Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Interrelationship Between Wnt/Beta-Catenin Pathway and Osteopontin in Liver Regeneration after Partial Hepatectomy in Mice
*Corresponding author: Alexander C Ufelle, Department of Public Health and Social Work, Slippery Rock University of Pennsylvania, Morrow Way Slippery Rock, USA.
Received: November 18, 2019; Published: December 02, 2019
DOI: 10.34297/AJBSR.2019.06.001049
Abstract
The burden of liver diseases is high and approximately 30 million people in the United States have liver disorders. The liver has a unique and intrinsic regenerative capacity that could be triggered by, but not limited to, hepatotoxins and surgical removal of liver tissue. Several animal studies have demonstrated spontaneous liver regeneration after partial hepatectomy. As of 2016, there are about 723 new active candidates added to the pediatric and 11,340 to adult waiting lists respectively, for liver transplantation. This numbers underscore the dire need for further research in the field of regenerative medicine. Several molecules and pathways, such as the Wnt/ β-catenin and osteopontin which are highly expressed in the liver, independently play important roles in liver regeneration after partial hepatectomy in mice. Furthermore, osteopontin is a Wnt target gene and mediates most of its biological activities through binding to integrins and CD44. Interestingly, AP-1 a transcription factor is activated within hours of partial hepatectomy in mice and it is independently regulated by osteopontin and β-catenin. Osteopontin and β-catenin also independently regulate apoptosis during cellular proliferation. This review discusses the potential interrelationship between β-catenin and osteopontin and subsequent integrin activation of AP-1 in regulating liver regeneration as one of the molecular mechanisms behind liver regeneration associated with partial hepatectomy. Finally, regeneration remains a major issue in liver transplantation for both recipients and donors, and this review will contribute to the advancement of the knowledge of the field of regenerative medicine.
Keywords: Regeneration; β–catenin; C-Jun; Integrins; Osteopontin; Partial hepatectomy
Introduction
According to the CDC, “From 2000 to 2015, death rates for chronic liver disease and cirrhosis in the United States increased 31% (from 20.1 per 100,000 to 26.4) among persons aged 45–64 years” [1]. Furthermore, the burden of liver diseases remains high despite advances in treatment options [2, 3, 4] and approximately 30 million people in the United States have liver disorders [5] .A 2016 study indicated that there are about 723 new active candidates added in pediatrics and 11,340 adults on the waiting list for liver transplantation and shortage of donors is the main reason for this development. Furthermore, about 80,000 persons are living with functioning liver grafts showing the importance of liver transplantation in saving peoples’ lives. The improvement in antiviral therapy for Hepatitis C virus infection decreased the number of people on the liver transplant waiting list associated with hepatocellular carcinoma (HCC) while cases of non-HCC liver diseases like non-alcoholic liver disease is on the rise [6] . Despiteyears of research much remains to be explored in the molecular mechanisms driving liver regeneration [7, 8]. These mechanisms are particularly important to understand because the number of patients with end stage liver condition is still on the rise [9].
It is estimated that only one-third of those on transplant waiting list will be transplanted highlighting the need for further research and approaches into liver regeneration. Liver transplantation pioneered by Starzl in 1963 remains the gold standard for the treatment of acute and chronic end stage liver failure. The long waiting list of liver transplant recipients partially drove the novel approach of providing living donor transplantation tissue which is increasingly used to address the lack of donor organs [10]. High mortality exists among patients with end stage liver disease unless they receive a transplant.
Partial Hepatectomy and Liver Regeneration
The liver has a unique and intrinsic regenerative capacity [7, 11, 12] that can be triggered by exposure to hepatotoxins [13][13], and or surgical removal of liver tissue [14]. A loss of regenerative function and capacity, especially in acute liver insult by chemicals or hepatotoxins, ultimately leads to liver failure, which leaves transplantation as the only viable therapeutic option[15]. Furthermore, liver regeneration is considered as a compensatory response of the organ to injury and represents an attempt of the organism to regain lost or compromised liver mass [16]. Several animal studies have demonstrated spontaneous liver regeneration after partial hepatectomy (PH) [17]. PH in animal models is comparable to that of a donor liver in living donor transplantation and this model has been successfully used to study liver regeneration. PH, a procedure first described more than 80 years ago by Higgins and Anderson is the surgical removal of three of the five lobes of mouse liver or three of four lobes of rat liver [18]. The liver is restored to former size approximately one week after PH. It is important to note that proliferation following PH must at some point cease, and cessation is promoted by transforming growth factor-β (TGF-β) and integrin signaling [19]. Donor safety is essential for a successful liver transplant, therefore continued research in liver regeneration is of the utmost importance to establish a balance between donor and recipient safety [20].
