Case Report 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Drug Induced Liver Injury and Lactic Acidosis Associated with Chronic Sustained Release Nicotinamide Exposure
*Corresponding author: Robert Goodnough, Department of Emergency Medicine, Baylor College of Medicine, USA
Received: September 11, 2019;Published: September 16, 2019
DOI: 10.34297/AJBSR.2019.05.000894
Keywords: Niacin; Nicotinamide; Hepatotoxicity; Lactate
Background
It is frequently difficult to identify an etiology in an individual case of acute liver injury. Xenobiotic exposures, infectious causes, and a broad differential of other potential contributors need to be considered. Pinpointing the specific cause of a patient’s liver injury helps to guide management, especially when xenobiotics are implicated, by indicating potential therapeutic interventions and by avoidance of recurrent injury from inadvertent re-challenge with an offending agent. We present a case of acute liver failure presenting with concomitant metabolic lactic acidosis and hypoglycemia associated with the therapeutic dosing of sustained-release niacin, confirmed by laboratory testing.
Case Report
A sixty-two-year-old male presented to the emergency department (ED) with complaints of malaise, abdominal pain, and nonbloody emesis. For the past 14 months, the patient had been taking “slo-niacin” (sustained-release nicotinamide), dosed at three grams per day. Other prescription medications the patient used on an ongoing basis were: metformin, glargine insulin, atorvastatin, gabapentin, metoprolol tartrate, lisinopril, aspirin, and levothyroxine. The patient also reported the use of cinnamon in both tea form and as a dietary supplement tablet. He denied excessive dosing or overdose with any prescription or over-the-counter medication. He had not drunk ethanol in recent decades. He also denied illicit drug use, wild mushroom ingestion, acetaminophen intake, iron supplementation, or the use of isoniazid. His past medical history was notable for diabetes mellitus type II, hypertension, coronary artery disease (with remote coronary artery bypass grafting), depression, and hypothyroidism. There was no history of previous liver disease.
One physical examination, the patient appeared unwell. Vital signs were: oral temperature 100.4 F; heart rate, 104 bpm; blood pressure, 95/49 mmHg; respiratory rate, 20 breaths per minute; and oxygen saturation, 96% on room air. Scleral icterus was present. Abdominal examination was unremarkable, without appreciable hepatomegaly. The patient was awake, alert without confusion, and did not display asterixis. The remainder of the neurologic exam was unremarkable. Initial liver function testing and coagulation profile demonstrated the following: AST, 406 IU/L; ALT, 294 IU/L; alkaline phosphatase, 346 IU/L; total bilirubin, 1.2 mg/dl; and INR, 3.4. The lactate was elevated to 17.0 mmol/L with a repeat value of 17.6 mmol/L. Other salient initial laboratory data included: WBC, 18,000 /mircoL; hemoglobin, 15.2 g/dl; platelets, 192,000/microL; Na, 141 mEq/L; K 4.7, mEq/L; Cl 98 mEq/L; C02, 13 mEq/L (anion gap, 30); BUN, 17 mg/dl; creatinine, 1.52 mg/dl; and glucose, 59 mg/dl. An elevated troponin enzyme level (0.96 ng/dl) and concurrent lateral electrocardiographic ST depressions were noted.
He received 3 liters of intravenous (IV) fluid resuscitation, with improvement of his lactate to 3 mmol/L and then subsequent normalization. He was treated empirically with IV vancomycin and piperacillin- tazobactam for suspected bacterial sepsis. Acyclovir was initiated IV for coverage of herpetic viral hepatitis. In addition, IV n-acetylcysteine was administered pending acetaminophen determination and as a non-specific intervention for liver failure. Over the course of hospitalization, the liver enzymes peaked above the limit of quantification of the laboratory, with AST and ALT >5000 IU/L; the bilirubin rose to a peak value of 9.9 mg/dl. The INR progressively prolonged to 5.8 and he developed thrombocytopenia (nadir 30,000/microL). The leukocytosis resolved and blood and urine cultures did not demonstrate the growth of pathogenic organisms. Further laboratory testing was negative for Wilson’s disease, acute syphilis, Epstein Barr virus, cytomegalovirus, hepatitis A, B, C, and E, human herpes Virus-6, HSV, and VZV. Serum from presentation did not show an elevated iron level and acetaminophen was non-detectable (<10 mg/L).
