Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Synthesis and Characterization of Hybrid Fiber Scaffold for Medical Implants
*Corresponding author: Abere DV, National Metallurgical Development Centre, Jos, Nigeria
Received: November 04, 2019; Published: January 16, 2020
DOI: 10.34297/AJBSR.2020.06.001099
Abstract
This work synthesized and characterized chitosan-silica based hybrid fiber from cowry shell and rice husk respectively with the aim of studying the behavior of a hybrid polymer-silicate composite via the electrospinning technique to produce nanofibers from natural polymer and agro waste, from which scaffolds can be produced which can be used for culturing and wound healing application in the biomedical field. The samples obtained were subjected to the following analyses: Energy Dispersive X-ray Florescent (ED-XRF), Fourier Transform Infrared Spectroscopy (FTIR), X-Ray Diffraction (XRD), Scanning Electron Microscopy (SEM) and Thermogravimetic Analysis (TA).
It was observed from the ED-XRF analysis that the major constituent of rice husk ash is silica (SiO2) with some associate metallic oxides. The peaks obtained from the FTIR analysis of the polymer confirm the characteristics features for the polysaccharide structure of chitosan while the characteristic and positions of the peaks observed in the extracted silica spectra showed similar position with that of the commercially available silica obtained from BDH chemicals UK. The Chitosan-silica hybrid has Si AOH and Si AO ASi with the respective bands within the regions of 3270– 3400cm-1 and 989–1300cm-1.The X-ray diffraction analysis indicates the presence of crystallite in polymers which usually results to improve mechanical properties, unique thermal behavior, and increased fatigue strength. These attributes make crystalline polymers desirable materials for biomedical applications. Also, amorphous components of the hybrid composite would present an improved biodegradation behavior of a biomedical material. It was observed from the morphological analysis that there was good interfacial interaction between the chitosan and the dispersion of the silica reinforcement material. The TA of the sample was carried out to investigate the effect of silica reinforcement on the thermal stability of the hybrid composite. It was discovered that the reinforcement of the silica matrix and its reaction with the polymer makes the thermal resistance of the hybrids to increase and hence, increase in thermal decomposition temperature.
Keywords: Polymer; Chitosan; Silica; Hybrid; Reinforcement; Amorphous; Polysaccharide
Introduction
The utilization of biomaterials in humans has experienced a great success in surgery, dentistry, orthopedics and the likes. For instance, heart valves prostheses are fabricated from carbons, metals, elastomers, fabrics and natural valves and other tissues chemically pretreated to reduce their immunologic response and to enhance durability [1] . Also, the human hip joints are fabricated from titanium, specific high strength alloys, ceramics, composites and ultrahigh molecular weight polyethylene. Dental implants fabricated from titanium materials to form an artificial tooth on which a crown is affixed. One of the advantages of the titanium implants is the bonding with the bone of the jaw. However, this attachment has been more accurately described as a tight apposition of mechanical fit and not true bonding [2] . Wear, corrosion and mechanical properties of titanium have also been of concern [3] . Several other materials have been used to fabricate these implants. Some of these include hydrogel, bioglass, hydroxylapatite, chitosan, and amorphous silica and others (Abere et al, 2017; Abere et al., 2018, Abere et al., 2019). This work attempts to produce a composite of bio glass and chitosan for biomedical application.
Chitosan is a unique polysaccharide produced from chitin (Nam et al, 2010). It has been globally used due to its excellent biocompatibility, low toxicity and biodegradability for diverse biomedical applications such as orthopedics, dental implants, drug delivery and others [4,5]. The application of chitin in the biomedical field started from the investigation of the reaction and chemical attributes of lysozyme; an enzyme in the human body fluids. It dissolves certain bacteria by cleaving to the chitinous material of the cell walls. It has been suggested that chitosan may be used to inhibit fibroplasias in wound healing and to stimulate tissue growth and differentiation in tissue culture [6] .