Wnt/β-catenin Pathway and Liver Regeneration
There are two Wnt pathways, the β-catenin-dependent (canonical) and β-catenin-independent(non-canonical) pathways [21]. For the canonical pathway, it is known that β-catenin is located in both the cytoplasm and on the hepatocyte membrane in adult liver [12, 22]. In the absence of Wnt signaling, β-catenin is phosphorylated to mark it for ubiquitination and degradation. During the activation of this pathway, soluble Wnts binds to Frizzled receptors to suppress β-catenin phosphorylation thus allowing nuclear translocation and binding to transcriptional partners for induction of genes related to cell proliferation and liver size determination [8, 23, 24]. Furthermore, T-cell factor (TCF) transcriptional regulation via lymphoid enhancer factor) (LEF) and TCF binding sites are regulated by Wnt/β-catenin pathway and have also been implicated in cancer development [25]. The Wnt/β- catenin pathway also plays an important role in liver regeneration. This pathway is activated within hours of PH. Additionally, overexpression of β-catenin protein promotes liver regeneration after PH in mice [17].
This is due to upregulation of cyclin D1, a β-catenin, target gene, which causes a drastic increase in the number of hepatocytes in S-phase in transgenic mice overexpressing β-catenin at 40 hours and 72 hours compared to wild-type. In addition, β-catenin conditional knockout mice showed delayed liver regeneration highlighting its role in liver proliferation [26]. Therefore, a controlled expression of β-catenin may be a useful tool to promote liver regeneration after PH and by extension in organ donation in liver transplantation in humans. β- catenin is under the regulation of numerous molecules such as Akt [27], Apc in mice liver, [28] and Axis inhibitory protein 2 (Axin2) [29]. Finally, β-catenin is important in biliary differentiation during development and is essential in the resolution of intrahepatic biliary cholestasis.
Osteopontin and Liver Regeneration
Osteopontin or Secreted phosphoprotein-1 (OPN or Spp1) is an extracellular matrix protein with important functional cleavage sites and post translational modifications[30]. OPN is an acidic arginine-glycine-aspartate containing adhesive glycoprotein with a molecular mass of approximately 44 kDa. It has a central section of the molecule that consists of sequences (RGD binding domain) that interact with seven integrins, such as αvβ3 and β5, a cryptic α9β1 and α4β1 region (SVVYGLR in humans and SLAYGLR in mice) that is only functional after protease cleavage and CD44 binding domain (v6 and v7 splice variants) [31, 32, 33]. αxβ2 integrin does not bind to either RGD or SVVYGLR sequences [34]. In addition, vimentin and MyD88 bind to OPN in coimmunoprecipitation studies. OPN is regulated by a number of pathways including G-protein coupled pathways, as well as Wnt/β-catenin, Hedgehog, NFkB and estrogen signaling pathways. OPN exhibits prosurvival and antiapoptotic signals to cells and its deficiency counters this effect [35]. OPN is chiefly expressed in bile duct epithelia in normal human liver [36, 37] and expression increases in macrophages during acute inflammation induced experimentally by carbon tetrachloride.
OPN is also highly expressed in many liver diseases and plays a critical role in liver regeneration following partial hepatectomy in mice. The roles of osteopontin in regeneration is controversial, however, as it may oppose or suppress liver repair under different pathological conditions. Hepatic and serum levels of OPN are markedly elevated in patients with alcoholic hepatitis, alcoholic cirrhosis, end stage alcoholic liver disease, hepatitis B and C and hepatocellular carcinoma. Higher levels of osteopontin indicates poorer prognosis compared with patients with lower values. This latter observation underscores its importance as an emerging biomarker [34]. On the other hand, OPN is thought to play a protective role in patients with acute liver failure, as patients who survived without transplantation had elevated serum osteopontin [38]. During partial hepatectomy in mice, osteopontin deficiency suppresses liver regeneration. OPN deficiency also inhibits diethylnitrosamine (DEN)-induced hepatocellular carcinoma demonstrating its role in liver cancer and liver proliferation[39].
