Opinion 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Plasma Growth Factors in Pain A New Dimension of Treatment
*Corresponding author: J Alcaraz Rubio, Henatologist and Medical Adviosry of Bridging Biosciences, Carretera De Aguilas, Buzon, 252-B, Cp: 30800, Lorca, Murcia, Spain
Received: September 22, 2019;Published: September 27, 2019
DOI: 10.34297/AJBSR.2019.05.000926
Opinion
Pain control has become a medical discipline that costs million dollars to administration in medication, health care and work disability every year. Analgesic units often have difficulty controlling the related pain symptoms in patients, that translates into repeated consultations and hospital admissions with disappointing results for the professionals involved and desperate for the patients who suffer. Without a doubt, the most frequent cause of pain related consultation and functional disability units is related to mechanical low back pain of both traumatic and degenerative origin. Only in the year 2018 it costed to coffers of the US administration about 500 million dollars. In this sense, although rehabilitation is one of the fundamental pillars for the therapeutic approach of these patients, residual pain is a symptom that is often difficult to treat.
Advance of science has placed in our hands a therapeutic tool with an extraordinary potential for application in this profile of patients as an adjunctive treatment to active rehabilitation. It is an autologous biotechnology through the management of the so-called Plasma Growth Factors”, (PRP). Although this therapy currently does not go through its best credibility moments due to the abusive lucrative desire that moves it; clinical studies suggest that it could be a safe, economical and very effective therapeutic option both in pain control and in the functional improvement of the patient. At this point it must be said that properly structured clinical trials are still necessary to determine and delimit the field of action of this type of medicine for this specific clinical application, since most scientific studies that we have in the literature are based on isolated clinical cases or series of clinical cases at most with obvious lack of a control group.
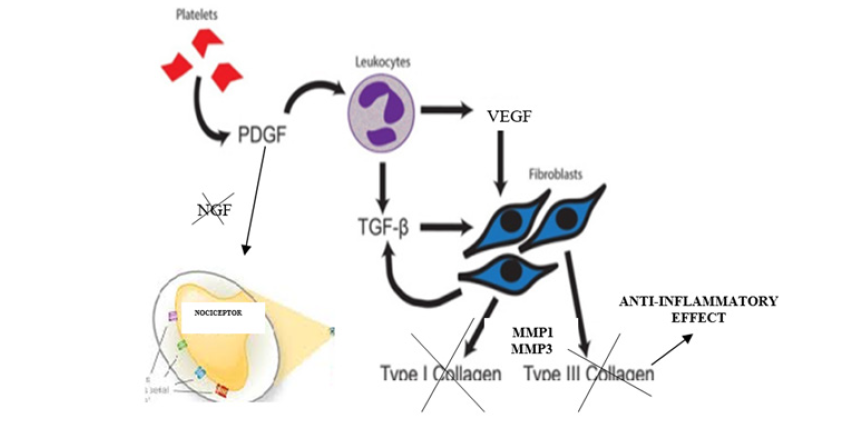
PRP are proteins from different cellular elements of peripheral blood, especially platelets, neutrophils and mononuclear with ample power of paracrine action on the cell cycle producing immunomodulation phenomena on cell apoptosis, which translates into a protection about the programmed death of the same, in addition intervening in processes of maturation and cell division, which opens up a range of extraordinary study possibilities, especially in the field of oncology and tissue repair. Similarly it has been observed that certain growth factors can also act on the inflammation pathway by inhibiting it, producing an indirect analgesic effect, in addition to acting directly by interrupting the release of the neurogenic growth factor (NGF) that would act on the nocicective nerve endings, involved in the pain path, producing a direct analgesic effect even 10 times more potent than opiates without their side effects. This type of therapy, given its immunomodulatory power over the cell cycle, would be contraindicated in patients with an active infectious or oncological process due to the in vitro possibility of exacerbating the base pathology. Similarly, it would be contraindicated in pregnant women and patients with some type of hemopathy.
The hypothetical route of action of this biological drug to produce an analgesic effect would come from the fact that it has been objectified in numerous preclinical and clinical studies that the platelet-derived growth factor (PDGF), which is found in the alpha granules of the platelets, induces the release of other types of cell growth factors such as: vascular-endothelial growth factor (VEGF) and transforming growth factor (TGF-A/B) which predominate in the lysosomes of mononclear cells from blood. These last 2 growth factors have an extraordinary anti-inflammatory function by inhibiting the release both 6 and 8 interleukines (IL-6 and 8) and MMP1 and MMP3 which are the collagenases that activate the fibroblasts that are activated during the process of tissue inflammation. On the other hand, both VEGF and A/B-TGF directly inhibit the release of neurogenic growth factor (NGF), thereby blocking nociceptive nerve endings that cause pain (Graphic 1).
We also know that the half-life of these proteins (growth factors) in serum is approximately during 4 months united to with their great capacity for tissue diffusion, makes them interesting from the therapeutic comfort point of view to dose spacing as well as being able to use a convenient route of administration. (infiltration, intravenous infusion or other routes of parenteral use). We are therefore facing a new dimension of using this biotechnology for a specific patient model, with an enormous potential for acting as an analgesic with an optimal tolerability profile because is an autologous product



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.