Silica based biomaterials, such as melt-derived bioactive glasses and sol-gel glasses, have been used for a long time in bone healing applications because of their ability to form hydroxyl apatite and to stimulate stem cell proliferation and differentiation [7] . They have also been reported to support the differentiation of osteoclasts. For these reasons silica based biomaterials have been suggested to be used as bone filling materials or as part of tissue engineering scaffolds in bone repair. Chemically, the most simple silicate material is silicon dioxide, or silica (SiO2). Structurally, it is a three-dimensional network that is generated when every corner oxygen atom in each tetrahedron is shared by adjacent tetrahedral. Thus, the material is electrically neutral and all atoms have stable electronic structures [3] . Silica fiber like every other fiber possesses a great strength, hence it is envisaged that if blended with chitosan and a hybrid fiber is made of the composite, it will enhance the strength of the material [8] .
It has been discovered that most medical problems cannot be corrected by either the natural healing or traditional surgical process [9] . In such cases, implants made from biomaterials can be successfully used to interface with living host tissue to give remedies [10]. Hence, this work attempt to synthesize, characterize and fabricate a hybrid, nanofiber composed of chitosan and bio-silicate glass for biomedical application by preparing an organic and inorganic hybrid cross linking network and electro spinning process to produce the nanofiber.
Materials and Method
Silica separation from rice husk ash
Rice husks were obtained from Owode, Ogun state, Nigeria and incinerated at 650 ˚C for a period of 4 hours to form rice husk ash. The ash was mixed with the solution of 1 molar concentration of sodium hydroxide (NaOH) solution. The result was sintered at 850˚C for 3 hours to form sodium silicate (NaSiO3)
Synthesis of chitosan
The cowry shell was washed thoroughly to get rid of impurities such as sand, dust, dirt and insect larva before sun drying. The subsequent product was pulverized to produce a powder of particle size of 250 microns which was subjected to the following chemical hydrolysis processes to form chitosan [11, 12].
Deproteinization: 80g of the powder was weighed into a conical flask and 100ml of 4% NaOH was added. NaOH was prepared by weighing 4g of NaOH pellet and dissolved and made up in 100ml to obtain 4% NaOH. The mixture was boiled and stirred at 100 ºC for 2hrs in a water bath. It was filtered and washed with deionized water. Red litmus paper was used to check if the base was completely washed away. After the washing, the mixture was filtered and the residue was scraped, gently into the Petri dishes, followed by oven drying at 110 ºC for 3hours. The weight of the sample was 62g after deproteinization.
Demineralization: 1M HCl was prepared by measuring 55ml of Conc. HCl and mixed with 1liter of distilled water in a cylindrical flask of 1000cm 3. 5% of the prepared solution was measured (30ml) and mixed with the deproteinized sample. The mixture was boiled and stirred for 60 minutes at 100 ºC in a water bath. Subsequent washing was done with distilled water followed by filtration. The mixture was examined with blue litmus to check the acidity of the mixture. The residue (chitin) obtained from above was scarped into the Petri dish and dried in the oven at 100 ºC for 2hrs.
Deacetylation: The isolated chitin was soaked in 510ml of 50% NaOH (weighing 50g of NaOH pellets and dissolved in 100ml of distilled water), boiled at 100 ºC for 2hrs in water bath and cooled for 30mins at room temperature. The mixture was placed on a magnetic stirrer at 30 ºC for 4hrs, filtered, washed and examined with red litmus to check if the base was completely washed away. The mixture was filtered to retain the solid matter which is chitosan. The chitosan was oven dried at 90 ºC for 24hrs.
