Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
M6A Methylation in Glioblastoma: From Molecular Mechanisms to Clinical Applications
*Corresponding author: Wei Liu, Department of Immunology, Hebei Medical University, China.
Jiankai Yang, Department of Neurosurgery, the Second Hospital of Hebei Medical University, China.
Received: June 25, 2025; Published: June 30, 2025
DOI: 10.34297/AJBSR.2025.27.003582
Abstract
Glioblastoma is the most common primary intracranial malignant tumor, characterized by treatment challenges and a poor prognosis. Despite standard treatment, including surgical resection combined with radiotherapy, outcomes remain unsatisfactory, with a median progression-free survival of 7 months and an overall survival of only 15 months. In recent years, RNA methylation has garnered significant attention, with N6-methyladenosine emerging as one of the most abundant epitranscriptional modifications in eukaryotic mRNAs. Regulated by methyltransferases, demethylases, and methylation reader proteins, it influences mRNA metabolism and plays a critical role in various physiological and pathological processes. It is widely believed to play a pivotal role in tumorigenesis and development by regulating various physiological and pathological processes. This paper reviews recent studies on the relationship between m6A methylation and glioblastoma, highlighting advances in its role in diagnosis, treatment, drug resistance, and prognosis. These findings aim to provide new insights for further research on m6A methylation and glioblastoma.
Keywords: M6A Methylation, M6A Regulatory Molecules, Glioblastoma, Molecular Mechanisms
Abbreviations: GBM: Glioblastoma; CNS: Central Nervous System; WHO: World Health Organization; m6A: N6-Methyladenosine; RNA: Ribonucleic Acid; METTL3: Methyltransferase-Like Protein 3; METTL14: Methyltransferase-Like Protein 14; WTAP: Wilms’ Tumor 1-Associating Protein; SAM: S-Adenosylmethionine; METTL16: Methyltransferase-Like Protein 16; FTO: Fat Mass And Obesity- Associated Protein; ALKBH3: AlkB Homolog 3; ALKBH5: AlkB Homolog 3; mRNA: Messenger RNA; YTHDFs: YTH Domain Family Proteins; YTHDCs: YTH Domain Containing Proteins; IGF2BPs: Insulin-Like Growth Factor 2 mRNA-Binding Proteins; HNRNPs: Heterogeneous Nuclear Ribonucleoproteins; HuR: Hu Family of RNA-Binding Proteins; VM: Vasculogenic Mimicry; GSCs: Glioma Stem Cells; CTNNBIP1: β-Catenin Interacting Protein 1; YY1: nonhistone Protein Yin Yang-1; ADAR1: Adenosine Deaminase Acting on RNA 1; EGR1: Early Growth Response Factor 1; A: Adenosine; PDGF: Platelet-Derived Growth Factor; CDK12: Cyclin-Dependent Kinases 12; ERK1/2: Extracellular Regulated Kinase 1/2; ROS: Reactive Oxygen Species; EMT: Epithelial-Mesenchymal Transition; HUVEC: Human Umbilical Vein Endothelial Cells; EGFR: Epidermal Growth Factor Receptor; GCLM: Glutamate Cysteine Ligase Modifier Subunit; CRM1: Chromosome Maintenance Protein 1; IRES: Internal Ribosome Entry Site; APA: Alternative Polyadenylation; TME: Tumor Microenvironment; HK2: Hexokinase 2; HMGCR: Hydroxymethylglutaryl-CoA Reductase; PD-L1: Programmed Death Ligand 1; PD-1: Programmed Cell Death Protein 1; TLC: Thin-Layer Chromatography; MeRIP-seq: Methylated RNA Immunoprecipitation Sequencing; dRNA-seq: Direct RNA-seq; lncRNA: Long Non-Coding RNA; NMD: Nonsense-Mediated Mrna Decay; NSC: Neural Stem Cell; IRES: Internal Ribosome Entry Site; TMZ: Temozolomide; IC50: Half-Maximal Inhibitory Concentration; UBE2D3: Ubiquitin- Binding Enzyme E2D3; MrGPS: m6A-Related Gene Pairs; HR: Hazard Ratio; OS: Overall Survival.
Introduction
Glioblastoma (GBM), the most common primary tumor of the Central Nervous System (CNS), is classified as grade IV by the WHO. It accounts for approximately 15.6% of primary brain tumors and 45.2% of malignant brain tumors, making it the most prevalent and aggressive primary brain tumor in adults [1-2]. GBM treatment primarily involves surgical resection combined with radiotherapy and concurrent temozolomide chemotherapy [3]. Despite receiving standard combination therapies, the prognosis for GBM patients remains dismal, with a 5-year survival rate below 5% [4].
Like DNA and histone modifications, RNA modification a branch of epigenetics plays a crucial role in various diseases, particularly tumors. N6-Methyladenosine (m6A) methylation, occurring at the nitrogen-6 position of the Adenine (A) base in RNA, is the most prevalent RNA modification in eukaryotes [5]. This modification process involves three key regulatory molecules: methyltransferases, demethylases, and m6A-binding proteins, which add, remove, and recognize m6A sites, respectively [6-7]. M6A methylation influences multiple aspects of RNA metabolism, such as splicing, miRNA processing, nuclear export, translation, and decay, and plays a significant role in GBM progression. This paper aims to systematically review recent studies on GBM and m6A methylation, offering valuable insights to guide future research in this field.
Overview of m6A Methylation
To understand the role of m6A regulatory molecules in GBM, it is essential to first clarify the regulatory mechanisms of m6A methylation. M6A methylation is a dynamic and reversible process regulated by methyltransferases, demethylases, and methylation reader proteins. Methyltransferases form transferase complexes to catalyze the methylation of specific RNA sites, while demethylases can promote the demethylation of methylated RNA, thus making m6A methylation modification exhibit dynamic and reversible characteristics. methylation reader proteins can specifically recognize and bind to RNA with m6A methylation modification, and further participate in the downstream effects of modifying RNA.
Methyltransferases
Methyltransferases Primarily Include Methyltransferase-Like Protein 3 (METTL3), Methyltransferase-Like Protein 14 (METTL14), and Wilms’ Tumor 1-Associating Protein (WTAP). METTL3 is the primary component of the m6A methyltransferase complex. It binds to S-Adenosylmethionine (SAM) and transfers a methyl group from SAM to a substrate adenosine [8]. METTL14 is highly homologous to METTL3 and does not have catalytic activity itself, but it can provide structural support for RNA binding and form a stable heterocomplex with METTL3 in a 1:1 ratio, thereby playing a key catalytic role [9-10]. WTAP acts as a regulatory subunit of the m6A methyltransferase complex, interacting with METTL3 and METTL14 and recruiting the complex to RNA [11]. Methyltransferase- Like Protein 16 (METTL16) is one of the homologs of METTL3. It mainly acts as a regulatory subunit to coordinate the activity of METTL3 and participates in the regulation of RNA biological func tions [12]. Methyltransferases are critical for m6A methylation. Dysfunctions or aberrant expression of these enzymes disrupt m6A regulation, impairing mRNA metabolism and gene expression.
Demethylases
Demethylases work exactly opposite to methyltransferases, removing the methyl group provided by S-adenosylmethionine from previously methylated RNA. Two demethylases have been identified in eukaryotes: Fat Mass and Obesity-Associated Protein (FTO), AlkB Homolog 3 (ALKBH3) and AlkB homolog 5 (AlkB homolog 5, ALKBH5). The mechanism of FTO demethylation is to catalyze the oxidation of m6A to generate intermediate products, which are gradually demethylated and finally form adenosine [13]. Different from FTO, ALKBH5 can interact with nuclear RNA and directly demethylate m6A to adenosine without generating intermediate products [14]. In addition to its role in mRNA stability, splicing and translation, ALKBH5 regulates m6A demethylation activity and also significantly affects the export of mRNA and the assembly of processing factors as well as RNA metabolism [15]. ALKBH3 is a newly discovered m6A demethylase that can induce m6A demethylation and significantly promote protein translation efficiency [16].
Methylation Reader Proteins
The interplay between m6A methyltransferases and demethylases underpins the dynamic and reversible nature of m6A modifications. For m6A modifications to exert specific biological functions, they must be recognized by diverse methylation reader proteins, which regulate downstream molecules and signaling pathways to elicit various effects [17]. Methylation reader proteins execute the regulatory functions of m6A modifications by specifically recognizing and binding to m6A-modified RNAs, thereby mediating processes essential for RNA metabolism homeostasis. Methylation reader proteins primarily include YTH Domain Family Proteins (YTHDF1-3), YTH Domain Containing Proteins (YTHDC1-2), Insulin- Like Growth Factor 2 mRNA-Binding Proteins (IGF2BPs), and Heterogeneous Nuclear Ribonucleoproteins (HNRNPs).
YTHDF Family: The YTH domain family includes three members: YTHDF1, YTHDF2, and YTHDF3, primarily located in the cytoplasm. These proteins play distinct roles in m6A modifications and exhibit a complex cooperative mechanism. Upon entering the cytoplasm, m6A-modified mRNAs are initially bound by YTHDF3 or YTHDF3-YTHDF1 complexes and subsequently recognized by YTHDF2, enabling distinct regulatory functions. YTHDF1 can regulate the translation initiation process by interacting with initiation factors, thereby improving the translation efficiency of targeted RNA [18]. YTHDF2 selectively binds to m6A-modified RNAs and directs them to decay sites, thereby facilitating RNA degradation [19]. YTHDF3 enhances mRNA translation efficiency through collaboration with YTHDF1. Additionally, it influences YTHDF2-mediated RNA decay; knockdown of YTHDF3 significantly reduces m6A-modified RNA decay [20]. These findings reveal a complex cooperative mechanism within the YTH domain family.
