Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
A DFT Study for Eliminating a Water Pollutant by Using an Adsorbent
*Corresponding author: Juan Horacio Pacheco Sánchez, Division of Postgraduate Studies and Research, Instituto Tecnológico de Toluc, México.
Received: February 10, 2020; Published: February 24, 2020
DOI: 10.34297/AJBSR.2020.07.001174
Abstract
A pollutant on the water is an azoic dye molecule (adsorbate) within its corresponding ˉO3S active site, and it can be relieved from water using chitosan copolymer molecule (adsorbent) within its active site, which is an ion NH+3 of ammonia from amino group protonated. After DFT geometry optimization, the reactivity between these two active sites in acidic aqueous solutions can become either neutral acid form or two products (sulfur dioxide SO2 and H2NOH). We infer the kind of adsorption established on the neutral acid form molecule.
Introduction
Our primary goal in here is to apply DFT to the interaction between chitosan (adsorbent) and azoic dye (adsorbate), through their reaction sites NH+3 (ion of ammonia from amino group protonated) and ˉO3S (sulfur trioxide ion of sulfonate group) respectively, just to infer possible existing adsorption. This is done first calculating its geometry optimization between NH+3 and ˉO3S . The reaction among these molecular ions produces sulfamic acid which ins zwitterion form +H3NSO3- is more stable than the neutral acid form H2NSO2OH in solid state [1, 2, [3, 4, 5]. Physical properties (structural and spectroscopic) and chemical reactions of sulfamic acid have been extensively reviewed [6, 7, 8]. The structure of both the zwitterion and neutral form has theoretically been studied previously [9, 10, [11, 12, 13]. We study here the neutral acid case as an isomer HSO3NH2.
Azo dyes are a class of synthetic dyes which when degraded in the bodies of water can cause the rupture of azo bond of amines, causing many harmful effects in some human organs such as the brain, liver, kidneys, central nervous system and reproductive system. Synthetic dyes also affect the photosynthetic activity of some aquifer’s plants due to the presence of aromatics, metals, chlorides, etc. [14] . The discovery of synthetic dyes has limited the role of natural dyes due to its characteristics such as low production cost, brighter colors, better resistance to environmental factors and easy application. However synthetic dyes can be often highly toxic and carcinogenic [15] . The dyes have become a major source of severe water pollution as a result of the rapid development of many industries that use them in order to colorize their products [16] . Effects described by the pollution of azoic dyes mean a problem that requires attention and treatment.
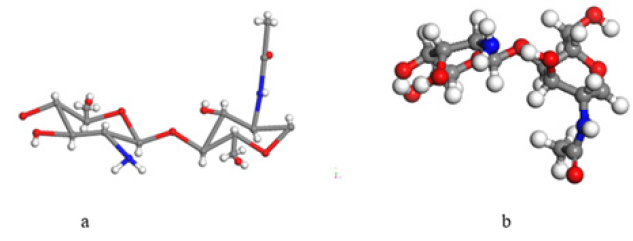
Chitosan is a product of chitin, which is the second most abundant natural polysaccharide in nature. Chitosan can be obtained from partial deacetylation of chitin [17] . Among the many uses of chitosan products are nutraceuticals, food protectors, medical uses, agricultural uses, and many others. Application to water purification is in research. Chitosan is a polymer, its chemical structure as copolymer is drawn in (s 1a), which is the input for applying geometry optimization, and its output can be seen in (Figure 1).
This copolymer chitosan is made up two units:
a. β-(1-4)-2-acetamide-2-deoxy-D-glucopyranose
b. β-(1-4)-2-amino-2-deoxy-D-glucopyranose.
The former has a molecular weight of 203.2, C8H13NO4formula, composition: C 47.3%, H 6.4%, N 6.9% and O 39.4%; and the latter has a molecular weight of 161.1, C6H11NO4formula, composition: C 44.7%, H 6.9 %, N 8.7%, O 39.7%. When chitosan is dissolved in an acidic medium the amino group is protonated, this fact generates a positive charge, while the azo dyes with sulfonate groups dissolved in water have a negative charge. Therefore, there are groups NH+3 and ˉO3S, which have attracted to each other, giving rise to adsorption of azo dyes with sulfonic groups in the chitosan.
It is known the use of ammonia (NH3) to remove sulfur dioxide (SO2) [18, 19]. Some researchers [20, 21] have also worked with interactions and reactions of sulfur trioxide and ammonia not alone.
In our case, we recognize an ion of ammonia NH+3 and ˉO3S in chitosan adsorbing an azoic dye with a sulfonate group ˉO3S We calculated the adsorption energy among NH+3 and ˉO3S molecular ions using molecular simulations.