Preparation of Silica-Polymer (chitosan- Polyetheleneoxide) Solutions
Polymer solutions were prepared with distilled water and glacial acetic acid. The concentration of the polymer solutions was prepared in wt/wt%.
i. Chitosan-PEO (Polyetheleneoxide) solution with total polymer concentration of 1.6% were prepared by dissolving chitosan and PEO in 50% glacial acetic acid in the ratio 3:1, 2:1, 1:1.
ii. The polymer solutions were stirred over night to allow homogeneity of the solutions.
iii. Silica solution was prepared by weighing 1.5g of the synthesized silica from RHA and dissolved in 25ml of 1M NaOH with continuous stirring at 60 ºC until a clear solution of Na- SiO3 (bioglass) was obtained.
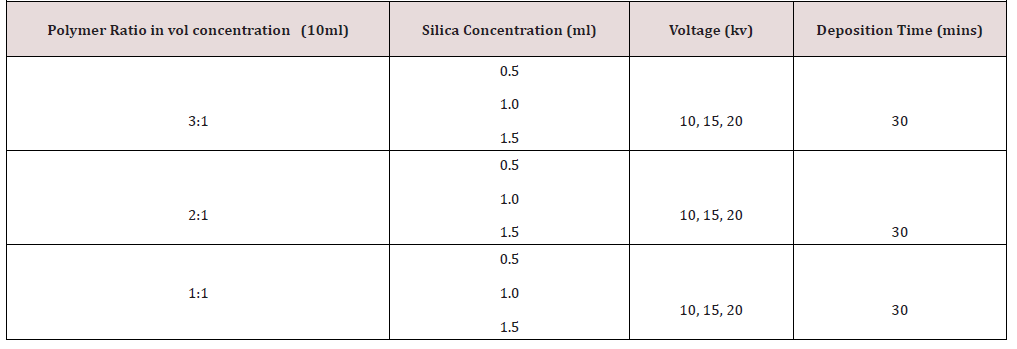
iv. The polymer solutions and silica concentration were prepared in vol/vol mixing. 10ml of each of the polymer solution in 3:1, 2:1, 1:1, with silica concentration of 0.5ml, 1ml and 1.5ml mixed together with each of the polymer solution in the given ratio. (Table 1) & (Table 2)
v. The solutions were used immediately for the electrospinning.
vi. Fiber deposits were collected on 1cm x 1cm glass slides and aluminum foil.
Electrospinning parameters; distance 10cm (between the tip of the spinneret and the aluminum collector) and deposition time is 30 minutes for each of the solution. Electrospinning parameters; distance 10cm (between the tip of the spinneret and the aluminum collector) and deposition time is 30 minutes for each of the solution.
Electrospinning of the solutions
The polymer solutions were poured into the spinneret (tilted at 10º) with the positive terminal of the voltage source dipped into the polymer solution and the negative terminal attached to the aluminum collector for the fibers. The target was placed 10cm from the tip of the spinneret. All operation was carried out at room temperature.
Characterization
The chemical composition of the rice husk ash produced was investigated through Energy Dispersive X-ray Fluorescence (EDXRF), (MiniPAL4 PANalytical B.V. Lelyweg1 7602 EA, Aimelo). Press powder technique was employed to prepare the sample before the analysis. The physical interactions between chitosan and silica were examined with the aid of Fourier Transform Infrared Spectrometer (FTIR). The samples were scanned at a wavelength of 400cm-1 to 4000cm-1. The surface and cross sectional morphology of the sample was investigated with Scanning Electron Microscopy (SEM). The samples were prepared in smaller pieces and sputter with gold before making the observation.
Thermogravimetry Analysis (TG)
12mg of the sample was used to perform the TGA from 50 to 800 ͦC with a heating rate of 10 ͦC/min in a dynamic synthetic air atmosphere with the aid of TGA-50 Shimadzu automatic analyzer.
Results and Discussion
Characterization
Fourier transform infra-red analysis (FT-IR): FT-IR (Model IR Prestige 21, Shimadzu) analysis was carried out on the extracted samples (chitosan and silica), electrospun nanofiber composites. The results obtained was compared to those of commercial silica and chitosan to identify the presence of hydrogen bond silanol group and the siloxane groups in silica and to evaluate the compositional and chemical features of the extracted chitosan and the nanofiber composite. The results are shown in (Figure 1) (Figure 2) (Figure 3) (Figure 4) (Figure 5) .