Molecular mechanism of OPN-induced Liver Regeneration
OPN mediate most of its biological activities through binding to integrins and the subsequent signal transduction pathway. β1 integrin is essential for hepatocyte proliferation and deleting β1 reduces hepatocyte proliferation and increases liver necrosis after PH [40]. Liver-specific integrin-linked kinase knockout (ILK KO) mice, shortly after birth showed increased hepatocyte and biliary cells proliferation and deposition of extracellular matrix. In addition, the same ILK-KO mice showed decreased expression of β1 and increased expression of β5, αv and α7 further supporting the role of integrins in liver regeneration [41]. Furthermore, integrins play contradicting roles in liver regeneration. This is supported by a study where deletion of αv β8 cause increased hepatocyte proliferation and increased liver regeneration after PH [42]. Several genes involved in integrin signaling pathway participate in the regulation of proliferation during rat liver regeneration [43].OPN binds to αv β3, in tumor cells and activates AP1 through nuclear factor-inducing kinase (NIK)– ERK (extracellular signal-related kinase) and MEKK1 (mitogen-activated protein kinase kinase 1)– JNK1 (c-Jun N-terminal kinase 1) signaling pathways [33].
Depressed expression of AP1, Myc and Cyclin D1 is associated with impaired liver regeneration associated with interleukin-6 deficiency in mice [44]. AP-1 transcription factor c-Jun is a key regulator of hepatocyte proliferation and lack of c-Jun impairs liver regeneration after PH in mice [45, 46]. JNK isoform (1) and AP-1 are activated within one hour of PH, JNK activates AP-1 which in-turn promotes the expression of cyclin D1 and initiation of G0 - G1 transition. c-Jun has been showed to be essential in liver regeneration as mice lacking this protein demonstrated impaired liver regeneration after PH. Finally, a study suggested that the CD44 isoform V6, may play a role in the proliferation of hepatocytes in rats after PH[47].
Apoptosis and Liver Regeneration
The ubiquitous expression of death receptor in the liver makes liver cells such as hepatocytes and cholangiocytes susceptible to apoptosis in response to injury [48]. A study using the Rat Genome 230 2.0 Array, showed that several genes in the apoptotic pathway regulate liver regeneration [49]. Inhibition of apoptosis plays a role in liver regeneration and the deficiency of apoptosis stimulating protein p53 promotes liver regeneration through the activation of mammalian target of rapamycin after PH in mice [50]. Decreased β-catenin appears to promote apoptosis, as Wnt/ β-catenin is inactive in apoptotic squamous epithelial cells and their non-apoptotic counterparts show intense membrane, cytoplasmic and nuclear β-catenin expression [51]. The expression of the Wnt antagonist Dkk-1 or a dominant negative LEF1 inhibits β- catenin activity, producing undersized livers with decreased cell proliferation and increased apoptosis [12, 52]. Furthermore, the direction of the association between OPN and apoptosis in the liver depends on the disease setting [53], suggesting that OPN may regulate apoptosis in the liver regeneration.
Wnt/ β-catenin pathway and OPN interaction
OPN has been shown to be a Wnt target gene and Wnt induces the OPN gene in rat kidney epithelial[54] and human breast cancer cells [55]. In addition, OPN expression is increased in colon cancer with deregulated β-catenin expression[56], suggesting Wnt/β- catenin pathway regulates OPN expression. OPN and Wnt/β-catenin pathway may have a synergistic effect in liver regeneration following PH in mice, but as with excess β-catenin expression, dysregulation of OPN contributes to the pathogenesis of liver disease. Since OPN and β-catenin independently support liver regeneration following partial hepatectomy, there is the possibility of a synergistic effect of both molecules in promoting liver regeneration after PH.
Future Direction
Demonstrate that Wnt/ pathway is essential for the induced OPN in liver regeneration after PH in mice. Research work to determine the inter-relationship between Wnt/ β-catenin pathway and OPN and the molecular pathways behind this interaction in liver regeneration after PH can be pursued with the assumption that “PH stimulates the Wnt/ β-catenin pathway to potentiate liver regeneration by inducing OPN”. This can be tested by determining the gene and protein expression of OPN in mouse model with the overexpression and deficiency of β-catenin. Apoptosis can also be assessed be assessed by TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) to determine its role in this web of interaction between OPN and β-catenin. Furthermore, it will also be beneficial to look at other markers of proliferation such as Ki67 as previous used in a study [57] and markers of cell survival such as NF-KB as alternative mechanism. Determine the effect of β-catenin induction of OPN on integrin and c-Jun expression and activation. This can be done by assessing the gene and protein expression of specific integrins and AP-1.