YTHDC Family: The YTHDC family represents another critical group of methylation reader proteins. YTHDC1 promotes mRNA splicing and facilitates mRNA export from the nucleus to the cytoplasm by recruiting splicing factors and nuclear export junction proteins. YTHDC2 can improve translation efficiency by directly recognizing mRNA with m6A modification, and can also promote the decomposition of ribosome-mRNA complex by modifying the head region of ribosome 40S subunit, thereby accelerating mRNA decay [21-22].
IGF2BPs Family: The IGF2BPs family consists of three members: IGF2BP1, IGF2BP2, and IGF2BP3. These proteins enhance the stability and translational efficiency of target mRNAs in an m6A-dependent manner. IGF2BPs can recruit cofactors such as HuR (an mRNA stabilizer) and MATR3 to prevent the degradation of m6A-modified mRNAs while promoting their translation [23]. Additionally, IGF2BPs regulate mRNA translation by influencing alternative splicing processes [24]. The IGF2BPs family plays crucial roles in tumor development, human development, and stem cell growth and differentiation. Dysregulation of IGF2BPs is associated with conditions such as diabetes, insulin resistance, lipid metabolism disorders, and neurological disorders [25-28]. IGF2BPs are also highly expressed in various aggressive malignancies, correlating with poor prognosis, metastasis, tumor progression, and tumor grading [29].
HNRNPs Family: The HNRNPs family comprises three members: HNRNPA2B1, HNRNPC, and HNRNPG. HNRNPA2B1 facilitates primary miRNA processing and maturation by binding to m6A-methylated transcripts, while HNRNPC and HNRNPG mediate alternative splicing to regulate mRNA abundance by processing m6A-modified transcripts [30-32]. In summary, the HNRNPs family possesses specific RNA-binding regions during transcription and plays key roles in cellular functions such as localization, translocation, alternative splicing, and mRNA translation [33-35].
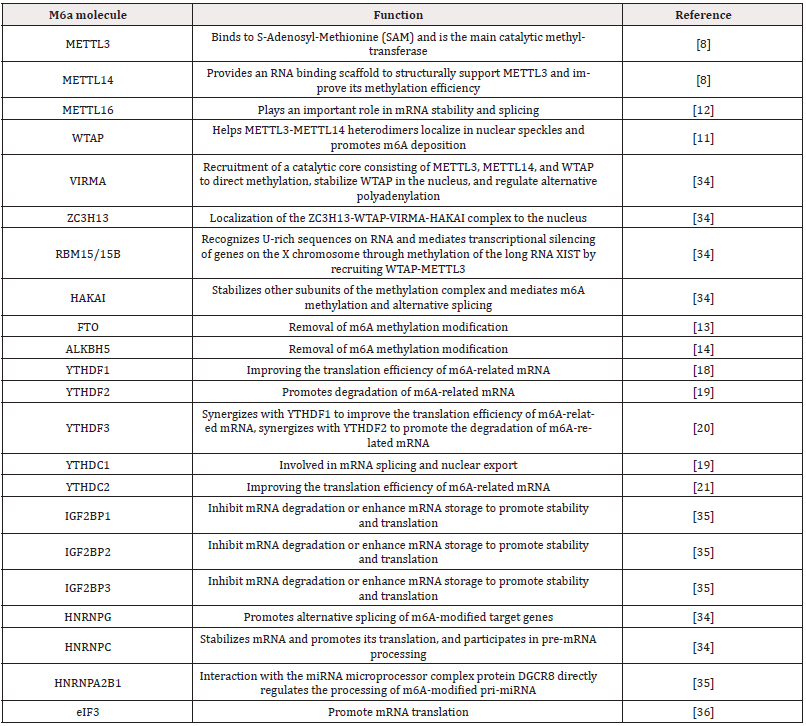
M6A methylation is a dynamic, reversible process co-regulated by methyltransferases, demethylases, and methylation reader proteins. The coordinated actions of these regulatory molecules enable m6A methylation to participate in various aspects of RNA metabolism, including splicing, translation, and decay. These three regulatory molecule types are structurally and functionally interconnected. Their mechanisms of action and interactions are illustrated below (Figure 1). Together, they constitute a complex network of m6A methylation regulation, laying the foundation for the subsequent understanding of the role of m6A methylation in GBM. At the same time, we have organized the m6A modification-related proteins and their main biological functions as follows (Table 1).
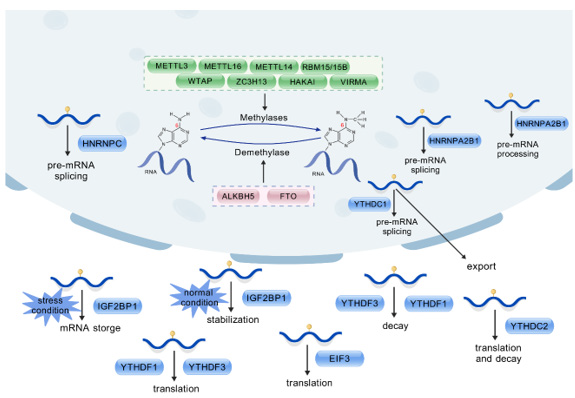
Note*: M6A modification is a dynamic and reversible post-transcriptional process that is biologically regulated by methyltransferases (METTL3, METTL14, METTL16, RBM15/15B, WTAP, ZC3H13, HAKAI, VIRMA) and demethylases (FTO, ALKBH5). Some methylation reading proteins (YTHDCs, YTHDFs, IGF2BPs, HNRNPA2B1) that bind to the corresponding bases of m6A sites enable m6a methylation modification to exert specific biological functions.
Figure 1: Coordinated action mechanism of m6a regulatory molecules.
Relationship Between m6A Regulatory Molecules and GBM
M6A methylation is closely associated with tumor development and progression. Building upon an understanding of the fundamental aspects of m6A methylation, investigating its regulatory molecules in GBM is crucial for comprehensively understanding the disease and identifying novel anti-tumor strategies.
Methyltransferases in GBM
Please keep the format consistent Currently, the role of METTL3 in GBM can be categorized into two contrasting perspectives. On one hand, certain studies indicate that METTL3 promotes GBM progression. For instance, METTL3-dependent m6A modification of HOTAIRM1 has been shown to promote glioma Vasculogenic Mimicry (VM), a tumor microcirculation mechanism independent of endothelial cells that ensures sufficient blood supply for tumor growth [36,37]. Additionally, Glioma Stem Cells (GSCs), known for their self-renewal and multidirectional differentiation abilities, are regarded as the initiating cells of GBM and a key reason for its therapeutic resistance. Recent studies have demonstrated that the transcription factor YY1 mediates GSC self-renewal by regulating the SENP1/METTL3/MYC axis, contributing to its high tumorigenic potential [38]. Conversely, other studies suggest that METTL3-mediated m6A modification exerts tumor-suppressive effects in GBM. For example, METTL3-mediated m6A modification upregulates circDLC1 expression, which promotes CTNNBIP1 transcription by sponging miR-671-5p, thereby inhibiting malignant GBM proliferation [39]. These findings highlight the complex bidirectional role of METTL3 in GBM, likely attributable to its interaction with distinct targets and signaling pathways.
Additionally, several targets and signaling pathways associated with METTL3 have been progressively identified. ADAR1, a newly identified target of METTL3, has been shown to exert oncogenic effects in GBM independent of its nucleic acid endonuclease activity, despite its typical immunomodulatory and antiviral roles in normal cells. Furthermore, the study revealed that ADAR1 knockdown significantly suppresses GBM growth in vivo, these findings underscore the critical role of ADAR1 in tumor progression and highlight its potential as a therapeutic target in GBM [40]. The PDGF–METTL3-OPTN signaling pathway has also been identified, with PDGF, an essential growth factor, promotes METTL3 expression by activating EGR1 in GBM, thereby enhancing GSC proliferation and self-renewal. METTL3 also regulates mitochondrial autophagy by modulating the m6A modification of OPTN, and forced OPTN expression mimics the effect of PDGF inhibition, suggesting a tumor-suppressive role for OPTN [41]. The BUD13/CDK12/MBNL1 axis is pivotal in regulating VM formation in GBM. METTL3 stabilizes BUD13 mRNA and upregulates its expression via m6A methylation, which subsequently stabilizes CDK12 mRNA, increases CDK12 expression, and ultimately phosphorylates MBNL1, thereby regulating VM formation in GBM [42]. Meng et al., reported that C5aR1 expression is significantly upregulated in GBM and correlates with prognosis, functioning through activation of the ERK1/2 signaling pathway [43]. ERK1/2 pathway activation increases METTL3 protein levels, which as a methyltransferase modulates the m6A modification of GPX4, maintains its m6A levels, and subsequently upregulates GPX4 expression.GPX4, a key anti-ferroptosis factor, reduces cytotoxic lipid peroxides by catalyzing the conversion of reduced glutathione to oxidized glutathione, thereby inhibiting ferroptosis and promoting GBM progression.