Discussion
The extracted chitosan (polymer) solution on electropinning did not form fibers; but drops and beads were formed with low degree of fiber deposition even at higher viscosity. This is in agreement with reports from other literatures that showed that electrospinning of chitosan in aqueous solvent was unsuccessful unless another electrospinning-inducing polymer [13] such as PEO is introduced into the solution. On addition of PEO, an increase in polymer viscosity was observed which led to the formation of nanofiber deposited on aluminum foil and glass surface.
ED-XRF analysis
The product of the incinerated process of rice husk was white rice husk ash whose chemical composition was investigated by ED- XRF analysis (Table 3). It can be observed from Table 3 that the major constituent of rice husk ash is silica (SiO2) which occurs with some other metallic oxides like alumina, ferric oxide, titanium oxide, calcium oxide, magnesium oxide, zinc oxide, manganese (II) oxide, phosphorus pent oxide and others [14] .
FTIR results analysis
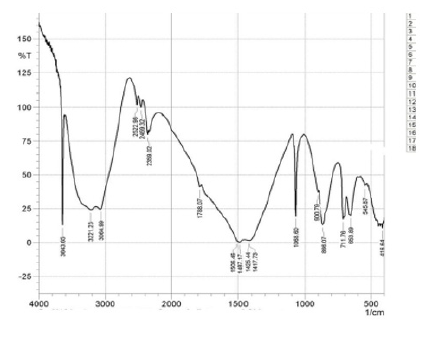
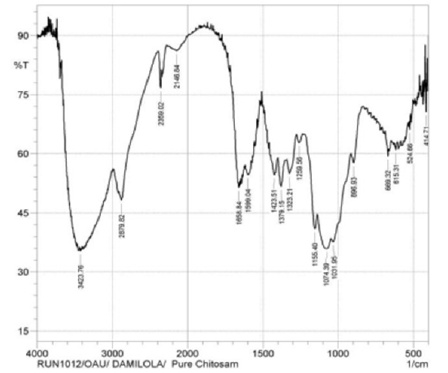
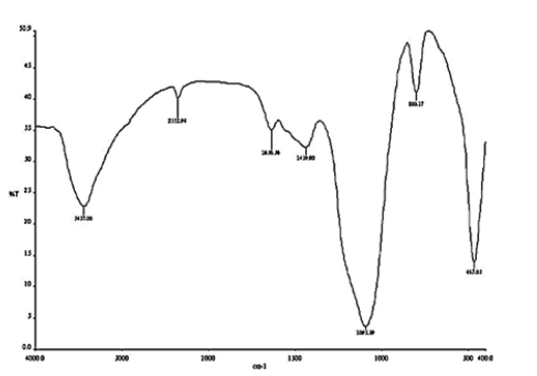
The characteristic absorption bands for extracted chitosan were observed as follows from (Figure 1) & (Figure 2); the peak on the band 3643.65cm-1 is attributed to the OH···O stretching vibration and N-H band which lies between 3221.23cm-1 and 3064.99cm-1 shows the intermolecular hydrogen bonding of the chitosan polymer molecules [9, 15]. The peaks at 2522.98cm-1, 2459.32cm-1, and 2359.02 can be attributed to the primary amine and –NH2 secondary amine absorption band respectively (IR correlation chart). Three peaks at 1487.17cm-1, 1425.44cm-1, 1417.73cm-1 assigned to the deformation of C-CH3 band and the band 1506.46cm-1 may be attributed to the carbonyl C=O-NHR band [9] . At 1068.60cm-1 is attributed to skeletal vibration involving C-O stretching. These peaks confirm the characteristics features for the polysaccharide structure of chitosan [16] extracted from cowry shells.