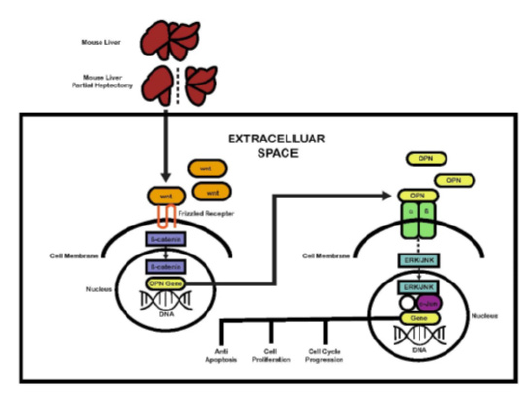
Figure 1: The schematic diagram outlining the interaction between Wnt/ β- catenin pathway and OPN, and the associated molecular pathways in the context of liver regeneration after PH, modified from Nejak-Bowen and Monga [12][12], Michalopoulos [8][8] & Zhao et al. [33][33].
It is also essential to determine nuclear expression of c-Jun and phospho-c-Jun to determine the activation state of the molecule in response to β-catenin induction of OPN. Furthermore, ILK or focal adhesion kinase (FAK) activation could also be assessed for their phosphorylated states respectively. In addition, the v6 and v7 splice variants of CD44 are alternative mechanism through which OPN mediate its biological function. c-Fos could be an alternative to c-Jun and phospho-c-Fos in the nucleus may be assessed. In addition to CD44 expression, nuclear expression of NFkB (p65 or p50) is an alternative molecular mechanism of behind β-catenin induction of OPN in liver regeneration [33]. (Figure 1)summaries the possible interaction between Wnt/ β-catenin and OPN in liver regeneration after partial hepatectomy in mice.
Conclusion
Despite advancement and success in liver transplantation carried out so far in the United States and around the world, more remain to be known about the molecular mechanism behind liver regeneration. Both Wnt/ β-catenin pathway and OPN promote liver regeneration after PH in mice. Therefore, further research to determine the interrelationship between Wnt/ β-catenin pathway and OPN in liver regeneration address a major knowledge gap in the understanding of liver regeneration and will promote both donor and recipient safety after liver transplant. In addition, it will open up other therapeutic options for patients with end stage liver disease. Finally, much needed research in regenerative medicine is necessary to determine the molecular mechanisms supporting liver regeneration through multiple pathways including OPN mediated liver regeneration through its interaction with Wnt/ β-catenin pathway.
Conflict of Interest
I do not have any conflict of interest
References
- CDC (2017) Quickstats: Death rates for chronic liver disease and cirrhosis, by sex and age group National Vital Statistics System, United States, 2000 and 2015.
- Byass P (2014) The global burden of liver disease: A challenge for methods and for public health. BMC Med 12:159.
- Neff GW, Duncan CW, Schiff ER (2011) The current economic burden of cirrhosis. Gastroenterol Hepatol 7: 661-671.
- Wang FS, Fan JG, Zhang Z, Gao B, Wang HY (2014) The global burden of liver disease: The major impact of china. Hepatology 60: 2099-2108.
- Collin de l'Hortet A, Takeishi K, Guzman-Lepe J, Handa K, Matsubara K, et al. (2016) Liver-regenerative transplantation: Regrow and reset. Am J Transplant 16: 1688-1696.
- Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, et al. (2018) Optn/srtr 2016 annual data report: Liver. Am J Transplant 18 Suppl 1: 172-253.
- Apte U, Gkretsi V, Bowen WC, Mars WM, Luo JH, et al. (2009) Enhanced liver regeneration following changes induced by hepatocyte-specific genetic ablation of integrin-linked kinase. Hepatology 50: 844-851.
- Michalopoulos GK (2017) Hepatostat: Liver regeneration and normal liver tissue maintenance. Hepatology 65: 1384-1392.
- Trapero-Marugan M, Little EC, Berenguer M (2018) Stretching the boundaries for liver transplant in the 21st century. Lancet Gastroenterol Hepatol 3: 803-811.
- Bozkurt B, Dayangac M, Tokat Y (2017) Living donor liver transplantation. Chirurgia 112: 217-228.
- Loforese G, Malinka T, Keogh A, Baier F, Simillion C, et al. (2017) Impaired liver regeneration in aged mice can be rescued by silencing hippo core kinases mst1 and mst2. EMBO Mol Med 9: 46-60.
