Opinion 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Preliminary ab initio Calculations on Pt (3D,1S) – O2 Interaction
*Corresponding author: Juan H Pacheco-Sánchez, Postgrade and Research Studies Division, Instituto Tecnológico de Toluca, México
Received: December 20, 2019; Published: January 09, 2020
DOI: 10.34297/AJBSR.2020.06.001091
Opinion
Potential energy surfaces (PES) of the 3D(5d96s1) ground state and 1S(3d10) lowest excited state of the Pt-O2 interaction were achieved by using variational and perturbative MRCI and pseudopotential relativistic methodologies in order to obtain distances and energies of adsorption. Platinum atom RECP xenon type was calculated through Gaussian basis set contracted to triple-zeta scheme (3s1p4d)/ (111/1/211), while oxygen atom RECP helium type was calculated through Gaussian basis set contracted to double- zeta scheme (4s4p1d)/ (111/1/211). These calculations have been carried out based on good results obtained for Pt-O and O-O interactions in agreement with experimental data, then we used the same basis sets to calculate Pt+O2 interactions.
In here, the reaction mechanisms between Pt and O2 are established by using symmetry C2v, by fixing platinum atom and bringing in O2 molecule perpendicularly to the axis of symmetry with distance as reaction coordinate. Single point step by step calculations have been accomplished, consequently each energy eigenvalue ob tained by means of the Shrödinger equation solution corresponds to the potential energy calculations, because kinetic energy is cero in each step. The equilibrium points obtained corresponds to chemisorption, due to a very high energy as shown in (Figure 1), where the solid curve minimum is located at (3.0Å,325 Kcal mol-1 (14.09 eV)) and the dashed line curve is at (3.1Å,840 Kcal mol -1 (36.43 eV)). According to literature [Atkins], these values correspond to chemical adsorption (chemisorption). Then, the generated bond is very strong. These attractive potential energy curves exhibit two avoided crossings, and their dissociation energies are hardly reached at room temperature, according to the conformations used to generate them. Oxygen reduction reaction (ORR) is still one of the main problems in PEM fuel cell designs [1, 2, 3]. The sluggish reaction kinetics of ORR requires an effective electrocatalyst to make fuel cell reach a practical level [4]. On the other hand, the most stable PtO2 intermediate molecule generated is located at the global minimum of these two potential energy curves, each one with different state.
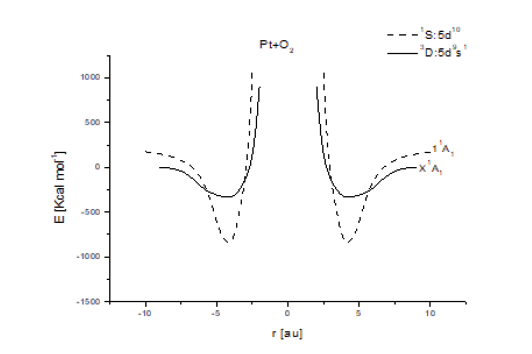
Figure 1: Potential energy curves of the Pt + O2 interaction at the 3D and 1S lowest states. The continuous line corresponds to 3D ground state, and dash line to 1S lowest exited state. The minimum correspond to PtO2 complex generated, here in the ground state, and the first excited state.
Actually, there is an intense research using platinum at least in four different complexes against hypoxia, which is low oxygenation in blood [5, 6, 7] . Then, ab initio calculations might help in designing intermediate molecule complexes for treatment of this kind of cancer. Our results do not match with ab initio DFT calculations supported on experimental data [8], because these are ten times below of our results that correspond to preliminary calculations. Then, there is more research to carry out when right basis sets will be got for new ab initio calculations.
References
- href="https://www.ncbi.nlm.nih.gov/pubmed/17218522">Zhang, K Sasaki, E Sutter, RR Adzic (2007) Stabilization of platinum oxygen-reduction electrocatalysts using gold clusters. Science 315(5809): 220-222.
- href="https://pubs.acs.org/doi/10.1021/ar300359w">J Wu, H Yang (2013) Platinum-Based Oxygen Reduction Electrocatalysts Acc Chem Res 46(8): 1848-1857.
- href="https://onlinelibrary.wiley.com/doi/abs/10.1002/fuce.200800089">TS Olson, B Blizanac, B Piela, JR Davey, P Zelenay, et al. (2009) Electrochemical Evaluation of Porous Non‐Platinum Oxygen Reduction Catalysts for Polymer Electrolyte Fuel Cells. Fuel Cells 9(5): 547-553.
- href="https://www.sciencedirect.com/science/article/abs/pii/S0927025618301678">X Wang, X Li, S Liao, B li (2018) DFT study of high performance Pt3Sn alloy catalyst in oxygen reduction reaction. Computational Materials Science 149: 107-114.
- href="https://pubs.rsc.org/en/content/articlelanding/2016/MT/C5MT00312A#!divAbstract">Ekaterina Schreiber Brynzak, Verena Pichler, Petra Heffeter, Buck Hanson, Sarah Theiner et al. (2016) Behavior of platinum(iv) complexes in models of tumor hypoxia: cytotoxicity, compound distribution and accumulation. Metallomics 8(4): 422-433.
- href="https://www.ncbi.nlm.nih.gov/pubmed/9861196">E Reed (1998) Platinum-DNA adduct, nucleotide excision repair and platinum based anti-cancer chemotherapy. Cancer Treat Rev 24(5): 331-344.
- href="https://www.ncbi.nlm.nih.gov/pubmed/24148385">A Martincic, M Cemazar, G Sersa, V Kovac, R Milacic, et al. (2013) A novel method for speciation of Pt in human serum incubated with cisplatin, oxaliplatin and carboplatin by conjoint liquid chromatography on monolithic disks with UV and ICP-MS detection. Talanta 116: 141-148.
- href="https://journals.aps.org/prl/abstract/10.1103/PhysRevLett.79.4481">A Eichler, J Hafner (1997) Molecular Precursors in the Dissociative Adsorption of O2 on Pt(111). Phys Rev Lett 79(22): 4481.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.