Case report 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Duodenal Schwannoma: Case Report and Literature Review
*Corresponding author: K Messoudi, Department of Medical Oncology, Hassan II University Hospital, Fez, Morocco.
Received: December 07, 2019; Published: December 16, 2019
DOI: 10.34297/AJBSR.2019.06.001068
Abstract
Schwannomas are rare benign tumors that arise from schwann cells of the nerve sheath. These tumors represent 0.4 to 1% tract of all mesenchymal tumors of gastrointestinal tract. The most common gastrointestinal site is the stomach. Duodenal schwannomas are extremely uncommon and poorly documented. In this case, we report the fatal outcome of a 52-year-old woman with unresectable duodenal schwannoma treated with adriamycin based chemotherapy.
Keywords: Malignant; Duodenal schwannoma; Metastases; Chemotherapy
Introduction
Schwannomas are benign tumors, that develop from Schwann cells of the nerve sheath. They account for approximately 5% of all mesenchymal tumors. Gastrointestinal schwannomas are rare and distinctive tumors from other schwannomas. The most frequent site of gastrointestinal (GI) schwannomas is the stomach followed by the colon and rectum [1] . There is no difference in the incidence between men and women. Individuals aged between are the most affected. Duodenal schwannomas are often asymptomatic and discovered incidentally. Usually, massive DS may cause nonspecific symptoms like abdominal pain and intestinal hemorrhage [2] . Endoscopic Ultrasound (EUS) and Immunohistochemistry (IHC) are essential tools for differential diagnosis with more common mesenchymal tumors of gastrointestinal tract. A marked PS100 expression on pathology examination is very suggestive of schwannomas [3] . Surgical resection remains the sole curative treatment [4] . Schwannomas are not sensitive to chemotherapy and radiotherapy. The following case highlights the diagnostic and therapeutic challenges posed by an unresectable duodenal schwannoma in a 52-year-old female.
Case Report
A 52-year-old female with no relevant prior medical history presented with a 4-month history of atypical epigastralgia with early satiety and recurrent vomiting. At the admission, the patient performance status was 2 on the ECOG scale. Physical examination did not reveal any specific signs. Performance status of the patient was 1 on the ECOG scale. Laboratory investigations were within normal limits. Duodenoscopy revealed a non-stenosing ulceroproliferative bulging mass extending over 20 cm of the second part of the duodenum. An abdominopelvic computed tomography scan showed a tissue lesion located in the lumen of the duodenum (2nd and 3rd portions) measuring 90mm X 75mm with a necrotic center, heterogeneously enhanced after contrasting medium injection (Figure 1). The tumor presented close contact with hepatic artery and portal vein. CT scan of the chest did not reveal any secondary lesions (Figure 2). Pathology examination of biopsy specimen revealed a fusiform tumor proliferation suggestive of a stromal tumor. Immunohistochemistry showed marked expression of S100 protein.
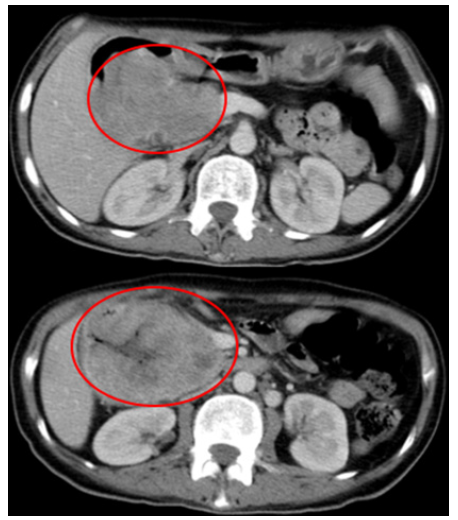
Figure 1: An abdominopelvic computed tomography scan showed a lesion located in the lumen of the duodenum with with double tissue and necrotic components enhanced after contrast.
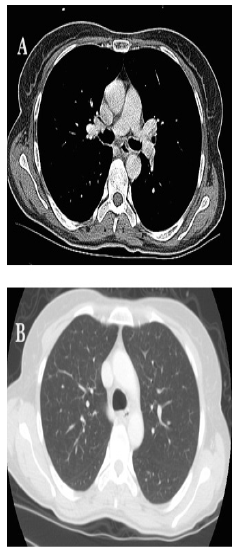
Figure 2: Thoracic CT scan: mediastinal window(A) and parenchymal window (B) showing no secondary thoracic localizations.