WTAP is highly expressed in GBM, and its role in ROS metabolism and tumor progression has been gradually discovered. In terms of ROS metabolism, the ROS level in the WTAP overexpression group increased significantly after being stimulated by ROS inducers; in terms of cell biological behavior, colony formation experiments, wound healing experiments, and Transwell experiments showed that WTAP can promote GBM cell proliferation and migration. and invasion; in terms of signaling pathways, Western blotting identified that WTAP regulates the phosphorylation of PI3K/Akt signal transduction [44]. UBE2D3, which is regulated by WTAP-mediated m6A modification, reduces the chemosensitivity of GBM to temozolomide [45]. Qiu et al., demonstrated through experiments that WTAP plays a key role in the mesenchymal transformation of glioblastoma stem cells and the formation of the tumor immunosuppressive microenvironment, highlighting the therapeutic potential of targeting WTAP and its downstream effectors to enhance the efficacy of immunotherapy [46]. In summary, WTAP mainly plays a role in promoting tumor progression in GBM, involving multiple biological processes and signaling pathways.
Demethylase in GBM
ALKBH5 is a key “eraser” of m6A methylation. Tao et al., reported that ALKBH5 was upregulated and METTL3 downregulated in U87-MG cells, promoting migration, invasion, Epithelial-Mesenchymal Transition (EMT), and VM by altering the levels of MMP2, CDH1, CDH2, and FN1 in GBM cells. Additionally, they found that overexpression of ALKBH5 aggravated EMT and VM progression in GBM in vivo, promoting GBM growth and metastasis [47]. In 2024, Fan et al., used Human Umbilical Vein Endothelial Cells (HUVEC) to conduct wound healing, tube formation analysis, and plasmid transfection in vivo and in vitro experiments to confirm the angiogenesis regulatory ability of ALKBH5 in GBM, which plays an important role in the formation and metastasis of GBM [48]. M6A methylated RNA immunoprecipitation sequencing and RNA sequencing revealed that ZDHHC3 is a direct target of ALKBH5.Mechanistically, ALKBH5 deficiency impairs ZDHHC3 mRNA stability via YTHDF2, leading to reduced PD-L1 expression by accelerating its degradation in GBM. Additionally, ALKBH5 deficiency increases cerebrospinal fluid lymphocyte infiltration and proinflammatory cytokines, inhibiting GBM formation and proliferation [49]. Growth factor receptors are one of the most important oncogenic pathways. Monotherapy based on drug inhibitors has little effect on them. ALKBH5 can be targeted to enhance the anti-tumor efficacy of Epidermal Growth Factor Receptor (EGFR) and Glutamate Cysteine Ligase Modifier Subunit (GCLM) inhibitors. The mechanism involves activated EGFR inducing ALKBH5 phosphorylation, inhibiting ALKBH5 nuclear export via Chromosome Maintenance Protein 1 (CRM1), leading to continuous m6A mRNA demethylation in the nucleus. ALKBH5 tightly regulates ferroptosis via m6A modification and YTHDF2-mediated GCLM decay, contributing to the EGFR-ALKBH5-GCLM oncogenic axis [50]. Tuoheti et al., found that ALKBH5 negatively regulates miR-124- 3p by hindering its processing. Knockdown of ALKBH5 promoted pri-miR-124-3p processing and increased mature miR-124-3p levels, thereby inhibiting the malignant behavior of GBM cells by targeting EPHA2 [51]. Zhang et al., found that ALKBH5 interacts with TAR DNA-Binding Protein 43 (TDP-43) in glioblastoma cells, and this complex then regulates CDC25A mRNA splicing through m6a demethylation to maintain the expression of its binding to cell division cycle 25A, ultimately promoting the G1/S transition and growth of GBM cells, ultimately promoting GBM growth [52].
In 2023, Du et al., found that FTO overexpression in vitro inhibits hypoxia-induced GBM proliferation, migration, and invasion, and reduces the percentage of m6A-methylated cells. In vivo, FTO overexpression inhibits tumor growth in xenograft models and decreases the protein levels of migration markers, including vimentin and Twist. The mechanism involves FTO overexpression promoting the nuclear translocation of FOXO3a and upregulating the expression of its downstream targets, including BIM, BNIP3, BCL-6, and PUMA [53]. Dong et al., also demonstrated through in vivo experiments that FTO induces cell apoptosis by inhibiting the m6A methylation of GSTO1, thereby slowing down the progression of GBM [54]. In clinical specimens from GBM patients, reduced FTO expression correlates with higher glioma grade and poor clinical outcomes. Mechanistically, FTO regulates the m6A modification of primiRNA- 10a, which is recognized by the methylation reader protein HNRNPA2B1, recruiting the miRNA microprocessor complex protein DGCR8 to mediate its processing. Additionally, the transcription factor SPI1 increases the tumor burden in GBM by inhibiting FTO’s transcriptional activity [55], while IDO1 facilitates GBM progression by inhibiting SLC7A11-dependent ferroptosis through an IDO1-AhR-FTO axis-mediated m6A methylation mechanism [56]. Lv et al., [34] found that MDH2 regulates FTO and ALKBH5 activity by modulating α-Ketoglutarate (αKG) levels, thus influencing m6A levels [57]. Specifically, MDH2 upregulation decreases αKG levels, weakening FTO and ALKBH5 activity and increasing m6A modification on RNA. MDH2 knockdown reduced m6A levels in GSCs, while MDH2 overexpression increased m6A levels, with αKG supplementation reversing this effect. MDH2 increases the m6A methylation of PDGFRB mRNA through this pathway, enhancing its stability and upregulating PDGFRB expression, which promotes GBM cell proliferation. This demonstrates that MDH2 influences GSC biological behavior through m6A modification, which plays a crucial role in GBM onset and progression, offering potential targets and strategies for treatment.
Overall, demethylases play a critical regulatory role in GBM, influencing tumor migration, invasion, and proliferation through various mechanisms, with their expression levels closely linked to GBM clinical characteristics and prognosis.
Methylation Reader Proteins In GBM
YTHDF Family: YTHDF1 plays a role in the development and progression of GBM and is associated with overall survival outcomes [58]. Furthermore, Yarmishyn et al., reported that among the five YTH family members, YTHDF1 exhibits the highest level of upregulation in GBM samples compared to normal brain tissue. It promotes GBM proliferation, chemoresistance, and cancer stem cell-like characteristics.MSI1 positively regulates YTHDF1 expression, and this upregulation is associated with increased tumor cell migration and decreased patient survival rates. YTHDF1 serves as a negative prognostic marker in GBM [59]. Studies indicate that YTHDF2 expression in diffuse gliomas rises in parallel with histological and molecular markers of malignancy, showing a positive correlation with greater tumor aggressiveness and poorer prognosis. Elevated YTHDF2 levels enhance glioma malignancy in both in vitro and in vivo models. Mechanistically, YTHDF2 facilitates UBXN1 mRNA decay in gliomas by recognizing METTL3-mediated m6A modifications, thereby promoting NF-κB activation [60]. Another study revealed that PRMT6 activates the Wnt/β-Catenin pathway by upregulating YTHDF2 transcription, highlighting the importance of the PRMT6-YTHDF2-Wnt/β-Catenin axis in driving GBM migration, invasion, and EMT in vitro and in vivo [61]. YTHDF3 is pivotal in promoting the proliferation, migration, and stemness of U87OSR cells. YTHDF3 significantly downregulates the EGFR-ITGA7-AKT and ERK signaling pathways in U87OSR cells, contributing to resistance against the EGFR inhibitor osimertinib. YTHDF3 also induces oncogenic senescence escape by downregulating p21 expression [62]. Additionally, YTHDF3 binds to the Internal Ribosome Entry Site (IRES) of m6A-modified cyclin D1 or the transcription factor c-myc, regulating IRES activity and GBM sensitivity to mTOR inhibitors in vitro and in xenograft models [63]. Collectively, members of the YTHDF family exhibit distinct roles in GBM progression, yet all are linked to poor patient prognosis.
YTHDC Family: Elevated YTHDC1 expression in GSCs facilitates the export of m6A-modified circPOLR2B from the nucleus to the cytoplasm, reducing nuclear circPOLR2B levels and subsequently decreasing the R-loop structures formed with its parental gene POLR2B. This alleviates transcriptional inhibition of POLR2B and, via Alternative Polyadenylation (APA), upregulates PBX1 expression, driving the malignant behavior of GSCs [64].