Methodology
To study some characteristics of this molecular system, density functional theory (DFT) is used. The main advantage of this technique is to predict the geometry, band structure and cohesive energies of extended systems, without the need for external parameters as for ab initio semiempirical techniques. It is based on the ground state, having major problems in the study of excited states. The calculations were accomplished using the DM ol3 computer program proposed by Delley [22]. DM ol3 was used to non-periodic structures with a generalized gradient approximation (GGA) to calculate the exchange-correlation potential and local potential gradient-corrected PW91. We use the DFT method with a set of DND numerical radial basis functions to calculate the interaction between groups NH+3 and ˉO3S [23]. Spin unrestricted orbital is used to solve Kohn-Sham equations when only one double bond is used in the sulfonate group ˉO3S. We accomplished calculations of connectivity which is a tool of DFT-DMol3 in order to get reactivity products.
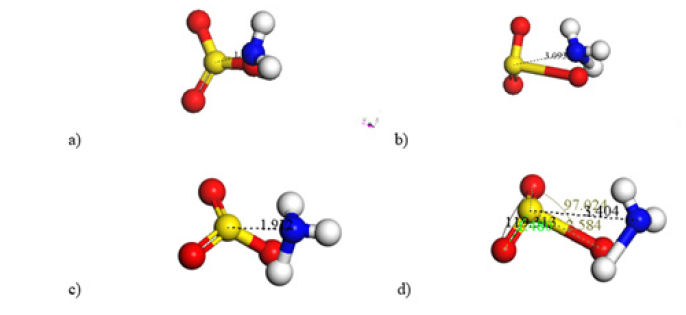
Figure 2: a) Input: Distance 1.912Å among ˉO3S sulfur oxide and ammonia ions in plane and pyramid geometry, respectively. b) Output: After the geometry optimization, growth of one OS bond and one NH+3 bond is observed. c) Input: Two double bonds of ˉO3S sulfur oxide in a plane structure, and 3 NH+ ammonia ions in pyramid geometry. d) Output: growth of one OS bond and one NH bond
Input-output geometry optimization of our molecules is observed in (Figure 2). In the Input of (Figure 2), the 3 NH+ molecule has a pyramidal shape with its three hydrogen atoms in the same plane, while the ˉO3S molecule has a planar shape; and an initial approach of 1.912Å among sulfur and nitrogen. The Output in Figure 2b provides a small repulsion among the ion molecules with a distance of 3.093Å between sulfur and nitrogen, showing an increment of 62%; an 83% increment of the SO bond length to 2.667Å and 94% of the NH bond length to 1.989Å. The other two SO bonds in the ˉO3S molecule, now in a pyramidal shape, have a length of 1.48Å, and the sulfur atom making SOO angles of 26.413°, 28.776° and 30.955° with the plane in which the three oxygen atoms are located, while the OSO angles are 117.923°, 100.302° and 91.536°. The molecule NH+3 has a pyramidal shape with three hydrogens in a plane making NHH angles of 27.836°, 36.047° and 26.863°, while HNH angles are 107.848°, 88.026° and 92.444° respectively. The sizes of the NH bonds are 1.032Å, 1.031Å and 1.989Å. The Output in Figure 2d provides a small repulsion among the ion molecules with the distance of 3.404Å between sulfur and nitrogen, showing an increment of 78%; an 68% increment of the SO bond length from 1.535Å to 2.584Å and 42% of the NH bond length from 1.362Å to 1.927Å.
After geometry optimization of 29 steps when there is only one double bond on ˉ, at a distance of 0.6816Å between H+ and ˉO ions, an equilibrium point at the energy of −654.471kcal/mol, and distance of 3.093Å between N and S was obtained. A natural bond elongation corresponding to the ions of each molecule is observed. The optimization energy of sulfonate group ˉNH+3 is -362.698kcal/ mol and the optimization energy of the amino group protonated
ˉNH+3 is -289.812kcal/mol. Then the adsorption energy is
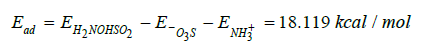
which corresponds to weak chemisorption [14, 24].
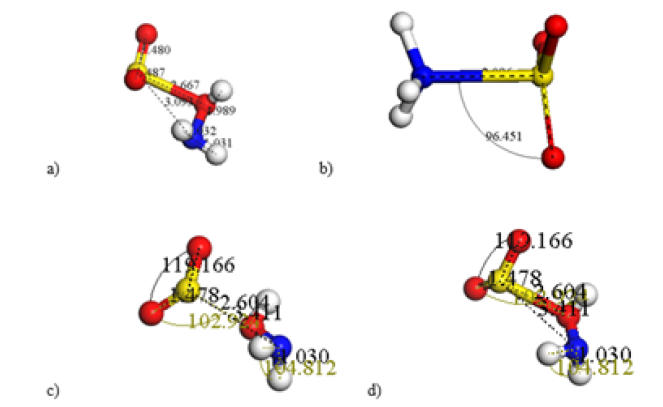
Figure 3: a) Neutral Acid form H2NOHSO2 given a connectivity 0.6 - 1.51Å. b) Zwitterion with connectivity 0.6 - 1.2Å. c) There are two products for connectivity 0.6 - 1.15Å. d) However, the Neutral Acid Form is again recovered for connectivity 0.6 – 1.51.