Tumor cells were negative for c-KIT, DOG1 and CD34; Molecular biology ruled out cKit-negative GI stromal tumor as c-KIT and PDGFR mutations were absent. Negative cytokeratin stains ruled out sarcomatoid carcinoma. Tumor was also negative for Melan A and smooth muscle markers thus eliminating a melanoma. (Figure 3a). Based on histology and immunochemistry patters, the diagnosis of duodenal schwannoma was considered. The case was discussed at a multidisciplinary meeting. Initial chemotherapy was indicated as complete surgical removing of the tumor would be difficult due to liver vessels close contact with the tumor.
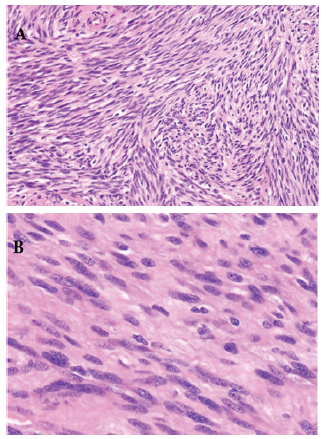
Figure 3a: H&E x 200: Schwannoma as a proliferation of spindle cells with wavy or oval nuclei. B): H&E x400: the Schwann cells with oval nuclei, eosinophilic cytoplasm, and indistinct cytoplasmic borders with nuclear palisading separated by fibrillary processes.
Adriamycin monotherapy at a dose of 60mg per m2 every 3 weeks was started. The patient succumbed to her disease after three cycles before we could assess the tumor response radiologically
Discussion
Schwannomas are rare tumors composed of Schwann cells that form the myelin sheath of peripheral nerves. They are often difficult to differentiate from other mesenchymal tumors. GI schwannomas represent 0.4 to 1% of submucosal tumors of the digestive tract [5, 6]. They are biologically benign tumors with good prognosis and to date, no evidence of malignant potential has been proved. Risk factors for malignant transformation and metastatic disease on pathology remain unclear although sporadic cases of malignant transformation of peripheral schwannomas have been reported previously in literature. This imposes close monitoring even after surgical excision of resectable tumors [7] . Gastric localization is the most common [8] . Duodenal schwannomas remain extremely rare [9] and frequently located in the 2nd and 3rd part of the duodenum [2, 10]. There is no difference in the incidence among the two sexes, and most occurred in the fifth to sixth decade (1). Our patient was 52 years old at the time of diagnosis, and the tumor was located in the second duodenal portion, which is consistent with literature data.
Duodenal schwannoma is often asymptomatic and incidentally discovered. Clinically, it can present as GI bleeding (hematemesis, melena) or even generalized abdominal pain [2]. Preoperative diagnosis of GI schwannoma is difficult due to the rarity of the tumor. More so this is compounded by the absence of pathognomonic features on oeso-gastro-duodenoscopy (OGD) [11, 15]. Duodenoscopy is essential in the workup of schwannoma. Endoscopic ultrasound (EUS), abdominal CT and magnetic resonance imaging (MRI) are used to locate the lesion, determine its links with surrounding vital organs and regional tumor extension. Extension is generally intramucosal [12, thus making endoscopy insufficient to differentiate it from other mesenchymal tumors like GIST, leiomyomas and leiomyosarcomas [13]. Nonetheless computed tomography remains the best imaging tool for disease staging. On Imaging, schwannoma presents as an oval or round, homogeneous mass, infiltrating intestinal mucosa [14].
MRI depicts gastrointestinal schwannomas as well defined, hypo intense on T1 and hyper intense lesions on T2 weighted sequences [15]. Pathology allows definitive diagnosis in most cases. Histologically, schwannoma consists of fusiform cells with poorly defined cytoplasm. Two histological subtypes have been previously described: Antoni type A presenting as congested fusiform cells whereas Antoni type B is defined as a group of fusiform cells well organized in a myxoid stroma[1, 16]. Expression of desmin and actin indicate leiomyoma or liposarcoma, while CD34 and CD117 suggest GISTs. S100 protein expression is in favor of schwannoma. In our case, the diagnosis of schwannomas was based on the PS100 expression and negative immunostaining for other mesenchymal tumor markers. Complete surgical resection with negative margins remains the only curative treatment for GI schwannoma, which depends largely on tumor location and size at the time of diagnosis. The role of chemotherapy and radiotherapy remains uncertain [17, 18].
Given the rarity of malignant schwannomas, there is no phase II or III trials in metastatic or locally advanced disease. In retrospective series, a combination of Doxorubicin with Ifosfamide was associated with satisfactory outcomes and a median PFS of 26.9 months (range 22.4-35.1) [18]. Our patient was placed solely on adriamycin monotherapy due to her poor general condition. Prognosis of advanced duodenal schwannomas is unknown. One study investigated prognostic factors and schwannoma survival in all locations [19]. After a median follow-up of 91 months, the 10- year disease-specific survival rate was 31.6% in localized disease, 25.9% for recurrent disease and 7.5% in metastatic disease [20]. The patients´ fatal outcome in this case suggest a potential aggressive behavior even in the absence of distant secondary lesions, thing which deserve to be studied in clinical trials.