IGF2BPs Family: Studies have shown that IGF2BP1 and IGF2BP3 are either absent or expressed at extremely low levels in most normal tissues. However, IGF2BP1-3 are re-expressed in various cancer types and cell lines, and their expression is generally linked to poor prognosis. Immunofluorescence staining and qRTPCR analysis further confirm that IGF2BP2 and IGF2BP3 expression levels are elevated in cancer tissues compared to adjacent normal tissues. Additionally, IGF2BPs are associated with the Tumor Microenvironment (TME) and cancer stemness. Notably, IGF2BP3 plays a critical role in maintaining and supporting the self-renewal of GSCs. IGF2BP3 knockout reduces the proliferation, invasion, and migration of GSCs and glioma cells [65]. IGF2BP1 is implicated in glioma initiation. Silencing LINC00689 suppresses glioma initiation via the miR-526b-3p/IGF2BP1 axis [66]. IGF2BP2 enhances RNA stability by recognizing m6A modifications. PBK, a serine/threonine protein kinase, is aberrantly overexpressed in various cancers and plays a key role in driving proliferation and migration, including in glioma. IGF2BP2 stabilizes endogenous PBK mRNA, with its regulatory effects on PBK stability relying on m6A modifications [67]. Another study demonstrates that IGF2BP2 recognizes m6A-modified CASC9, enhancing its stability. CASC9 subsequently interacts with IGF2BP2 to form the IGF2BP2/CASC9 complex, which stabilizes Hexokinase 2 (HK2) mRNA and promotes aerobic glycolysis in GBM [68]. High IGF2BP3 expression is strongly associated with poor glioma prognosis, and its levels rise significantly with increasing WHO glioma grades. IGF2BP3 expression is elevated in GBM patients compared to other glioma subtypes. High IGF2BP3 expression is positively correlated with IDH wild-type status and 1p/19q non-co-deletion. Furthermore, IGF2BP3 is highly expressed in patients over 60 years old and in those with Progressive Disease (PD) as their primary treatment outcome. IGF2BP3 expression is also closely linked to the mutation status of cancer-related genes [69]. CircRNF10 is significantly overexpressed in GBM and is associated with poor patient prognosis. IGF2BP3 enhances circRNF10 stability in GSCs, while the transcription factor ZBTB48 upregulates IGF2BP3 transcription. circRNF10 forms a positive feedback loop with ZBTB48 and IGF2BP3, upregulating HSPB1, reprogramming GSC iron metabolism, and conferring resistance to ferroptosis. Additionally, circRNF10 functions as a competitive inhibitor of the E3 ubiquitin ligase MKRN3, blocking its enzymatic activity and protecting ZBTB48 from degradation. Amplification of oncogenic signaling and resistance to ferroptosis drive GBM progression [70]. Another study reveals that IGF2BP3 regulates GPX4 protein expression by directly binding to a specific motif on its mRNA, thereby influencing ferroptosis. The m6A modification of this motif is essential for GPX4 mRNA stability and translation. IGF2BP3 deletion induces ferroptosis in glioma by modulating GPX4 expression [71].
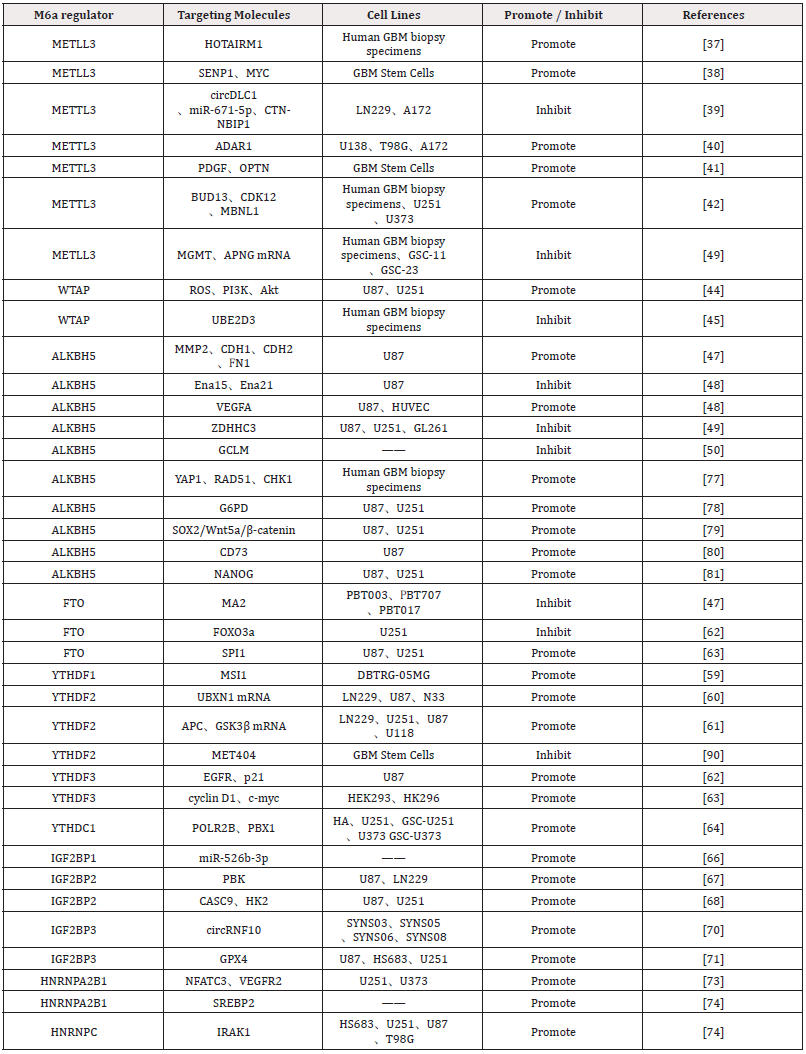
HNRNPs Family: HNRNPA2B1 and HNRNPC are broadly expressed in the GBM microenvironment, exhibiting heterogeneity across various cell types and cell cycle phases. They are expressed at higher levels in M1 macrophages, GBM cells, and T/NK cells. In cells within the S phase, except for M2 macrophages, HNRNPC exhibits higher expression compared to other m6A regulators. HNRNPA2B1 expression is notably elevated in cells during the G2/M phase. For cells in the G1 phase, the m6A regulators with higher expression levels are HNRNPA2B1, HNRNPC, WTAP, and YTHDC1 [72]. Studies reveal that HNRNPA2B1 is significantly upregulated in GBM and positively correlates with VM-like cell behaviors in U251 and U373 cell lines. HNRNPA2B1 binds NFATC3 mRNA, enhancing intracellular NFATC3 protein levels. NFATC3 interacts with the VEGFR2 promoter to upregulate VEGFR2 expression, driving VM and modulating tumor blood supply to sustain continuous growth [73]. Zhang et al., identify HNRNPA2B1 as a critical independent prognostic risk factor for glioma. HNRNPA2B1 induces Hydroxymethylglutaryl- CoA Reductase (HMGCR) expression by stabilizing SREBP2 mRNA, thereby promoting de novo cholesterol synthesis. HNRNPA2B1 ablation significantly suppresses glioma cell proliferation, GSC self-renewal, and tumorigenesis [74]. HNRNPC expression is upregulated in glioma tissues compared to normal brain tissues, with glioma malignancy increasing alongside HNRNPC expression levels, independent of age, gender, or tumor location. However, studies examining the relationship between HNRNPC levels and Overall Survival (OS) suggest that elevated HNRNPC expression may be associated with better prognosis [75]. Conversely, Chen et al., [35] report that overexpression of HNRNPC mRNA and protein correlates with poor prognosis in glioma patients. Mechanistically, HNRNPC stabilizes IRAK1 mRNA in an m6A-dependent manner, thereby activating the Mitogen-Activated Protein Kinase (MAPK) signaling pathway. The HNRNPC–IRAK1–MAPK axis represents a critical oncogenic pathway in glioma [76-81]. The mechanisms and targets of m6A regulators affecting the biological behaviors of GBM cells are summarized in (Figure 2 & Table 2).
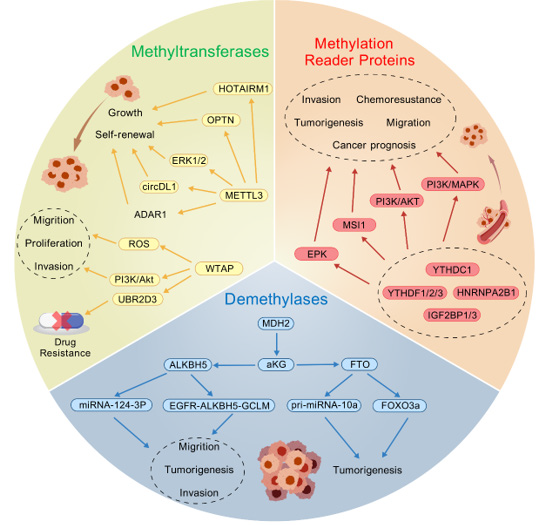
Note*: M6A methylation-related regulators in glioma affect the growth, self-renewal, proliferation, differentiation, autophagy, apoptosis, migration, invasion, drug resistance and prognosis of Glioma Stem Cells (GSCs) through various cellular pathways to regulate the occurrence and development of gliomas.
Figure 2: Mechanisms of M6A regulators to regulate GBM cell biological functions.
Clinical Significance of m6A Regulatory Molecules
Given the close relationship between m6A regulatory molecules and GBM, studying their clinical significance is crucial for the diagnosis and treatment of GBM.
Diagnosis
M6A methylation plays a critical role in GBM, prompting increasing research on its diagnostic value in GBM. Studies have shown that YWHAG and ALKBH5 serve as independent prognostic markers for GBM patients. Additionally, several m6A methylation regulators play key roles in regulating PD-L1/PD-1 expression and immune infiltration, thereby influencing the tumor immune microenvironment of GBM. For instance, HNRNPC, YWHAG, and ALKBH5 are positively correlated with PD-L1, suggesting their potential as predictors of immunotherapeutic responses in GBM patients. These regulators were significantly upregulated in GBM tissues, leading to a marked increase in immune scores, monocytes, M1 macrophages, activated mast cells, and eosinophils in the GBM tumor immune microenvironment. Meanwhile, immune cell invasiveness dynamically changed with variations in the copy number of three m6A regulators: HNRNPC, YWHAG, and ALKBH5 [82].
Common methods for detecting m6A modifications include liquid chromatography-mass spectrometry (LC-MS/MS), Thin-Layer Chromatography (TLC), colorimetric methods, and spot blotting. The most established method for m6A detection is methylated RNA Immunoprecipitation Sequencing (MeRIP-seq). Recently, Oxford Nanopore Technologies developed a novel Direct RNA-seq (dRNAseq) method, which identifies m6A with much higher resolution. dRNA-seq, with its higher lncRNA coverage, provides a clear advantage for in-depth analysis of m6A number and precise location, while MeRIP-seq is better suited for preliminary m6A screening studies [83]. In this study, m6A modifications in lncRNAs from the glioblastoma U87-MG cell line were analyzed using MeRIP-seq and dRNA-seq techniques. Twenty-four relevant lncRNA molecules were identified, with some showing correlations with glioma grade or patient survival prognosis, making them potential biomarkers.A comparison of the two techniques reveals that MeRIP-seq is suitable for preliminary screening, while dRNA-seq offers greater accuracy in identifying specific sites. This study provides technical support for further exploration of m6A’s role in cancer diagnosis, including GBM, and related therapeutic studies.