Considering a nuclear NS equilibrium separation R = 3.093Å for ˉNH+3 molecule, and assuming that ˉNH+3 ions possess spherically symmetric charge distributions, which up to that point no overlap has occurred, even if an elongation of the corresponding bonds to ions of each molecule is significant, where the spacing among such H+ and ˉO ions is about 0.6816Å. This value is obtained by measuring the bond distances: ˉOH+, H+ N, and N ˉO of a triangle of sides 0.987Å, 1.989Å and 1.446Å respectively, with ˉOH+N angle 43.676°.
Since SO bond, before geometry optimization is 1.46Å, when making a connectivity calculation with a tolerance of 0.6 to 1.507Å for the size of the bond, we see an oxygen of ˉO3S molecular ion inserted into an NH bond of the ion of ammonia molecule without breaking molecules, therefore one H2NOHSO2 complex molecule is to be created through the complex SO3NH3 shown in Figure 2b, still needing connectivity. The entire dye molecule will be adsorbed on chitosan molecule without altering the distances at which they grew 3.093Å SO of the dye and 1.989Å NH of chitosan after a geometry optimization; evidently after a connectivity calculation the NH bond becomes two bonds: one 1.446Å NO and another 0.987Å OH with 108.2° NOH angle. Then NH and OH molecules share one electron occupying a stable orbital and making a covalent bond. This is the only case in which we have a neutral acid form H2NOHSO2 through the isomer HSO3NH2 of sulfamic acid with covalent bonds as shown in (Figure 3), therefore there is chemisorption due to the electronegativity of H, N, and O atoms. With the formation of a zwitterion as in (Figure 3) is not possible to clean water-pollution when dealing with chitosan, due to the orientation of the ammonia ion ˉNH+3 as part of it. In (Figure 3) we applied connectivity 0.6 - 1.15Å and two products are obtained losing the double bond previously used in (Figure 2), however when connectivity 0.6 – 1.51Å is applied the neutral acid form is recovered with new characteristics. The most important are a reduction of SO bond to 2.604Å and new adsorption energy, now with optimization energy in which the sulfonate group ˉO3S gives -362.698kcal/ mol, the amino group protonated ˉNH+3gives -289.812kcal/mol, and the neutral acid form gives -658.388kcal/mol. Then:
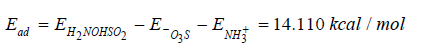
Discussion
We have two structural models of ˉO3S because we initially started working with only one double SO bond by considering satisfied the octet rule in this way. Then, we realized that several articles [25, 26, [27, 28, 29] have been working azo dye red 2 molecule using two SO double bonds.
In these articles have been made investigation about:
a. Degradation of amaranth dye by using both TiO2-Zeolite Y catalyst and ion exchange sonocatalytic [25].
b. Amaranth adsorbed onto papaya seeds [26].
c. Pea and peanuts have been used to adsorb amaranth dye pollutant [27].
d. Embryotoxicity of amaranth red 2 in rats [28].
e. Adsorption removal of amaranth by nanoparticles-composed Cu2O microspheres [29].
The importance of removing this pollutant is evident after these statements, one more reason is that it is a histamine liberator and may intensify symptoms of asthma.
Conclusion
Applying DFT geometry optimization first to ˉNH+3 ion, then to ˉO3S ion and finally to both molecular ions in a particular position take us to the isomer HSO3NH2 complex molecule which is neutral acid form NH2SO2OH of sulfamic acid, and it is chemisorption.
We present two possibilities of ˉO3S ion, one of them is because we found it in this way in literature, and the other because when applying DFT connectivity it is considered with a single double bond, and in this case the adsorption energy is more chemisorbed. However, when this has two double bonds, the adsorption energy is less chemisorbed. These two adsorption energies remain in the threshold among physisorption and chemisorption, and we can say that the lowest adsorption energy is closer to physisorption than to chemisorption. s
These two ions work as adsorbent and adsorbate active sites of chitosan and azoic dye molecules, respectively. Furthermore, chitosan has nutritious properties as food. Consequently, chitosan has the right properties to be used as natural food, favoring cleaning of pollutants in both water and the human body.
References
- MA Wong, KB Wiberg, MJ Frisch (1992) Solvent effects. 2. Medium effect on the structure, energy, charge density, and vibrational frequencies of sulfamic acid. J Am Chem Soc 114(2): 523-529.
- FA Kanda, A King (1951) Masthead. J Am Chem Soc 73: 2315.
