Mini Review 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Metabolic basis of trained immunity
*Corresponding author: Chaofeng Han, National Key Laboratory of Medical Immunology & Institute of Immunology, Second Military Medical University, Shanghai, China.
Received: July 11, 2019; Published: July 18, 2019
DOI: 10.34297/AJBSR.2019.04.000762
Introduction
Innate immunity is considered to be unable to construct immunological memory in the traditional concept. However, in recent years, there is growing evidence to dispute this idea. Plants and invertebrates lacking an adaptive immune system can mount resistance to secondary infections [1,2]. NK cells and monocytes are also found have memory characteristics [3,4] In 2011, Netea et al. [5] proposed a new term “trained immunity” for describing innate immune memory responses. In 2012, Quintin et al. [6] further demonstrate trained immunity even occurred in mice lacking T and B cells, serving as an increased response to secondary infections which is monocyte- dependent after priming with β-glucan from Candida albicans.
Further molecular mechanisms studies of trained immunity suggest that epigenetic reprogramming plays a key role in mediating innate immune memory. Quintin et al. [6] demonstrated that β-glucans from C. albicans cell wall induced monocyte training associated with epigenetic changes in H3K4me3, which requires receptor dectin-1 and the non-canonical Raf-1 pathway. Another work demonstrated that BCG (bacille calmette guerin) has similar trained immunity inducing function through the NOD2 receptor and also mediated by increased H3K4me3 [7]. Ostuni et al. [8] suggested that latent enhancers, a group of stimulus-specific expanded cis-regulatory repertoire associated with a steady H3K4me1 mark could provide an epigenomic memory of the exposure to environmental agents. This new founding may further describe the mechanisms of trained immunity.
However, there is poorly insight on the specific cellular processes that mediate trained immunity. Recently, glucose metabolism switch from oxidative phosphorylation to aerobic glycolysis has turned out to be crucial for activation of immune cells [9]. There is a growing concern over metabolism switch and innate host defense.
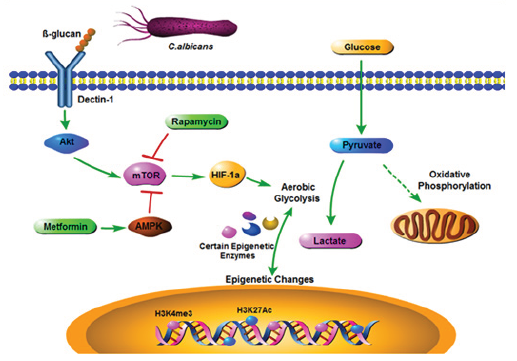
Figure 1: Model of metabolic changes in trained monocytes. In monocytes, dectin-1 receptor can recognize β-glucan from C. albicans cell wall and initiate downstream activation of AKT-mTOR-HIF-1α pathway, which shifts the glucose metabolism from oxidative phosphorylation to aerobic glycolysis. Aerobic glycolysis is the metabolic basis for trained immunity. Rapamycin and metformin would inhibit mTOR activation and abrogate trained immunity effect. Glucose metabolism changes may interact with the epigenetic changes that may mediate trained immunity though certain epigenetic enzymes and the detail mechanisms are still unclear. AKT, Protein kinase B; mTOR, mammalian target of rapamycin; HIF-1α, Hypoxia-inducible factor 1-alpha; AMPK, 5’ adenosine monophosphate-activated protein kinase; H3K4me3, histone 3 Lys 4 trimethylation; H3K27Ac, histone 3 Lys 27 acetylation.
In a recent issue of Science, Cheng et al. [10] reported that aerobic glycolysis mediated by mTOR- and HIF-1α is the metabolic basis for trained immunity (Figure 1). The authors have established an in vitro model of trained immunity with human monocytes. The monocytes induced by the β-glucan show nonspecific protection against both infections and malignancies 7 days later after the initial priming [6]. They used ChIP-seq to monitor trained monocytes how the epigenome was affected with H3K4me3 and H3K27Ac, two classic promoter epigenetic markers. According to the epigenetic signals in promoter region, the authors found upregulated genes in trained monocytes and many of those genes are associated with central metabolism. Especially there is an increase in active genes involved in glycolysis and mTOR pathways. The interrelating transcription factor HIF-1α level was also increased in β-glucan trained monocytes. In addition, the authors used total RNA sequencing to analyze splenocytes of mice challenged with β-glucan and found glycolysis genes were also up-regulated in vivo. These findings implicated that glycolysis may have a role to play in the training phenomenon.
Next, the authors investigated how glucose metabolism changes in trained monocytes. Trained immunity phenomenon was blockaded by incubation of cells with 2-deoxy-D-glucose, a glycolysis inhibitor, suggesting that glycolysis is the main energy source of trained monocytes. Consistent with the hypothesis that trained monocytes undergo a shift from oxidative metabolism toward glycolysis, the authors observed that there are increased glucose consumption, lactate production, and ratio of NAD+/NADH in trained monocytes on day 7 relative to naïve cells. Moreover, by high-resolution respirometry, trained monocytes showed a reduced baseline oxygen consumption, a decreased maximal rate of oxygen consumption and a reduction of the capacity of the mitochondrial electron transport chain (ETC). These observations reinforced the hypothesis.