To overcome the limitation of quantifying m6A modifications at precise sites, Ma has screened the Bst 2.0 hot-start DNA polymerase, which can distinguish between m6A and Adenosine (A), and developed an ultrasensitive m6A RNA assay capable of isothermal quantification at single-base resolution. This method offers a highly sensitive tool for site-specific m6A detection and quantification, expected to serve as a foundation for precise diagnosis and epigenetic regulation of diseases such as GBM [84]. These assays offer technical support for further research into the application of m6A methylation in GBM diagnosis and facilitate the screening of more accurate biomarkers.
Clinicopathologic Features
A 2019 study by Chai et al., found that most of the 13 major m6A RNA methylation regulators were differentially expressed in 904 gliomas, stratified by clinicopathological features (such as WHO classification, IDH status, and 1p/19q deletion). The expression levels of most m6A regulators were closely associated with WHO histologic grading and corresponding classifications. They also found that the expression levels of WTAP, YTHDF, and ALKBH5 were positively correlated with histologic grading, while FTO expression was negatively correlated with grading [85]. Additionally, Li et al., showed that METTL3 expression could be used to differentiate molecular profiles between histologically similar GBM subtypes. They established a novel mechanism in which m6A modifications near mRNA splicing factors regulate Nonsense-Mediated mRNA Decay (NMD) [86]. These findings suggest that m6A regulator expression levels are closely linked to the clinicopathological features of GBM, providing a foundation for further understanding of its pathogenesis.
Treatment
M6A Modification-Related Enzymes: Designing inhibitors targeting demethylases is a therapeutic strategy based on m6A methylation modification. By inhibiting demethylase activity, the m6A modification level of RNA molecules increase, which subsequently regulates gene expression and inhibits tumor cell growth, self-renewal, and tumorigenesis, exerting an anti-tumor effect. In 2017, Cui et al., reported that the FTO inhibitor MA2 significantly inhibited the growth and self-renewal of GSCs, showing dose-dependent effects on various GSC lines, with minimal or no effects on the normal Neural Stem Cell (NSC) line NSC006, brain astrocytes, and HeLa cells. Additionally, MA2 inhibited GSC self-renewal, reduced stem cell frequency, and reversed the increased sphere formation rate caused by METTL3 or METTL14 knockdown. In in vivo experiments, the tumor volume in the MA2 treatment group was significantly smaller than in the control group, with reduced tumor luciferase activity and prolonged mouse survival. This suggests that MA2 may have therapeutic potential in inhibiting GSC tumorigenesis [87]. Takahashi et al., identified two novel ALKBH5-selective inhibitors, Ena15 and Ena21, knockdown of ALKBH5 or treatment with Ena15 and Ena21 inhibited GBM cell line proliferation, reduced S-phase cell cycle progression, increased m6A-modified RNAs, and stabilized FOXM1 mRNAs, promoting GBM progression. In conclusion, Ena15 and Ena21 are potential candidates for further studies on the biological functions of ALKBH5 [88]. In addition, Huff et al., optimized a previously discovered FTO small molecule inhibitor. This new drug lead FTO-43 showed efficacy comparable to that of the clinical chemotherapy drug 5-fluorouracil, increased the level of m6A methylation modification in a manner comparable to FTO knockdown in cancer cells, and regulated the Wnt/PI3KAkt signaling pathway, showing a strong anti-proliferative effect in tumors such as GBM [89]. Increasing m6A methylation by inhibiting demethylases offers a novel approach to GBM treatment, with potential synergistic effects when combined with other therapies. Further investigation into combined therapeutic strategies is needed.
Discovery of new targets: This approach aims to identify improved m6A modification-related immune checkpoints and targeting strategies. A previous study demonstrated that METTL3- dependent m6A modification of HOTAIRM1(a long non-coding RNA) promotes glioma VM formation [37]. Therefore, targeting HOTAIRM1 could disrupt mitochondrial oxidative phosphorylation and serine metabolism, potentially ameliorating GBM [59]. Ji et al., found that the m6A-modified lncRNA LINREP (a long intergenic non-coding RNA) promotes GBM progression by recruiting the PTBP1/HuR complex, suggesting that the HuR/LINREP/PTBP1 axis may be a potential therapeutic target for GBM [90]. Similarly, the m6A-modified lncRNA WEE2-AS1 (a novel lncRNA reported to promote GBM progression) promotes GBM progression by stabilizing RPN2, suggesting that WEE2-AS1 may serve as a prognostic biomarker and therapeutic target for GBM [91]. In addition to targeted therapies, new developments in combination therapies have emerged. mTOR inhibitor resistance hinders GBM treatment, and m6A-modified Internal Ribosome Entry Site (IRES) mRNAs promote resistance to mTOR inhibitors. Therefore, co-targeting m6A-methyl vesicles and mTOR may be an effective strategy against GBM [64].
Drug Resistance
Temozolomide (TMZ) is a first-line chemotherapeutic agent for gliomas, and mTOR inhibitors also play a role in glioma treatment. However, the high resistance of GSCs severely limits the efficacy of these agents in treating GBM, and the underlying mechanisms remain unclear. New evidence suggests that METTL3-mediated m6A modification contributes to self-renewal and radioresistance in GSCs. Shi et al., found that METTL3 knockdown attenuated GSC self-renewal and proliferation, decreased the IC50 (half-maximal inhibitory concentration) of TMZ, and increased TMZ-induced γH2AX levels, suggesting upregulation of double-stranded DNA damage. METTL3-mediated m6A modification also regulated the mRNA stability of two key DNA repair genes: MGMT and APNG [92]. In another study, YTHDF2 was found to be upregulated in TMZ-resistant tissues and cells, and the survival rate of patients with high YTHDF2 expression was lower than that of those with low YTHDF2 expression. Mechanistically, YTHDF2 binds to the m6A site in the 3’UTR of EPHB3 and TNFAIP3, reducing mRNA stability. YTHDF2 activates the PI3K/Akt and NF-κB signaling pathways by inhibiting the expression of EPHB3 and TNFAIP3, thereby enhancing TMZ resistance. Inhibition of these two pathways attenuated YTHDF2-mediated TMZ resistance [93]. In addition, stimulation of IRES-mediated mRNA translation through activation of the salvage pathway, which drives the synthesis of proteins promoting GBM resistance, can confer resistance to mTOR inhibitors in GBM cells. After m6A modification, the DRACH motif in these IRES RNAs is enhanced by HNRNPA1 binding upon mTOR inhibitor exposure, leading to increased IRES activity and corresponding resistance to mTOR inhibitors [64].
Wei et al., found that circSPECC1 was modified with m6A, and its m6A modification level was reduced in TMZ-resistant GBM cell lines [94]. IGF2BP1 binds to circSPECC1, and under normal conditions, this binding enhances circSPECC1 stability and increases its expression level. However, in recurrent GBM and TMZ-resistant GBM cell lines, the low m6A modification level of circSPECC1 restricts IGF2BP1 binding, resulting in decreased circSPECC1 stability and expression. CircSPECC1 encodes a protein, SPECC1-415aa, which binds to ANXA2 and prevents ANXA2 from binding to EGFR, thus inhibiting EGFR phosphorylation. This in turn inhibits EGFR phosphorylation and AKT signaling pathway activation, ultimately restoring the sensitivity of TMZ-resistant GBM cells to TMZ. Together, these molecular interactions influence the sensitivity of GBM cells to TMZ and the development of GBM.
Bao et al., reported that the expression level of the Ubiquitin- Binding Enzyme E2D3 (UBE2D3) was elevated in GBM tissues compared to normal brain tissues and was closely associated with DNA repair signaling pathways [47]. In both in vitro and in vivo studies, TMZ treatment combined with reduced UBE2D3 expression was shown to further suppress U87 cell viability and tumor growth, significantly increasing the apoptosis rate and DNA damage. Conversely, the overexpression of UBE2D3 exerted the opposite effect. Furthermore, findings indicated that WTAP promotes m6A modification of UBE2D3 via an IGF2BP1-dependent mechanism. The WTAP-IGF2BP1 axis regulates UBE2D3 stability in an m6A-dependent manner, influencing tumor malignancy and TMZ chemo-sensitivity in GBM by modulating DNA repair signaling pathways. These findings suggest that m6A methylation plays a crucial role in drug resistance in GBM and highlight m6A methylation as a potential new avenue for overcoming drug resistance in GBM.
