Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Iron Chelation, Hydroxyl and DPPH Radical Scavenging Efficacies of Cobalt (II)- 2- Guanidinobenzimidazole Complex
*Corresponding author: Waseem Hassan (Ph.D), Institute of Chemical Sciences, University of Peshawar, Peshawar -25120, Khyber Pakhtunkhwa, Pakistan.
Received: February 20, 2020; Published: March 05, 2020
DOI: 10.34297/AJBSR.2020.07.001215
Abstract
The guanidine and imidazole derived moieties have gained significant attention in nucleophilic organocatalysis, anion recognition, coordination and pharmaceutical chemistry. The present study was designed to understand the biochemical efficacy of the Cobalt (II)- 2- Guanidinobenzimidazole [Co (II)-2GBI] complex. For the purpose, we measured the Fe (II) chelation efficacy of the stated complex. Co (II)-2GBI exhibited significantly (p< 0.005) higher potential at 10, 20 & 40 uM as compared with the standard, EDTA. Infact, 95% chelation was recorded at 50 ug/mL. One of the products of Fenton reaction is hydroxyl radical. Significant hydroxyl radical scavenging potential was noted. Infact 61% activity was the highest recorded at 50μg/mL. It was further confirmed by DPPH scavenging potential, where the complex inhibited 85 % (DPPH) at 50 μg/mL. The IC50 values of the complex are 25.01, 29 and 40.63μg/mL against, Fe (II) chelation, DPPH and hydroxyl quenching assay. The presence of nitrogen containing moieties in complex may contribute towards the broad antioxidant potential. However, further molecular studies are needed to decode the exact biochemical mechanism of action.
Keywords: Co (II)-2GBI, Iron Chelation and Radical Scavenging Assays
Introduction
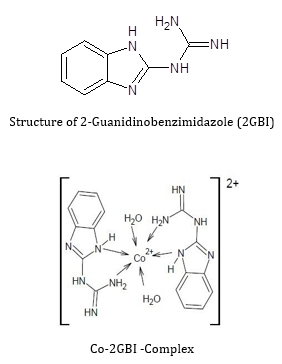
Coordination compounds play a pivotal role in chemical and pharmaceutical industries [1]. 2-Guanidinobenzimdazole (2-GBI) has been extensively studied for its coordination complexes with transition metals ions [2]. The structure of 2-Guanidinobenzimdazole consists of guanidine and benzimidazole group. It is a poly functional planar molecule with a delocalized π system. It contains five nitrogen atoms that act as basic Centre and five labile N-H groups forming a planer structure with sp2 hybridization [3]. These metal complexes interact through the lone pair of nitrogen of imidazole and can replace one of the acidic N-H of the guanidine group. This compound can act both as a mono- or bidentate ligand to form strong complexes with transition metals [4]. The structure of 2-GBI is shown in the (Figure 1) Extensive literature has been reported on coordination complexes of 2 GBI with transition metals ions such as Zn (II), Cu (II), Mn (II), Ni (II), Cr (II), Fe (II), Co (II), Sn (IV). The characterization of all these metal complexes were confirmed by FT-IR, NMR, UV-Visible spectroscopy. The interaction of 2 GBI with metal ions occurs through donor nitrogen atoms of guanidine and imidazole moieties [5,6].
Benzimidazole with its many analogues plays a major role in a vast number of biological activities like antibacterial and antifungal agents. They also play a major role in catalyzing the topological reactions through the breakage of DNA or reunion mechanism using topoisomerase II enzyme. The benzimidazole molecule also showed strong lipophilic character which reveals its bioactive effect [7]. Guanidine plays a very crucial role in binding the transition state due to their non-covalent specific binding nature. Various novel drugs were synthesized which acts at CNS, as antithrombotic, inhibitors of Na+/H+ exchangers and NO synthase [8,9]. 2GBI shows biological properties as a Na+ and K+ transport regulator in the apical membrane of the skin, reduces the gastric acid secretions, and serves as a hypoglycemiant and hypotensor. It also performed an important role in the activity of photosynthesis and can act as a mild uncoupler in photophosphorylation.