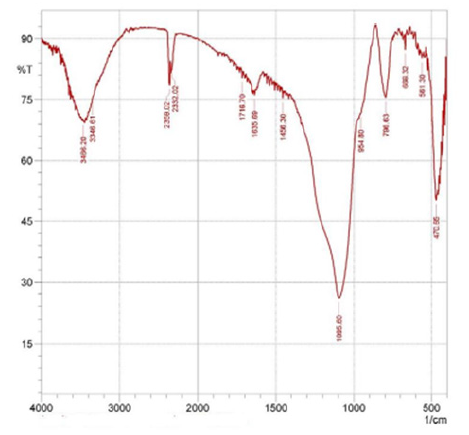
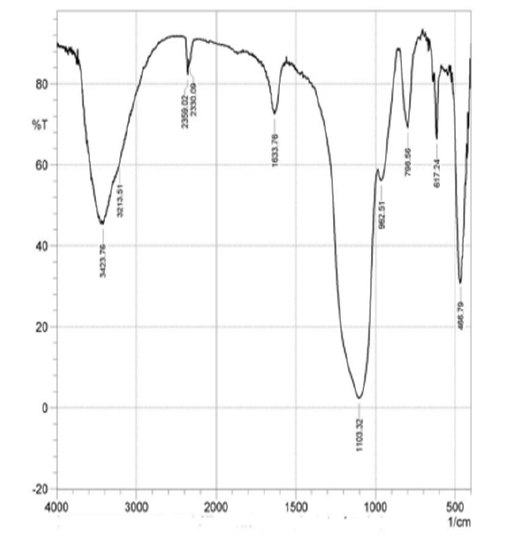
After the extraction of silica from rice husk ash, the major chemical and functional groups were identified from the FTIR spectra as shown in (Figure 3) & (Figure 4). The highest peak observed at wave number 1103.32cm-1 was due to the siloxane bonds (Si-O-Si) and (Si-O-Si) vibration between 1000cm-1 and 500cm-1 band. The peak at wave number 3423.76cm-1 and 3213.51cm-1 indicate the presence of silanol OH functional group. Also, the characteristic and positions of the peaks observed in the extracted silica spectra showed similar position with that of the commercially available silica obtained from BDH chemicals UK as shown by the FTIR spectra results. The silanol group gives the extracted silica a significant potential for application as matrices of many chemical species such as organic molecules, metals and polymeric materials.
The electrospinning of composite solutions may lead to differences in composition of the pre-spun solutions and the deposited fibers [13]. As a result of this the composition of the electrospun fibers was analyzed by FT-IR. From (Figure 5) the characteristic band in 3650–3180cm-1 is attributed to the overlapping of Si-OH stretch and amine band. Also, the spectral bands in 2110–2400cm-1 are as a result of Si-H stretch which overlaps C O vibration. There was an AC at O band at 1636cm-1 [17] . The adsorption peak at 1442cm-1 is similar to in-plane OH bending. The peak observed at 1075cm-1 is related to Si AO ASi stretch [17] whereas the peak at 780cm-1 is associated to the overlapping of Si AC stretch and NH2wag. The peak at 459cm-1 is assigned to O-Si- bending [18] . The large intensity of the peak at 1080cm-1 might be due to the overlapping of the Si AO ASi and the AC AO AC A of glycosidic linkage [17, 19]. Chitosan-silica hybrid has Si AOH and Si AO ASi with the respective bands within the regions of 3270–3400cm-1 and 989–1300cm-1 [16] .