The authors then investigated the mechanisms by which glucose metabolism shifts. It is known that mTOR is a master regulator of glucose metabolism in activated lymphocytes [11]. Based on the findings that epigenetic signals at promoters of genes in the mTOR pathway were significantly increased and mTOR phosphorylation was up-regulated in trained monocytes, the authors demonstrated that the mTOR pathway was activated in β-glucan trained monocytes. Then, they found that dectin-1 C-type lectin receptor was the sensor for β-glucan and mTOR activation was mediated through Akt-PI3K (phosphatidylinositol 3-kinase) pathway in trained monocytes. In addition, the authors demonstrated that histonemethylation also could partially modify the induction of glycolysis because MTA, a methyltransferase inhibitor reduced lactate production in the trained monocytes.
Finally, the authors clarified whether glucose metabolism shifts toward glycolysis induce trained immunity. Inhibition of mTOR and HIF-1α led to a dose-dependent inhibition of β-glucan induced training effect in monocytes. In vivo, metformin, an indirect mTOR inhibitor could abrogate the protective effects in β-glucan induced experimental mice models. They further used myeloid cell specific HIF-1α conditional knockout mice to elucidate the effects of mTORHIF- 1α were mediated only at the level of innate immunity. β-glucan induced trained immunity was completely abrogated in mHIF- 1α KO mice infected with S. aureus compared to wild-type mice. Therefore, mTOR and HIF-1α mediated glycolysis in myeloid cells is crucial for mounting trained immunity both in vitro and in vivo.
The authors provided compelling evidence demonstrating the basic role of aerobic glycolysis in trained immunity and shed new light on the relationship between metabolism and innate immune memory which was previously ignored. However, several questions remain to be answered. First, whether and how glucose metabolism changes interact with the epigenetic changes that may mediate trained immunity? As it is known that many metabolites of glucose cellular metabolism act as co-factors for epigenetic enzymes, [12] the authors supposed that NAD+-dependent histone deacetylase sirtuins family may play a role in modulating monocyte function. Sirtuin-1 and sirtuin-6 histone deacetylases can induce immune tolerance [13,14] rather than training and the authors found that the expression of sirtuin1 was inhibited in trained monocytes. Moreover, sirtuin-1 activator resveratrol partially inhibited the enhanced cytokines production in vitro trained model. These results indicated that sirtuin1 may inhibit trained immunity. However, a series of issues are raised by these findings. How might metabolism shift influence sirtuins? How sirtuins modify chromatin in trained immune cells? Is there any other epigenetic enzymes play roles in this process? It would be interesting to investigate the complex interaction between the metabolites and immune memory responses through epigenetic enzymes.
Secondly, trained immunity was proposed by Netea et al. [5] for describing innate immunological memory of past insults. However, the β-glucans induced cell model they established was only found valid in monocytes, not in NK cells, which were also well reported of adaptive characteristics [3]. Nonetheless, the role of metabolism shift in other innate immune cells still needs further study.
Last but not least, kinetic study of metabolites indicated the metabolic shift happened in the day3-7 after β-glucan treatment. Further investigation on kinetic change of mTOR and HIF-1α activation is necessary for finding out the details of the metabolic shift.
IL-1β is an important factor in autoinflammatory and autoimmune diseases [15]. The authors found IL-1β also could induce trained immunity in monocytes in vitro. Long lasting in vivo effects of trained immunity induced by BCG have also been demonstrated in humans [7]. These works indicated trained immunity effects would be clinically useful. Collectively, Cheng et al. [10] defined glycolysis as a fundamental process in trained immunity. Further studies of metabolic regulatory mechanism in innate immune memory responses may benefit therapeutic designs for both infectious and inflammatory diseases.
References
- Durrant WE, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42: 185-209.
- Kurtz J, Franz K (2003) Innate defence: evidence for memory in invertebrate immunity. Nature 425(6953): 37-38.
- Sun JC, Beilke JN, Lanier LL (2009) Adaptive immune features of natural killer cells. Nature 457(7229): 557-561.
- vant Wout JW, Poell R, van Furth R (1992) The role of BCG/PPD-activated macrophages in resistance against systemic candidiasis in mice. Scand J Immunol 36(5): 713-719.
- Netea MG, Quintin J, van der Meer JW (2011) Trained immunity: a memory for innate host defense. Cell Host Microbe 9(5): 355-361.
- Quintin J, Saeed S, Martens JHA, Giamarellos Bourboulis EJ, Ifrim DC, et al. (2012) Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12(2): 223-232.
- Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, et al. (2012) Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A 109(43): 17537-17542.
- Ostuni R, Piccolo V, Barozzi I, Polletti S, Termanini A, et al. (2013) Latent enhancers activated by stimulation in differentiated cells. Cell 152(1-2): 157-171.
- Neill LA, Hardie DG (2013) Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 493(7432): 346-355.
- Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, et al. (2014) mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345(6204): 1250684.
- Hongbo Chi (2012) Regulation and function of mTOR signalling in T cell fate decisions. Nature Reviews Immunology 12: 325-338.
- Donohoe DR, Bultman SJ (2012) Metaboloepigenetics: interrelationships between energy metabolism and epigenetic control of gene expression. J Cell Physiol 227(9): 3169-3177.
- Liu TF, Vachharajani VT, Yoza BK, McCall CE (2012) NAD+-dependent sirtuin 1 and 6 proteins coordinate a switch from glucose to fatty acid oxidation during the acute inflammatory response. J Biol Chem 287(31): 25758-25769.
- Tannahill GM, Curtis AM, Adamik J, Palsson McDermott EM, McGettrick AF, et al. (2013) Succinate is an inflammatory signal that induces IL- 1beta through HIF-α Nature 496(7444): 238-242.
- Dinarello CA, Simon A, van der Meer JW (2012) Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov 11(8): 633-652.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.