The patient underwent a liver biopsy. On pathologic examination, the tissue showed a ductular reaction with associated confluent hepatic necrosis and cholestasis. A few scattered acidophil bodies were also seen in the hepatic lobules, and a moderate neutrophilic and lymphocytic infiltrate was noted.
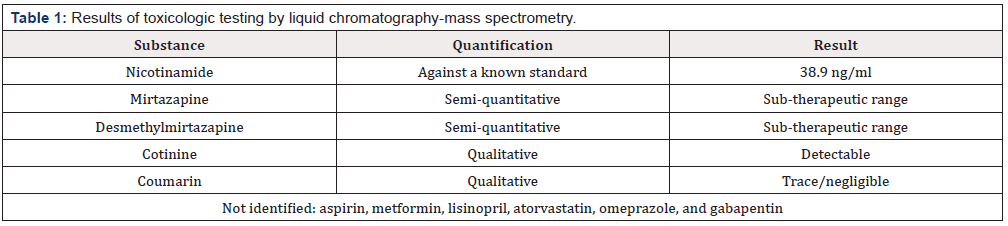
Additional toxicologic testing (Table 1) was carried out by liquid chromatography-mass spectrometry (LC-MS) performed on the patient’s serum at presentation, assaying a broad range of illicit, prescribed, or over the counter supplemental xenobiotics, including his reported medication list. Analysis showed a peak consistent with nicotinamide, quantified against a known standard at 38.9 ng/ ml. Following improvement in his liver function and with resolution of coagulopathy, the patient was discharged. At a two-week post-discharge follow up visit, the patient was found to be clinically stable, although he continued to display persistent thrombocytopenia.
Discussion
Niacin is a water-soluble vitamin commonly used to treat hyperlipidemia, chronically dosed at relatively high levels. The association of niacin with hepatotoxicity is well documented, especially at higher dosing and, in particular, when sustained-release preparations are prescribed. For example, a trial comparing sustained release (SR) to standard formulations found that 52% receiving SR niacin had to discontinue the medication due aminotransferase elevations; none treated with standard formulation discontinued for this reason [1]. Acute liver injury due to niacin, however, is uncommon. This is supported by a review by the Drug Induced Liver Injury Network of 300 cases of severe liver injury that found only one case in that series associated with niacin use [2]. Nonetheless, multiple case reports have implicated niacin in acute liver injury. In one such report, a 35-year-old man who had increased his own dosing of niacin from 3 grams to 9 grams daily manifested hepatic injury that resolved then recrudesced upon re-challenge [3]. With SR niacin, injury has been reported even at 3 grams per day, the therapeutic dosing level in our case, which was confirmed by LC-MS (and which excluded a surreptitious overdose) [4].
In humans, niacin is biotransformed to nicotinamide, and then undergoes further metabolism. [5] The mechanism of toxicity for niacin-induced liver injury is unclear, as is the differential toxicity of SR preparations compared to standard (crystalline) preparations at similar therapeutic dosing. One proposed mechanism is that nicotinamide is produced via a low capacity pathway, compared to other oxidative pathways for niacin, and that the accumulation of hepatotoxic metabolites via the nicotinamide-pathway may become greater with SR preparations [5]. Patients with acute liver injury from SR preparations of niacin dosed between 2 to 4g per day, later have tolerated standard niacin preparations [6]. In our case, as in others, it may be the formulation rather than the absolute dosing of niacin that led to hepatotoxicity.