Prognosis
Zhang et al., identified 25 regulators significantly associated with glioma prognosis. Of these, 14 regulators were identified as risk factors, including 3 methylation transferases (METTL5, WTAP, ZCCHC4), 1 demethylase (ALKBH5), and 10 methylation reader proteins (YTHDF1, YTHDF2, YTHDF3, HNRNPC, IGF2BP1, IGF2BP2, IGF2BP3, ELAVL1, G3BP1, RBMX), while an additional 11 regulatory factors were identified as protective factors, including 5 methyltransferases (METTL3, METTL14, METTL16, VIRMA, ZC3H13), 1 demethylase (FTO), and 5 methylation reader proteins (YTHDC1, YTHDC2, FMR1, EIF3A, PRRC2A). Using the iPAGE (An algorithm), they established the m6A-Related Gene Pairs (MGPs) and subsequently developed the MrGPS to predict Overall Survival (OS) in glioma patients. This model leveraged the relative expression of gene pairs, demonstrating significant improvement over singlegene- based models by integrating data and mitigating batch effects. Notably, the IGF2BP3–CYP17A1 gene pair was identified as a risk factor with a Hazard Ratio (HR) exceeding 1, highlighting its significance within the model. The MrGPS demonstrated excellent performance in glioma prognosis, achieving an average C-index of 0.76 and an average AUC of 0.82 for 1-year, 3-year, and 5-year OS predictions across five independent cohorts [95]. Wang et al., found that ALKBH5 expression was dramatically increased in glioma, and high expression of ALKBH5 predicted poor prognosis. Overexpression of ALKBH5 promotes cell proliferation, migration, and invasion in vitro and accelerates tumor growth in vivo [96]. Deng et al., identified YWHAG and ALKBH5 as independent prognostic indicators of GBM through risk analysis and validated these findings experimentally using clinical samples. Both YWHAG and ALKBH5 serve as prognostic markers for GBM patients [59]. Fan et al., further suggested that ALKBH5 is a promising prognostic factor for GBM patients and plays a critical role in GBM progression by promoting angiogenesis [49]. In summary, m6a regulatory molecules have important clinical effects. Therefore, it is of great significance to realize the transition from basic research to clinical application of m6a regulatory molecules.
Discussion
GBM is the most aggressive primary brain tumor. The effectiveness of surgical interventions and adjuvant radiotherapy or chemotherapy are still unsatisfactory, and new treatment methods are urgently needed. M6A methylation is a post-transcriptional modification regulated by three classes of enzymes: methyltransferases, demethylases, and methylation reader proteins. Recent studies have increasingly emphasized the key role of m6A methylation in GBM, this article reviews these latest advances and elaborates on the relationship between m6A regulatory molecules and GBM as well as the clinical significance of m6A regulatory molecules. For example, in terms of diagnosis: HNRNPC, YWHAG and ALKBH5 are significantly upregulated in GBM tissues, suggesting that they can be used as diagnostic indicators for GBM patients; in terms of clinical pathological characteristics: the expression of METTL3 can be used to classify GBM with similar histology; in terms of treatment: reducing the expression of demethylase-FTO to enhance the level of m6A methylation modification, shows a significant anti-proliferative effect in GBM. This method of increasing the level of m6A methylation by inhibiting demethylase provides a new idea for the treatment of GBM; in terms of drug resistance: high expression of YTHDF2 means that GBM patients are resistant to TMZ, while the WTAP-IGF2BP1 axis can affect the sensitivity of GBM to TMZ by regulating the stability of UBE2D3; in terms of prognosis: many m6A regulatory factors have been identified as prognostic indicators for GBM, with YWHAG and ALKBH5 being the most representative. Various signs indicate that the occurrence and development of GBM is accompanied by changes in m6A modification levels and the expression of m6A-related proteins.
However, despite progress in understanding the regulatory role of m6A modification in GBM, several limitations remain. The role of m6A regulatory factors in GBM remains controversial, and their influence on GBM progression requires further investigation. Some regulatory elements identified in recent studies have been linked to the prognosis of GBM patients, highlighting the need for additional clinical experiments to assess their therapeutic value. Moreover, novel prognosis-related molecules are yet to be discovered. Modulating the expression or activity of m6A modification-related enzymes in animal models has shown potential in improving GBM’s pathological characteristics. However, translating these findings into effective treatments presents significant challenges particularly in overcoming the blood-brain barrier, which remains a major unresolved issue. Additionally, m6A methylation can influence ferroptosis- related RNAs, suggesting that these processes may jointly regulate key physiological mechanisms in GBM. However, the relationship between them requires further clarification. The progression of GBM is also likely to involve multiple epigenetic modifications, but the interactions and synergistic mechanisms between m6A methylation and other epigenetic pathways are still poorly understood. Furthermore, improving m6A detection technology is urgent. Although antibody-based immunoprecipitation sequencing can identify m6A modification sites, variations in antibody specificity and affinity may lead to false positives or negatives. These limitations hinder the comprehensive analysis of m6A modifications in GBM samples and impede our ability to study their roles in depth.
Beyond m6A methylation, other RNA modification mechanisms also play critical roles in glioma biology. For instance, 5-Methylcytosine (m5C) influences cell proliferation by regulating the expression of proliferation-related genes and affects apoptosis by modulating apoptosis-associated gene expression. Similarly, 7-Methylguanosine (m7G) primarily regulates mRNA translation initiation to promote cell proliferation and influences the cell cycle by modulating cell cycle-related mRNAs. In terms of diagnosis and treatment, the enzymes or modification sites associated with m5C and m7G hold potential as diagnostic markers. Moreover, targeted therapies designed to regulate these enzymes or modification sites may serve as effective treatments for GBM. Conducting in-depth research on these RNA modification mechanisms is essential for advancing GBM diagnostic and therapeutic strategies.
Conclusion
In summary, RNA modification holds significant potential for advancing the diagnosis and treatment of GBM. However, the role of m6A in GBM remains largely unexplored comparable to a vast ocean still awaiting thorough investigation. Current research into this area is in its early stages, and numerous aspects require further exploration. Given the limited effectiveness of conventional treatments such as surgery, radiotherapy, and chemotherapy, in-depth studies on m6A methylation may pave the way for novel therapeutic approaches. This article reviews the relationship between m6A and GBM, highlights recent advances across various domains, and anticipates future research directions. It offers a fresh perspective and strategic insight into the evolving landscape of GBM diagnosis and therapy.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The work was supported by Central Guidance for Local Science and Technology Development Fund Projects of Department of Science and Technology of Hebei Province (Project Number: 246Z7743G); Yanzhao Golden Platform Talent Gathering Project of Hebei Provincial Department of Human Resources and Social Security (Project Number: B2024025); Provincial Clinical Medical Talents Project in 2025 (ZF2025087) and Hebei Natural Science Foundation (H2024206010).
Conflicts of Interest
All authors declare that they have no conflict of interest.
References
- Lu YB, Sun TJ, Chen YT, Cai ZY, Zhao JY, et al. (2020) Targeting the Epithelial-to-Mesenchymal Transition in Cancer Stem Cells for a Better Clinical Outcome of Glioma. Technol Cancer Res Treat 19: 1533033820948053.
- Qiu J, Shi Z, Jiang J (2017) Cyclooxygenase-2 in glioblastoma multiforme. Drug Discov Today 22(1): 148-156.
- Liu Y, Zhou F, Ali H, Lathia JD, Chen P (2024) Immunotherapy for glioblastoma: current state, challenges, and future perspectives. Cell Mol Immunol 21(12): 1354-1375.
- Huang B, Li X, Li Y, Zhang J, Zong Z, et al. (2020) Current Immunotherapies for Glioblastoma Multiforme. Front Immunol 11: 603911.
- Huang W, Chen TQ, Fang K, Zeng ZC, Ye H, et al. (2021) N6-methyladenosine methyltransferases: functions, regulation, and clinical potential. J Hematol Oncol 14(1): 117.
- You Cai Yi, Xiao Yu Chen, Jing Zhang, Jin Shui Zhu (2020) Novel insights into the interplay between m6A modification and noncoding RNAs in cancer. Mol Cancer 19(1): 121.
- Han J, Kong H, Wang X, Zhang XA (2022) Novel insights into the interaction between N6-methyladenosine methylation and noncoding RNAs in musculoskeletal disorders. Cell Prolif 55(10): e13294.
- Luan J, Kopp JB, Zhou H (2023) N6-methyladenine RNA Methylation Epigenetic Modification and Kidney Diseases. Kidney Int Rep 8(1): 36-50.
- Huang J, Dong X, Gong Z, Qin LY, Yang S, et al. (2019) Solution structure of the RNA recognition domain of METTL3-METTL14 N6-methyladenosine methyltransferase. Protein Cell 10(4): 272-284.
- Liu J, Yue Y, Han D, Wang X, Fu Y, et al. (2014) A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol 10(2): 93-95.
- Xie L, Zhang X, Xie J, Xu Y, Li XJ, et al. (2023) Emerging Roles for DNA 6mA and RNA m6A Methylation in Mammalian Genome. Int J Mol Sci 24(18): 13897.
- Pendleton KE, Chen B, Liu K, Hunter OV, Xie Y, et al. (2017) The U6 snRNA m6A Methyltransferase METTL16 Regulates SAM Synthetase Intron Retention. Cell 169: 824-835.e14.
- Fu Y, Jia G, Pang X, Wang RN, Wang X, et al. (2013) FTO-mediated formation of N6-hydroxymethyladenosine and N6-formyladenosine in mammalian RNA. Nat Commun 4: 1798.
- Yue Y, Liu J, He C (2015) RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev 29(13): 1343-1355.
- Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, et al. (2013) ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell 49(1): 18-29.
- Ueda Y, Ooshio I, Fusamae Y, Kitae K, Kawaguchi M, et al. (2017) AlkB homolog 3-mediated tRNA demethylation promotes protein synthesis in cancer cells. Sci Rep 7: 42271.
- Allis CD, Jenuwein T (2016) The molecular hallmarks of epigenetic control. Nat Rev Genet 17(8): 487-500.
- Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, et al. (2015) N (6)-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell 161(6): 1388-1399.
