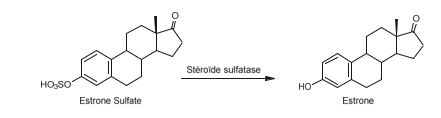
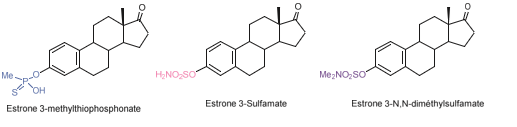
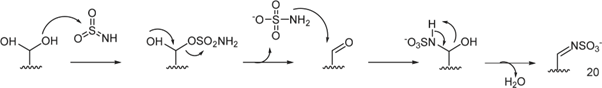
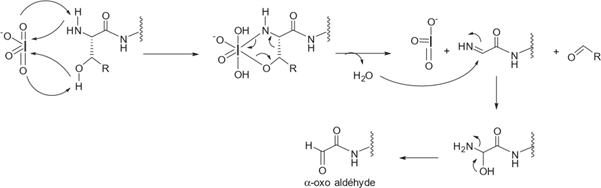


 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.