- Monga SP (2014) Hepatic regenerative medicine: Exploiting the liver's will to live. Am J Pathol 184: 306-308.
- Mehendale HM UU (2010) Liver regeneration and tissue repair In: Comprehensive toxicology 9: 2nd Part (CA M, edn): Elsevier, pp: 339-367.
- Tao Y, Wang M, Chen E, Tang H (2017) Liver regeneration: Analysis of the main relevant signaling molecules. Mediators Inflamm 2017: 4256352.
- Ghouri YA, Mian I, Rowe JH (2017) Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J Carcinog 16: 1.
- Fausto N, Campbell JS, Riehle KJ (2006) Liver regeneration. Hepatology 43: S45-S53.
- Nejak-Bowen KN, Thompson MD, Singh S, Bowen WC, Jr., Dar MJ, et al. (2010) Accelerated liver regeneration and hepatocarcinogenesis in mice overexpressing serine-45 mutant beta-catenin. Hepatology 51: 1603-1613.
- Nevzorova YA, Tolba R, Trautwein C, Liedtke C (2015) Partial hepatectomy in mice. Lab Anim 49: 81-88.
- Gilgenkrantz H, Collin de l'Hortet A (2018) Understanding liver regeneration: From mechanisms to regenerative medicine. Am J Pathol 188(6):1316-1327.
- Kwon YJ, Lee KG, Choi D (2015) Clinical implications of advances in liver regeneration. Clin Mol Hepatol 21(1): 7-13.
- Shang S, Hua F, Hu Z (2017) The regulation of beta-catenin activity and function in cancer: Therapeutic opportunities. Oncotarget 8(20): 33972-33989.
- Nejak-Bowen KN, Monga SP (2011) Beta-catenin signaling, liver regeneration and hepatocellular cancer: Sorting the good from the bad. Semin Cancer Biol 21(1): 44-58.
- MacDonald BT, Tamai K, He X (2009) Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell 17(1): 9-26.
- Zeng G, Awan F, Otruba W, Muller P, Apte U, et al. (2007) Wnt'er in liver: Expression of wnt and frizzled genes in mouse. Hepatology 45(1): 195-204.
- Cadigan KM, Waterman ML (2012) Tcf/lefs and wnt signaling in the nucleus. Cold Spring Harb Perspect Biol 4(11).
- Tan X, Behari J, Cieply B, Michalopoulos GK, Monga SP (2006) Conditional deletion of beta-catenin reveals its role in liver growth and regeneration. Gastroenterology 131: 1561-1572.
- Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, et al. (2007) Phosphorylation of beta-catenin by akt promotes beta-catenin transcriptional activity. J Biol Chem 282: 11221-11229.
- Colnot S, Decaens T, Niwa-Kawakita M, Godard C, Hamard G, et al. (2004). Liver-targeted disruption of apc in mice activates beta-catenin signaling and leads to hepatocellular carcinomas. Proc Natl Acad Sci U S A 101: 17216-17221.
- Alison MR, Lin WR (2016) Regenerating the liver: Not so simple after all? F1000Res 5: 1818.
- Wang KX, Denhardt DT (2008) Osteopontin: Role in immune regulation and stress responses. Cytokine Growth Factor Rev 19: 333-345.
- Lund SA, Giachelli CM, Scatena M (2009) The role of osteopontin in inflammatory processes. J Cell Commun Signal 3: 311- 322.
- Shevde LA, Samant RS (2014) Role of osteopontin in the pathophysiology of cancer. Matrix Biol 37: 131-141.
- Zhao H, Chen Q, Alam A, Cui J, Suen KC, et al. (2018) The role of osteopontin in the progression of solid organ tumour. Cell Death Dis 9: 356.
- Wen Y, Jeong S, Xia Q, Kong X (2016) Role of osteopontin in liver diseases. Int J Biol Sci 12(9): 1121-1128.
- Denhardt DT, Noda M, O'Regan AW, Pavlin D, Berman JS (2001) Osteopontin as a means to cope with environmental insults: Regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest 107(9): 1055-1061.
- Lorena D, Darby IA, Gadeau AP, Leen LL, Rittling S, et al. (2006) Osteopontin expression in normal and fibrotic liver. Altered liver healing in osteopontin-deficient mice. J Hepatol 44(2): 383-390.