- Liu S, Li G, Li Q, Zhang Q, Zhuo L, et al. (2020) The roles and mechanisms of YTH domain-containing proteins in cancer development and progression. Am J Cancer Res 10(4): 1068-1084.
- Shi H, Wang X, Lu Z, Zhao BS, Ma H, et al. (2017) YTHDF3 facilitates translation and decay of N6-methyladenosine-modified RNA. Cell Res 27(3): 315-328.
- Hsu PJ, Zhu Y, Ma H, Guo Y, Shi X (2017) Ythdc2 is an N6-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res 27(9): 1115-1127.
- Kretschmer J, Rao H, Hackert P, Sloan KE, Höbartner C, et al. (2018) The m6A reader protein YTHDC2 interacts with the small ribosomal subunit and the 5’-3’ exoribonuclease XRN1. RNA 24(10): 1339-1350.
- Huang H, Weng H, Sun W, Qin X, Shi H, et al. (2018) Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol 20(3): 285-295.
- Zhou KI, Pan T (2018) An additional class of m6A readers. Nat Cell Biol 20(3): 230-232.
- Christiansen J, Kolte AM, Hansen Tv O, Nielsen FC (2009) IGF2 mRNA-binding protein 2: biological function and putative role in type 2 diabetes. J Mol Endocrinol 43(5): 187-195.
- Li X, Allayee H, Xiang AH, Trigo E, Hartiala J, et al. (2009) Variation in IGF2BP2 interacts with adiposity to alter insulin sensitivity in Mexican Americans. Obesity (Silver Spring) 17(4): 729-736.
- Dai N, Zhao L, Wrighting D, Krämer D, Majithia A, et al. (2015) IGF2BP2/IMP2-Deficient mice resist obesity through enhanced translation of Ucp1 mRNA and other mRNAs encoding mitochondrial proteins. Cell Metab 21(4): 609-621.
- Ravanidis S, Kattan FG, Doxakis E (2018) Unraveling the Pathways to Neuronal Homeostasis and Disease: Mechanistic Insights into the Role of RNA-Binding Proteins and Associated Factors. Int J Mol Sci 19(8): 2280.
- Yaniv K, Yisraeli JK (2002) The involvement of a conserved family of RNA binding proteins in embryonic development and carcinogenesis. Gene 287(1-2): 49-54.
- Alarcón CR, Goodarzi H, Lee H, Liu X, Tavazoie S, et al. (2015) HNRNPA2B1 Is a Mediator of m (6)A-Dependent Nuclear RNA Processing Events. Cell 162(6): 1299-1308.
- Liu N, Dai Q, Zheng G, He C, Parisien M, et al. (2015) N (6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature 518(7540): 560-564.
- Liu N, Zhou KI, Parisien M, Dai Q, Diatchenko L, et al. (2017) N6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res 45(10): 6051-6063.
- Wu B, Su S, Patil DP, Liu H, Gan J, et al. (2018) Molecular basis for the specific and multivariant recognitions of RNA substrates by human hnRNP A2/B1. Nat Commun 9(1): 420.
- Lv J, Xing L, Zhong X, Li K, Liu M, et al. (2023) Role of N6-methyladenosine modification in central nervous system diseases and related therapeutic agents. Biomed Pharmacother 162: 114583.
- Chen F, Xie X, Chao M, Cao H, Wang L (2022) The Potential Value of m6A RNA Methylation in the Development of Cancers Focus on Malignant Glioma. Front Immunol 13: 917153.
- Long S, Yan Y, Xu H, Wang L, Jiang J, et al. (2023) Insights into the regulatory role of RNA methylation modifications in glioma. J Transl Med 21(1): 810.
- Wu Z, Lin Y, Wei N (2023) N6-methyladenosine-modified HOTAIRM1 promotes vasculogenic mimicry formation in glioma. Cancer Sci 114(1): 129-141.
- You J, Tao B, Peng L, Peng T, He H, et al. (2023) Transcription factor YY1 mediates self-renewal of glioblastoma stem cells through regulation of the SENP1/METTL3/MYC axis. Cancer Gene Ther 30: 683-693.
- Wu Q, Yin X, Zhao W, Xu W, Chen L (2022) Molecular mechanism of m6A methylation of circDLC1 mediated by RNA methyltransferase METTL3 in the malignant proliferation of glioma cells. Cell Death Discov 8(1): 229.
- Tassinari V, Cesarini V, Tomaselli S, Ianniello Z, Silvestris DA, et al. (2021) ADAR1 is a new target of METTL3 and plays a pro-oncogenic role in glioblastoma by an editing-independent mechanism. Genome Biol 22(1): 51.
- Lv D, Gimple RC, Zhong C, Wu Q, Yang K, et al. (2022) PDGF signaling inhibits mitophagy in glioblastoma stem cells through N6-methyladenosine. Dev Cell 57: 1466-1481.e6.
- Liu M, Ruan X, Liu X, Dong W, Wang D, et al. (2022) The mechanism of BUD13 m6A methylation mediated MBNL1-phosphorylation by CDK12 regulating the vasculogenic mimicry in glioblastoma cells. Cell Death Dis 13(12): 1017.
- Meng X, Wang Z, Yang Q, Liu Y, Gao Y, et al. (2024) Intracellular C5aR1 inhibits ferroptosis in glioblastoma through METTL3-dependent m6A methylation of GPX4. Cell Death Dis 15(10): 729.
- Ji Q, Guo Y, Li Z, Zhang X (2024) WTAP regulates the production of reactive oxygen species, promotes malignant progression, and is closely related to the tumor microenvironment in glioblastoma. Aging (Albany NY) 16: 5601-5617.
- Bao J, Sun R, Pan Z, Wei S (2025) UBE2D3 regulated by WTAP-mediated m6A modification inhibits temozolomide chemosensitivity in glioblastoma. Naunyn Schmiedebergs Arch Pharmacol 398: 919-931.
- Qiu J, Zhao R, Ma C, Wang Q, Li B, et al. (2025) O-GlcNAcylation stabilized WTAP promotes GBM malignant progression in an N6-methyladenosine-dependent manner. Neuro Oncol 27: 900-915.
- Tao M, Li X, He L, Rong X, Wang H, et al. (2022) Decreased RNA m6A methylation enhances the process of the epithelial mesenchymal transition and vasculogenic mimicry in glioblastoma. Am J Cancer Res 12(2): 893-906.
- Fan Y, Yan D, Ma L, Liu X, Luo G, et al. (2024) ALKBH5 is a prognostic factor and promotes the angiogenesis of glioblastoma. Sci Rep 14: 1303.
- Tang W, Xu N, Zhou J, He Z, Lenahan C, et al. (2022) ALKBH5 promotes PD-L1-mediated immune escape through m6A modification of ZDHHC3 in glioma. Cell Death Discov 8(1): 497.
- Lv D, Zhong C, Dixit D, Yang K, Wu Q, et al. (2023) EGFR promotes ALKBH5 nuclear retention to attenuate N6-methyladenosine and protect against ferroptosis in glioblastoma. Mol Cell 83: 4334-4351.e7.
- Tuoheti M, Li J, Zhang C, Gao F, Wang J, et al. (2024) MiR-124-3p inhibits cell stemness in glioblastoma via targeting EPHA2 through ALKBH5-mediated m6A modification. Hum Cell 38: 10.
- Zhang Y, Xie S, Li W, Gu J, Zhang XA, et al. (2025) TDP-43/ALKBH5-mediated m6A modification of CDC25A mRNA promotes glioblastoma growth by facilitating G1/S cell cycle transition. MedComm (2020) 6(3): e70108.
- Du P, Meng L, Liao X, Liu Y, Mo X, et al. (2023) The miR-27a-3p/FTO axis modifies hypoxia-induced malignant behaviors of glioma cells. Acta Biochim Biophys Sin (Shanghai) 55(1): 103-116.
- Dong J, Mao J, Wu W, Qian X, Yu Z (2025) FTO Suppresses Proliferation and Induces Apoptosis of T98G Glioblastoma Cells via N6-methyladenosine Modification of GSTO1. Neurochem Res 50(2): 83.
- Zhang S, Zhao S, Qi Y, Li B, Wang H, et al. (2022) SPI1-induced downregulation of FTO promotes GBM progression by regulating pri-miR-10a processing in an m6A-dependent manner. Mol Ther Nucleic Acids 27: 699-717.
- Tian Q, Dan G, Wang X, Zhu J, Chen C, et al. (2025) IDO1 inhibits ferroptosis by regulating FTO-mediated m6A methylation and SLC7A11 mRNA stability during glioblastoma progression. Cell Death Discov 11(1): 22.
- Lv D, Dixit D, Cruz AF, Kim LJY, Duan L, et al. (2024) Metabolic regulation of the glioblastoma stem cell epitranscriptome by malate dehydrogenase 2. Cell Metab 36: 2419-2436.e8.
- Deng X, Sun X, Hu Z, Wu Y, Zhou C, et al. (2023) Exploring the role of m6A methylation regulators in glioblastoma multiforme and their impact on the tumor immune microenvironment. FASEB J 37(9): e23155.
- Yarmishyn AA, Yang YP, Lu KH, Chen YC, Chien Y, et al. (2020) Musashi-1 promotes cancer stem cell properties of glioblastoma cells via upregulation of YTHDF1. Cancer Cell Int 20(1): 597.
- Chai RC, Chang YZ, Chang X, Pang B, An SY, et al. (2021) YTHDF2 facilitates UBXN1 mRNA decay by recognizing METTL3-mediated m6A modification to activate NF-κB and promote the malignant progression of glioma. J Hematol Oncol 14(1): 109.