- RL Sass (1960) A Neutron Diffraction Study on the Crystal Structure of Sulfamic Acid. Acta Crystallogr 13: 320-324.
- JW Bats, P Coppens, TF Koetzle (1977) Acta CrAystallogr B 37: 1333.
- A Reuven, D Marcellus, RS Parker, AL Kwiram (1981) J Chem Phys 74: 179.
- LF Audrieth, M Sveda, HH Sisler, MJ Butler (1940) Sulfamic Acid, Sulfamide and related aquo-ammonosulfuric acids. Chem Rev 26: 49-94.
- GA Benson, WJ Spillane (1980) Sulfamic acid and its N-substituted derivatives. Chem Rev 80(2): 151-186.
- R Santmeyer, R Aarons (1969) in Kirk-Othmer Encyclopedia of Chemical Technology, A. Standem Interscience, New York, USA. 19: 242.
- SJ Hickling, RG Woolley (1990) An ab initio Hartree-Fock study of the zwitterion of sulphamic acid, +H3NSO3-. Chem Phys Lett 166(1): 43-48.
- JE Douglas, GL Kenyon, PA Kollman (1978) The ammonia-sulfur trioxide interaction. An ab initio study. Chem Phys Lett 57(4): 553-556.
- P Kaliannan, S Vishveswara, VSR Rao (1983) Ab initio SCF—MO study of the molecular structures of aminomethanol, aminesulfonic acid and N-methyl-sulfamate. J Mol Struct 105(3-4): 359-374.
- DWJ Cruickshank (1985) A reassessment of dπ—pπ bonding in the tetrahedral oxyanions of second-row atoms. J Molec Struct Eisentein 130(1-2): 177-191.
- P Kaliannan, S Vishveswara, VSR Rao (1987) Current Sci 54: 111.
- G Crini, PM Badot (2008) Application of Chitosan, a Natural Aminopolysaccharide, for Dye Removal from Aqueous Solutions by Adsorption Processes Using Batch Studies: A Review of Recent Literature. Prog Polym Sci 33: 399.
- Q Liu, B Yang, L Zhang, R Huang (2015) Adsorption of an Anionic Azo Dye by Cross-Linked Chitosan/Bentonite Composite. Int J Biol Macromol 72: 1129-1135.
- Kyzas GZ, Siafaka PI, Pavlidou EG, Chrissafis KJ, Bikiaris DN (2015) Synthesis and adsorption application of succinyl-grafted chitosan for the simultaneous removal of zinc and cationic dye from binary hazardous mixtures. Chem Eng J 259: 438-448.
- NA Travlou, GZ Kyzas, NK Lazaridis, EA Deliyanni (2013) Chem Eng J 217: 256.
- FR Alix, JL Duncan, Ch R McLamon (2003) US Patent No. US6605263 B2.
- EM Hartley Jr, MJ Matteson (1975) Ind Eng Chem Fundam 14(1): 67.
- M Solimannejad, A Boutalib (2004) G2 Molecular Orbital Investigation of OC H+−XH, OCH+−X2, and OCH+−XY(YX) (X = Y = F, Cl, and Br) Proton Bond Complexes. J Phys Chem 108: 10342.
- P Antoniotti, S Borocci, F Grandinetti (2005) Comment on “Computational Investigation of SO3−NH3-nXn (n = 0−3; X = F, Cl) Interactions”. J Phys Chem 109(10): 2410-2411.
- Delley (2000) From molecules to solids with the DMol3 approach. J Chem Phys 113(18): 7756.
- A Pastor de Abram (2004) Quitina y Quitosano: obtención, caracterización y aplicaciones, Pontificia Universidad Católica del Perú / Fondo Editorial, Chile.
- P Atkins, J Paula, (2010) Physical Chemistry. WH Freeman and Company, Novena Edició New York, USA.
- AH Alwash, AZ Abdullah, N Ismail (2013) TiO2-zeolite Y catalyst prepared using impregnation and ion-exchange method for sonocatalytic degradation of amaranth dye in aqueous solutionInternational Scholarly and Scientific Research & Innovation 7(6): 375.
- CT Weber, GC Collazzo, MA Mazutti, EL Foletto, GL Dotto (2014) Removal of hazardous pharmaceutical dyes by adsorption onto papaya seedsWater Science & Technology 70(1): 102.
- R Rehman, A Afzal (2015) Batch Scale Removal of an Organic Pollutant Amaranth Dye from Aqueous Solution using Pisum sativum Peels and Arachis hypogaea Shells as Adsorbents J Chem Soc Pak 37(5): 930.
- TFX Collins, J McLaughlin, C Gray (1972) Fd Cosmet Toxicol 10: 619.
- H Liao, Z Wang (2018) Adsorption removal of amaranth by nanoparticles composed Cu2O microspheres. Journal of Alloys and Compounds 769: 1088-1095.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.