XRD results analysis
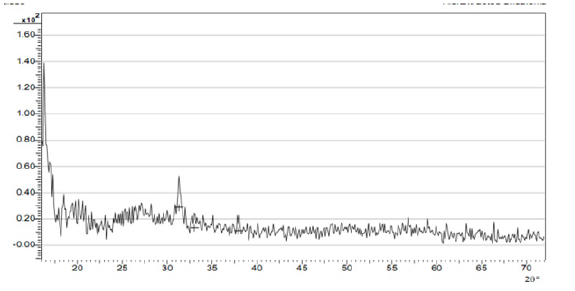
The diffraction pattern from amorphous materials (including many polymers) is devoid of the sharp peaks characteristic of crystals and consists of broad features or halos. The X-ray diffraction pattern of chitosan twelve peaks were identified at 2θ with the peaks showing an hydrated partially crystalline material with amorphous background identified between 55º to 70º at 2θ. This result suggests that the extracted chitosan is a semi crystalline polymeric material.
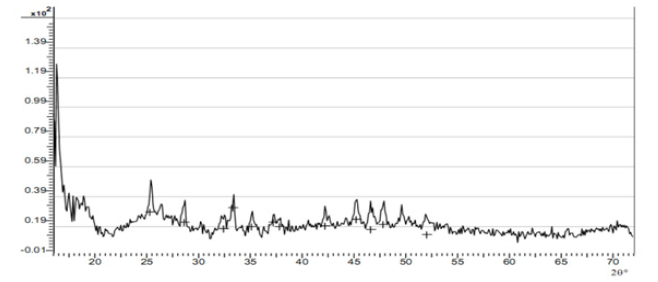
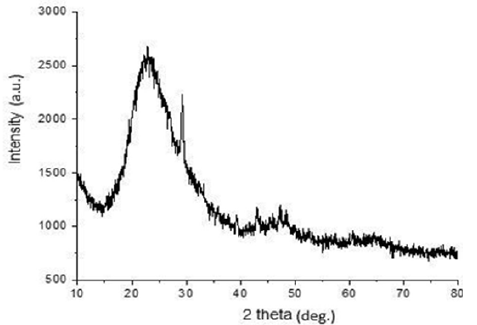
The XRD spectra of 2θº diffraction between 30º and 40º showed three specific peaks at 31.27º, 32.98˚ and 37.89˚. These features of extracted silica spectrum suggested the characteristic of amorphous micro porousilica [18] . The XRD result is presented in Figure 6. From the pattern, on comparison with known standards in the JCPDS Powder Diffraction File for silica (Joint Committee on Powder Diffraction Standards) (Figure 7). Amorphous components would present an improved biodegradation behavior of a material [3] . The presence of crystallite in polymers usually leads to enhanced mechanical properties, unique thermal behavior, and increased fatigue strength. These properties make semi crystalline polymers (often referred to simply as crystalline polymers) desirable materials for biomedical applications [3] .
The XRD analysis of chitosan -silica hybrid was also carried out to examine the crystal phase, orientation and their structural properties. It can be observed from (Figure 8), that the peak of the most crucial amorphous curve is at 23.3 in the 2h angle that reveals the state of the amorphous of the silica network while chitosan occur in the anhydrous crystalline form. It had been stated that the anhydrous crystalline chitosan and amorphous silica is at 2h = 23 while a highly discrete crystalline projection found at 2h angle of 29.3 reveals the impact of the silica form [20, 21].
SEM analysis
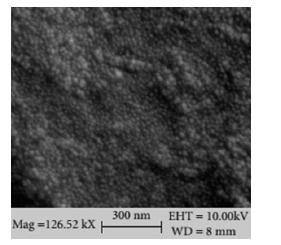
The particles of silica in the composite occur in the form of white round beads, and their dispersion within the matrix is clearly visible as in (Figure 9). The homogeneous and dense structure of silica in the matrix of chitosan can be observed in the hybrid composite as the concentrations of SiO2 increases. This indicates good interfacial interaction within the chitosan and the dispersion of the silica reinforcement material. The bright spots indicate the silicon element clearly reflect a uniform distribution of silica in Chitosan-Silica hybrid domains. The nanometer rate of dispersion of silica in chitosan shows the influence on the thermal properties of the hybrid.