Conclusion
The above case report is one of the fewest cases of malignant schwannomas reported in the literature that highlights the diagnostic and therapeutic challenges associated with unresectable malignant disease. Their management imply a multidisciplinary approach in a reference center to confirm the diagnosis and to better define therapeutic approach as no specific guidelines are available regarding medical treatment.
Consent
Written informe consent was obtained from the patient’s mother for publication of this case report and accompanying images.
Competing Interest
The authors declare that they have no competing interests.
References
- Miettinen M, Shekitka KM, Sobin LH (2001) Schwannomas in the colon and rectum: a clinicopathologic and immunohistochemical study of 20 cases. Am J Surg Pathol 25(7): 846-855.
- Meynen P, Van Holsbeeck B, Seynaeve P, Mortelmans L (1989) Duodenal schwannoma. Rofo 151(5): 621-622.
- Mysorekar VV, Rao SG, Jalihal U, Sridhar M (2010) Schwannoma of the ascending colon. Indian J Pathol Microbiol 53(1): 198-200.
- lamo JM, Lo ́ pez F, Galindo A, Guerra JA, Sousa JM, Cruz C (2003) Small duodenal GIST as a cause of massive digestive bleeding in a young patient. Cir Esp 73: 262.
- Fujii Y, Taniguchi N, Hosoya Y, Yoshizawa K, Yasuda Y, et al. (2004) Gastric schwannoma : sonographic findings. J Ultrasound Med. 23(11): 1527-1530.
- Inagawa S, Hori M, Shimazaki J, Matsumoto S, Ishii H, et al. (2001) Solitary schwannoma of the colon: report of two cases. Surg Today 31(9): 833-838.
- Woodruff JM, Selig AM, Crowley K, Allen PW (1994) Schwannoma (neurilemoma) with malignant transformation. A rare, distinctive peripheral nerve tumor. Am J Surg Pathol 18(9): 882-895.
- Whitehead R (1989) Gastrointestinal and Oesophageal Pathology. 2nd edition, Churchill Livingston,New York.
- Seno K, Itoh M, Endoh K, Joh T, Yokoyama Y, et al. (1994) Schwannoma of the duodenum causing melena. Intern Med 33(10): 621-623.
- Nilsson B, Jonsson I (1957) Malignant neurinoma of the duodenum; report of one case and review of the literature. Acta Chir Scand 113(5): 357-363.
- Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M (2003) Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics23(2): 283-304.
- Kazerooni EA, Quint LE, Francis IR (1992) Duodenal neoplasms : predictive value of CT for determining malignancy and tumor resectability. AJR Am J Roentgenol 159(2): 303-309.
- Levy AD, Quiles AM, Miettinen M, Sobin LH (2005) Gastrointestinal schwannomas: CT features with clinicopathologic correlation. AJR Am J Roentgenol 184(3): 797-802.
- Takeda M, Amano Y, Machida T, Kato S, Naito Z, et al. (2012) CT, MRI, and PET findings of gastric schwannoma. Jpn J Radiol 30(7): 602-605.
- Miettinen M, Shekitka KM, Sobin LH (2001) Schwannomas in the colon and rectum: a clinicopathologic and immunohistochemical study of 20 cases. Am J Surg Pathol 25(7): 846-855.
- Pollock J, Morgan D, Denobile J, Williams J (2001) Adjuvant radiotherapy for gastrointestinal stromal tumor of the rectum. Dig Dis Sci 46(2): 268-272.
- Trojanowski JQ, Kleinman GM, Proppe KH (1980) Malignant tumors of nerve sheath origin. Cancer 46(5): 1202-1212.
- Kroep JR, Ouali M, H Gelderblom, A Le Cesne, TJA Dekker, et al. (2011) First-line chemotherapy for malignant peripheral nerve sheath tumor (MPNST) versus other histological soft tissue sarcoma subtypes and as a prognostic factor for MPNST: an EORTC Soft Tissue and Bone Sarcoma Group study. Annals of Oncology 22(1): 207-214.
- C Zou, KD Smith, J Liu, Lahat G, Myers S, et al. (2009) Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Annals of Surgery 249(6): 1014-1022.
- M Anghileri, R Miceli, M Fiore, Mariani L, Ferrari A, et al. (2006) Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer 107 (5): 1065-1074.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.