We excluded other common causes of hepatic injury. Because of the history of cinnamon dietary supplement use, we did consider this as a potential exotic hepatotoxin. The coumarin component of cinnamon bark is a recognized hepatotoxin [7]. Notably, the coumarin content of Cinnamomum verum (true, or Ceylon cinnamon) is far less than that of cheaper Cassia cinnamon [7]. The trace/ negligible LC-MS value in this patient and low coumarin content of U.S. marketed cinnamon make this highly unlikely to have been a contributor to the liver injury in this case. This present case also highlights the recognized phenomenon of lactic acidosis associated with niacin toxicity. Lactic acidosis was also reported in a 16-yearold presenting with a lactate of 4.7 mmoL/L after intentionally ingesting 13 grams of niacin in an attempt to obscure the anticipated results of urine drug screening [8]. In another case of lactic acidosis, a 44-year-old man taking three grams per day of “generic niacin,” in the setting of chronic ethanol abuse, presented with paranoia, hypoglycemia to 30 mg/dl, and an anion gap metabolic lactic acidosis with a lactate level of 9.5 mmol/L, a serum bicarbonate of 11 mmol/L, and an AST and ALT 528 U/L and 230 U/L, respectively9. After resuming niacin at half the previous dose while abstaining from ethanol, symptoms did not recur [9].
Lactic acidosis also has been documented after short-term use of SR niacin supplementation. A 41-year-old man taking three grams daily of SR niacin for one month presented with nausea, vomiting, and elevated transaminase values [10]. After restarting SR niacin treatment post-hospital discharge, he represented one week later with a nausea, weakness, and dehydration, associated with a serum lactate of 13 mmol/L and a serum bicarbonate of 15 mEq/L, although the AST at that time was only 46 U/L [10]. Use of metformin was considered as a possible confounding factor in our patient’s lactic acidosis but was excluded by the toxicology testing results.
Conclusion
Niacin administration, in the SR formulation at therapeutic dosing, may lead to liver injury. Concomitant metabolic lactic acidosis also may occur. Practitioners should recognize early signs of hepatic injury and metabolic derangement in the setting of SR niacin even at therapeutic dosing and consider its potential causal association with potentially life-threatening illness.
References
- McKenney JM, Proctor JD, Harris S, Chinchili VM (1994) A Comparison of the Efficacy and Toxic Effects of Sustained- vs Immediate-Release Niacin in Hypercholesterolemic Patients. JAMA 271(9): 672-677.
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, et al. (2008) Causes, Clinical Features, and Outcomes from a Prospective Study of Drug-Induced Liver Injury in the United States. Gastroenterology 135(6): 1924-1934.
- Winter SL, Boyer JL (1973) Hepatic toxicity from large doses of vitamin B3 (nicotinamide). N Engl J Med. 289(22):1180-1182.
- Bassan M (2012) A case for immediate-release niacin. Hear Lung 41(1): 95-98.
- McKenney J (2004) New Perspectives on the Use of Niacin in the Treatment of Lipid Disorders. Arch Intern Med 164(7): 697-705.
- Henkin Y, Johnson KC, Segrest JP (1990) Rechallenge With Crystalline Niacin After Drug-Induced Hepatitis From Sustained-Release Niacin. JAMA 264(2): 241-243.
- Lungarini S, Aureli F, Coni E (2008) Coumarin and cinnamaldehyde in cinnamon marketed in Italy: A natural chemical hazard? Food Addit Contam Part A Chem Anal Control Expo Risk Assess 25(11): 1297-1305.
- Arcinegas Rodriguez S, Gaspers MG, Lowe MC (2011) Metabolic Acidosis, Hypoglycemia, and Severe Myalgias: an attempt to mask urine drug screen results. Pediatr Emerg Care 27(4): 315-317.
- Schwab RA, Bachhuber BH (1991) Delirium and lactic acidosis caused by ethanol and niacin coingestion. Am J Emerg Med 9(4): 363-365.
- Earthman TP, Odom L, Mullins CA (1991) Lactic acidosis associated with high-dose niacin therapy. South Med J 84(4): 496-497.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.