Thermogravimetic Analysis
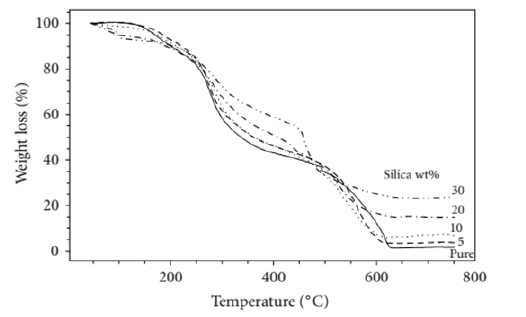
Thermogravimetric analysis of the chitosan silica nano-composites was performed in synthetic air within the range of 50–750 ˚C to investigate the influence of silica reinforcement on the thermal stability of the hybrid composite. It could be observed from (Figure 10) that the weight loss which could be observed within 105 and 170 ˚C might be as a result of the loss of moisture content present in chitosan and subsequent product of condensation of the Si–OH groups [22] . Chitosan has low rate of weight loss in the axis ranging between 175 ˚C and 290 ˚C because of the decomposition of low molecular weight species [22] . The rate of thermal decomposition is much around 180 ˚C and 465 ˚C due to the complex dehydration of the saccharide rings, depolymerization, and decomposition of the acetylated and deacetylated units of the polymer [23] . Therefore, the incorporation of silica and its reaction with the polymer makes the thermal resistance of the hybrids to increase and hence, increase in thermal decomposition temperature [24, 25].
Conclusion
Silica was obtained through the incinerated process of rice husk and subjected to chemical analysis to examine the chemical composition of the rice husk ash while chitosan was synthesized through chemical hydrolysis process from cowry shell. The products obtained from the incinerated and hydrolysis processes were made into fiber through electrospinning technique. The hybrid composite formed was subjected to various characterization techniques. It was observed that the major constituent of rice husk ash is silica (SiO2) with some associate metallic oxides. The crystalline nature of polymers will improve mechanical properties, unique thermal behavior, and increase fatigue strength. These attributes make crystalline polymers desirable materials for biomedical applications. Whereas, the amorphous component of the hybrid composite would present an improved biodegradation behavior of a biomedical material. It was observed from the morphological analysis that there was good interfacial interaction between the chitosan matrix and the dispersion of the silica reinforcement material.
References
- Park SB, You JO, Park HY, Haam SJ, Kim WS (2001) Biomaterials, Park (22nd edn), France.
- Anderson DG, Burdick JA, Langer R (2004) Smart Biomaterials Science. 305: 1923-1924.
- Ratner BD, Hoffman AS, Schoen FJ, Lemons JE (2004) Osteoconduction at Porous Hydroxyapatite with Various Pore Configurations. Biomaterials, Volume 21(12): 1291-1298.
- Abere DV, Oyatogun GM, Akinwole IE, Abioye AA, Rominiyi AL, et al. (2017) Effects of Increasing Chitosan Nanofibre Volume Fraction on the Mechanical Property of Hydroxyapatite. American Journal of Materials Science and Engineering 5(1): 6-16.
- Akinwole IE, Alebiowu G, Oyatogun GM, Abere DV, Adenigba AE, et al. (2018) Synthesis and Characterization of Cowry and Crab Shells Based Chitosan for Drug Delivery. Bioceram Dev Appl 8: 107.
- Betre H, Setton LA, Meyer DE, Chilkoti (2002) A Characterization of a genetically engineered elastin-like polypeptide for cartilaginous tissue repair. Biomacromolecules 3: 910-916.
- Chang B, Lee C, Hong K, Youn H, Ryu H, et al. (2000) Osteoconduction at Porous Hydroxyapatite with Various Pore Configurations. Biomaterials 21(12): 1291-1298.
- Langer R., Tirrell DA (2004) Designing materials for biology and medicine. Nature 428: 487- 492.