- Wen Y, Feng D, Wu H, Liu W, Li H, et al. (2015) Defective initiation of liver regeneration in osteopontin-deficient mice after partial hepatectomy due to insufficient activation of il-6/stat3 pathway. Int J Biol Sci 11: 1236-1247.
- Srungaram P, Rule JA, Yuan HJ, Reimold A, Dahl B, et al. (2015) Plasma osteopontin in acute liver failure. Cytokine 73(2): 270-276.
- Lee SH, Park JW, Woo SH, Go DM, Kwon HJ, et al. (2016) Suppression of osteopontin inhibits chemically induced hepatic carcinogenesis by induction of apoptosis in mice. Oncotarget 7(52): 87219-87231.
- Speicher T, Siegenthaler B, Bogorad RL, Ruppert R, Petzold T, et al. (2014) Knockdown and knockout of beta1-integrin in hepatocytes impairs liver regeneration through inhibition of growth factor signalling. Nat Commun 5: 3862.
- Gkretsi V, Apte U, Mars WM, Bowen WC, Luo JH, et al. (2008) Liver-specific ablation of integrin-linked kinase in mice results in abnormal histology, enhanced cell proliferation, and hepatomegaly. Hepatology 48: 1932-1941.
- Greenhalgh SN, Matchett KP, Taylor RS, Huang K, Li JT, et al. (2019) Loss of integrin alphavbeta8 in murine hepatocytes accelerates liver regeneration. Am J Pathol 189(2): 258-271.
- Xu C, Yang Y, Yang J, Chen X, Wang G. (2012) Analysis of the role of the integrin signaling pathway in hepatocytes during rat liver regeneration. Cell Mol Biol Lett 17(2): 274-288.
- Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, et al. (1996) Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 274(5291): 1379-1383.
- Seki E, Brenner DA, Karin M. (2012) A liver full of jnk: Signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology 143(2): 307-320.
- Stepniak E, Ricci R, Eferl R, Sumara G, Sumara I, et al. (2006) C-jun/ap-1 controls liver regeneration by repressing p53/p21 and p38 mapk activity. Genes Dev 20(16): 2306-2314.
- Della Fazia MA, Pettirossi V, Ayroldi E, Riccardi C, Magni MV, et al. (2001) Differential expression of cd44 isoforms during liver regeneration in rats. J Hepatol 34(4):555-561.
- Guicciardi ME, Malhi H, Mott JL, Gores GJ (2013) Apoptosis and necrosis in the liver. Compr Physiol 3(2): 977-1010.
- Xing XK, Li MH, Guo ZW, Xu CS (2016) Expression profiles of genes associated with mitochondria-mediated apoptosis and their roles in liver regeneration. Genet Mol Res 15.
- Shi H, Zhang Y, Ji J, Xu P, Shi H, et al. (2018) Deficiency of apoptosis-stimulating protein two of p53 promotes liver regeneration in mice by activating mammalian target of rapamycin. Sci Rep 8: 17927.
- Donmez HG, Demirezen S, Beksac MS. (2016) The relationship between beta-catenin and apoptosis: A cytological and immunocytochemical examination. Tissue Cell 48(3):160-167.
- Lade AG, Monga SP (2011) Beta-catenin signaling in hepatic development and progenitors: Which way does the wnt blow? Dev Dyn 240(3): 486-500.
- Iida T, Wagatsuma K, Hirayama D, Nakase H (2017) Is osteopontin a friend or foe of cell apoptosis in inflammatory gastrointestinal and liver diseases? Int J Mol Sci 19(1).
- Vinas JL, Sola A, Jung M, Mastora C, Vinuesa E, et al. (2010) Inhibitory action of wnt target gene osteopontin on mitochondrial cytochrome c release determines renal ischemic resistance. Am J Physiol Renal Physiol 299(1): 234-242.
- Ravindranath A, Yuen HF, Chan KK, Grills C, Fennell DA, et al. (2011) Wnt-beta-catenin-tcf-4 signalling-modulated invasiveness is dependent on osteopontin expression in breast cancer. Br J Cancer 105(4): 542-551.
- Rohde F, Rimkus C, Friederichs J, Rosenberg R, Marthen C, et al. (2007) Expression of osteopontin, a target gene of de- regulated wnt signaling, predicts survival in colon cancer. Int J Cancer 121(8): 1717-1723.
- Minocha S, Villeneuve D, Rib L, Moret C, Guex N, et al. (2017) Segregated hepatocyte proliferation and metabolic states within the regenerating mouse liver. Hepatol Commun 1(9): 871-885.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.