- Yu P, Xu T, Ma W, Fang X, Bao Y, et al. (2024) PRMT6-mediated transcriptional activation of ythdf2 promotes glioblastoma migration, invasion, and emt via the wnt-β-catenin pathway. J Exp Clin Cancer Res 43(1): 116.
- Lee HH, Hsieh CC, Chang CC, Liao WT, Chi HC (2023) YTHDF3 Modulates EGFR/ATK/ERK/p21 Signaling Axis to Promote Cancer Progression and Osimertinib Resistance of Glioblastoma Cells. Anticancer Res 43(12): 5485-5498.
- Benavides Serrato A, Saunders JT, Kumar S, Holmes B, Benavides KE, Bashir MT, et al. (2023) m6A-modification of cyclin D1 and c-myc IRESs in glioblastoma controls ITAF activity and resistance to mTOR inhibition. Cancer Lett 562: 216178.
- Lin H, Cui Z, E T, Xu H, Wang D, et al. (2024) M6A-methylated circPOLR2B forms an R-loop and regulates the biological behavior of glioma stem cells through positive feedback loops. Cell Death Dis 15(8): 554.
- Shao W, Zhao H, Zhang S, Ding Q, Guo Y, et al. (2022) A pan-cancer landscape of IGF2BPs and their association with prognosis, stemness and tumor immune microenvironment. Front Oncol 12: 1049183.
- Zhan WL, Gao N, Tu GL, Tang H, Gao L, et al. (2021) LncRNA LINC00689 Promotes the Tumorigenesis of Glioma via Mediation of miR-526b-3p/IGF2BP1 Axis. Neuromolecular Med 23(3): 383-394.
- Cun Y, An S, Zheng H, Lan J, Chen W, et al. (2023) Specific Regulation of m6A by SRSF7 Promotes the Progression of Glioblastoma. Genomics Proteomics Bioinformatics 21(4): 707-728.
- Liu H, Qin S, Liu C, Jiang L, Li C, et al. (2021) m6A reader IGF2BP2-stabilized CASC9 accelerates glioblastoma aerobic glycolysis by enhancing HK2 mRNA stability. Cell Death Discov 7(1): 292.
- Chen P, Xu J, Cui Z, Wu S, Xie T, et al. (2023) Multi-omics analysis of N6-methyladenosine reader IGF2BP3 as a promising biomarker in pan-cancer. Front Immunol 14: 1071675.
- Wang C, Zhang M, Liu Y, Cui D, Gao L, et al. (2023) CircRNF10 triggers a positive feedback loop to facilitate progression of glioblastoma via redeploying the ferroptosis defense in GSCs. J Exp Clin Cancer Res 42(1): 242.
- Deng L, Di Y, Chen C, Xia J, Lei B, et al. (2024) Depletion of the N6-Methyladenosine (m6A) reader protein IGF2BP3 induces ferroptosis in glioma by modulating the expression of GPX4. Cell Death Dis 15(3): 181.
- Yuan F, Cai X, Cong Z, Wang Y, Geng Y, et al. (2022) Roles of the m6A Modification of RNA in the Glioblastoma Microenvironment as Revealed by Single-Cell Analyses. Front Immunol 13: 798583.
- Wang H, Shi Y, Zhou X, Zhang L, Yang A, Zhou D, et al. (2024) HNRNPA2B1 stabilizes NFATC3 levels to potentiate its combined actions with FOSL1 to mediate vasculogenic mimicry in GBM cells. Cell Biol Toxicol 40(1): 44.
- Zhang J, Liu B, Xu C, Ji C, Yin A, et al. (2024) Cholesterol homeostasis confers glioma malignancy triggered by hnRNPA2B1-dependent regulation of SREBP2 and LDLR. Neuro Oncol 26(4): 684-700.
- Wang LC, Chen SH, Shen XL, Li DC, Liu HY, et al. (2020) M6A RNA Methylation Regulator HNRNPC Contributes to Tumorigenesis and Predicts Prognosis in Glioblastoma Multiforme. Front Oncol 10: 536875.
- Chen JJ, Lu TZ, Wang T, Yan WH, Zhong FY, et al. (2024) The m6A reader HNRNPC promotes glioma progression by enhancing the stability of IRAK1 mRNA through the MAPK pathway. Cell Death Dis 15(6): 390.
- Kowalski Chauvel A, Lacore MG, Arnauduc F, Delmas C, Toulas C, et al. (2020) The m6A RNA Demethylase ALKBH5 Promotes Radioresistance and Invasion Capability of Glioma Stem Cells. Cancers (Basel) 13(1): 40.
- Liu Z, Chen Y, Wang L, Ji S (2021) ALKBH5 Promotes the Proliferation of Glioma Cells via Enhancing the mRNA Stability of G6PD. Neurochem Res 46(11): 3003-3011.
- Liu B, Zhou J, Wang C, Chi Y, Wei Q, et al. (2020) LncRNA SOX2OT promotes temozolomide resistance by elevating SOX2 expression via ALKBH5-mediated epigenetic regulation in glioblastoma. Cell Death Dis 11(5): 384.
- Malacrida A, Rivara M, Di Domizio A, Cislaghi G, Miloso M, et al. (2020) 3D proteome-wide scale screening and activity evaluation of a new ALKBH5 inhibitor in U87 glioblastoma cell line. Bioorg Med Chem 28(4): 115300.
- Ding C, Yi X, Chen Xiangrong, Wu Z, You H, et al. (2021) Warburg effect-promoted exosomal circ_0072083 releasing up-regulates NANGO expression through multiple pathways and enhances temozolomide resistance in glioma. J Exp Clin Cancer Res 40(1): 164.
- Deng X, Sun X, Hu Z, Wu Y, Zhou C, et al. (2023) Exploring the role of m6A methylation regulators in glioblastoma multiforme and their impact on the tumor immune microenvironment. FASEB J 37(9): e23155.
- Krusnauskas R, Stakaitis R, Steponaitis G, Almstrup K, Vaitkiene P (2023) Identification and comparison of m6A modifications in glioblastoma non-coding RNAs with MeRIP-seq and Nanopore dRNA-seq. Epigenetics 18(1): 2163365.
- Ma X, Xia Y, Wang S, Yang Z, Lei X, et al. (2023) One-Base-Gap Circular Probe-Mediated Dual Amplification for Isothermal Detection of N6-Methyladenosine Modifications. Anal Chem 95(48): 17595-17602.
- Chai RC, Wu F, Wang QX, Zhang S, Zhang KN, et al. (2019) m6A RNA methylation regulators contribute to malignant progression and have clinical prognostic impact in gliomas. Aging (Albany NY) 11(4): 1204-1225.
- Li F, Yi Y, Miao Y, Long W, Long T, et al. (2019) N6-Methyladenosine Modulates Nonsense-Mediated mRNA Decay in Human Glioblastoma. Cancer Res 79(22): 5785-5798.
- Cui Q, Shi H, Ye P, Li L, Qu Q, et al. (2017) m6A RNA Methylation Regulates the Self-Renewal and Tumorigenesis of Glioblastoma Stem Cells. Cell Rep 18(11): 2622-2634.
- Takahashi H, Hase H, Yoshida T, Tashiro J, Hirade Y, et al. (2022) Discovery of two novel ALKBH5 selective inhibitors that exhibit uncompetitive or competitive type and suppress the growth activity of glioblastoma multiforme. Chem Biol Drug Des 100: 1-12.
- Huff S, Kummetha IR, Zhang L, Wang L, Bray W, et al. (2022) Rational Design and Optimization of m6A-RNA Demethylase FTO Inhibitors as Anticancer Agents. J Med Chem 65(16): 10920-10937.
- Ji X, Liu Z, Gao J, Bing X, He D, et al. (2023) N6-Methyladenosine-modified lncRNA LINREP promotes Glioblastoma progression by recruiting the PTBP1/HuR complex. Cell Death Differ 30(1): 54-68.
- Li B, Zhao R, Qiu W, Pan Z, Zhao S, et al. (2022) The N6-methyladenosine-mediated lncRNA WEE2-AS1 promotes glioblastoma progression by stabilizing RPN2. Theranostics 12: 6363-6379.
- Shi J, Zhang P, Dong X, Yuan J, Li Y, et al. (2023) METTL3 knockdown promotes temozolomide sensitivity of glioma stem cells via decreasing MGMT and APNG mRNA stability. Cell Death Discov 9(1): 22.
- Chen Y, Wang YL, Qiu K, Cao YQ, Zhang FJ, et al. (2022) YTHDF2 promotes temozolomide resistance in glioblastoma by activation of the Akt and NF-κB signalling pathways via inhibiting EPHB3 and TNFAIP3. Clin Transl Immunology 11(5): e1393.
- Wei C, Peng D, Jing B, Wang B, Li Z, et al. (2024) A novel protein SPECC1-415aa encoded by N6-methyladenosine modified circSPECC1 regulates the sensitivity of glioblastoma to TMZ. Cell Mol Biol Lett 29: 127.
- Zhang N, Yang F, Zhao P, Jin N, Wu H, et al. (2023) MrGPS: an m6A-related gene pair signature to predict the prognosis and immunological impact of glioma patients. Brief Bioinform 25(1): bbad498.
- Wang C, Xu N, Zhong X, Liu B, Tang W, et al. (2025) ALKBH5 facilitates tumor progression via an m6A-YTHDC1-dependent mechanism in glioma. Cancer Lett 612: 217439.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.