- Raif EM, Harmand MF (1993) Molecular interface characterization in human bone matrix: Biochemical and IR Spectroscopic Studies. Biomaterials 14: 978-984.
- Meinel L, Holfman S, Karageorgion V, Zichnew L (2004) Engineering cartilage-like tissue using human mesenchymal stem cells and silk protein scaffolds. Biotechnological engineering 88(3): 379-391.
- Nam YS, Won HO, Park Daewoo I, Samuel M, Hudson (2010) Effect of the degree of deacetylation on the thermal decomposition of chitin and chitosan nanofibres. Carbohydrate Polymers 80: 291-295.
- Luo XL, Xu JJ, Wang JL, Chen HY (2005) Chitosan composite. Chem Commun 21: 69-72.
- Li Z, Yubao L, Aiping Y, Xuelin P, Xuejiang W, et al. (2005) Preparation and in vitro investigation of chitosan/nano-hydroxyapatite composite used as bone substitute materials. J Mater Sci Mater Med 16: 213-219.
- Battegazzore D, Bocchini S, Alongi J, Frache A (2014) Rice Husk as Bio-source of Silica: Preparation and Characterization of PLA-Silica Bio-composites. RSC Adv 4: 54703-54712.
- Li H, C Zhou, M Zhu, J Tian, J Rong (2010) Preparation and Characterization of Homogeneous Hydroxyapatite/Chitos an Composite Scaffolds via In-Situ Hydration. Journal of Biomaterials and Nanobiotechnology 1(1): 42-49.
- Al-Sagheer F, Muslim S (2010) Thermal and mechanical properties of chitosan/SiO2 hybrid composites. J Nanomater 1-7.
- Prerna D, Ramanand J (2012) Comparative study of physical and thermal properties of Chitosan - Silica hybrid coatings prepared by sol-gel method. Pelagia Reseach Library Der Chemica Sinica 3(3): 589-601.
- Azadeh T, Marzieh H, Vahid M, Masoud R (2013) Synthesis and characterization of hydrophobic silica aerogel by two step (acid-base) sol-gel process. JNS 3: 181-189.
- Yeh JT, Chen CL, Huang KS (2007) Synthesis and Properties of Chitosan/ SiO2 Hybrid Materials. Mater Lett 61: 1292-1295.
- Todorova EV, Chernev GE, Djambazov SP (2014) Structure and properties of functionalized porous silica hybrid materials. Open J Inorg Non-Metal Mater 4: 35-43.
- Kow, Kien Who, Yusoff, Rozita, Abdul Aziz, et al. (2014) From bamboo leaf to aerogel: Preparation of water glass as a precursor J Non-Cryst Solids 386: 76-84.
- Budnyak TM, Tertykh VA, Yanovska ES, Kolodyńska D, Bartyzel A (2015) Adsorption of V(V), Mo (VI) and Cr (VI) Oxoanions by Chitosan-silica Composite Synthesized by Mannich Reaction. Adsorption Sci Tech 33: 645-657.
- Mahatmanti FW, Nuryono AO, Narsito BG (2016) Adsorption of Ca (II), Mg (II), Zn (II) and Cd (II) On Chitosan Membrane Blended with Rice Hull Ash Silica and Polyethylene Glycol. Indones J Chem 16: 45-52.
- Abere DV, Oyatogun GM, Oluwasegun KM, Ojo SA, Akinwole IE, et al. (2019) Development and Characterization of Cowry Shell Based Hydroxyapatite for Dental and Orthopaedic Applications. Arch Biomed Eng & Biotechnol 2(4): 2019.
- Abere DV, Oyatogun GM, Oluwasegun KM, Ayodele TJ, Ajayi SV, et al. (2018) Synthesis and Characterization of Alumina-Chitosan-Hydroxyapatite Bio composites for Load Bearing Application. European Scientific Journal (ESJ) 14(30): 145-165.





 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.