Another important property of 2 GBI is that it can act as a stimulator or inhibitor of different ions in the dermis of the body [10]. The molecules with odd number of electrons and high instability can be termed as free radicals. They are produced by a variety of cellular enzymes, non-enzymes and electron transport processes [11]. Mitochondria, endoplasmic reticulum, diamine oxidase, B5 enzymes and cytochrome p450, proxisomes, phagocytic cells, xanthine oxidase, NADPH oxidase, lipoxygenases, cyclooxygenases and peroxisomal oxidase are some of the fundamental endogenous and/ or intrinsic sources of free radical production. The exogenous and/ or extrinsic sources includes but not limited to radiations (X-rays, UV light) transition metals, infection, smoking, ozone, heat, industrial waste, cigarette smoke, therapeutic drugs and herbicides. Excessive production of free radical can cause oxidative stress which has been implicated in a variety of diseases like diabetes, cardiovascular disease, cancer, neurodegenerative disorders and other chronic conditions [12-14]. The role of metals and specially Iron (Fe) cannot be neglected in causing molecular damage. Fenton and Haber-Weiss reactions are reported to be involved in initiating oxidative stress, both of them cause production of radicals. Therapeutically, metal chelation or hydroxyl quenching may be of strong interest in the field of drug designing [12,13]. However, there is no literature data available of the 2GBI and its stated metal complex on the Fe (II) chelation. This led us to design the present project. For the purpose, the Fe (II) chelation ability of complex was performed. We also investigated the hydroxyl-radical scavenging ability of the stated complex. Furthermore, the radical scavenging potential of complex was also evaluated by DPPH method.
Material and Method
Preparation of [Co (II) (2GBI)2Cl2] Complex
By using 1:2 ratio, the cobalt (II) complex with 2GBI was synthesized. Briefly, in 30 mL ethanol, the solution of 2GBI was prepared. A dehydrated cobalt (II) chloride (dehydration was carried out at a temperature of 120 ⁰C in electric oven for 3-4 hours) was added to the same solution. The mixture was stirred and heated for 30 minutes. It was then kept aside until the formation of crystalline compound takes place (mostly for 1 to 2 days). The crystals were separated through filtration, washed with diethyl ether and dried [6].
Fe (II) Chelation Assay
The Fe (II) chelation ability of the compound was determined by the method of[15].The reaction was started by the addition of 500 μM FeSO4 (150 μL) to a reaction media, which contained (168 μL) 0.1 M Tris–HCl (pH 7.4), 218 μL saline. The volume of the tested compound was used in the region of 0-25μL. Total incubation time was 5 minutes, before the addition of 13 μL 0.25% 1, 10-phenanthroline (w/v).The net absorbance of the reaction was measured at 510 nm in UV-Spectrophotometer[15].
OH, Radical Scavenging Ability
We also decoded the OH radical scavenging ability of the synthesized complex. The principal idea was to inhibit Fe2+/H2O2-induced decomposition of deoxyribose. We used the method of Halliwell and Gutteridge (1989).We started the reaction by adding (0-100 ul) of the complex to a mixture containing (120 μL) 20 mM deoxyribose, (400μL) 0.1 M phosphate buffer, (40 μL) 20 mM hydrogen peroxide, and (40μL) 500 μM FeSO4. Total volume was 800μL with double distilled water. The reaction incubation time was 30 min and the temperature were 37°C. It was stopped by adding 0.5ml of 2.8% trichloroacetic acid. Lastly, 0.4ml of 0.6% thiobarbituric acid (TBAR) was added to the solution. It was further incubated for 20 min in boiling water. Data was recorded at 532 nm in a UV Spectrophotometer. The following equation was used for percent inhibition calculation [16].
Percent Inhibition= {(A0 –A1)/A0) *100}.
DPPH Radical Scavenging Assay
The scavenging ability of the complex was performed against the DPPH• radical. [17] method was followed for the purpose. 100 μM DPPH• was added to a reaction medium which contained different concentrations of the complex. After 30 min incubation (37 ⁰C), the absorbance was measured at 518 nm against DPPH. This was the principal depiction of their scavenging ability. We used Ascorbic acid as a positive control. Infact, it was considered 100% of inhibition [17].
Statistical analysis
The data was analyzed by one-way analysis of variance (ANOVA), followed by tukey’s multiple comparison test or Bonferroni’s multiple comparison test, when appropriate. Differences between groups were considered significant when p <0.05. Detail descriptive statistical analysis were performed, and graphics were created using Graph Pad Prisma 6.0.
Results
The current study reveals, for the first time, the ferrous ion chelating potential of CoII)-2GBI complex. The Fe (II) chelation assay was performed for serial concentrations (10 to 50 ug/mL) as shown in (Table 1). Its worthy to note that the complex exhibited significantly (p< 0.005) higher potential at each concentration as compared with EDTA, the standard. Precisely, at 30μg/mL the sample exhibited 60.56 % while the standard (EDTA) showed 55.99 % chelation. Similarly, at higher concentrations of 40μg/mL and 50μg/mL the Fe+2 chelation potential of the complex is 80.98% and 95.23 % respectively. While, at same concentrations the standard EDTA presented 80.98% and 92.54 % chelation capacity. The highest potential was noted at 50 ug/mL, where almost 95 chelation was recorded. 25.01ug/mL was the IC50 of Co(II)-2GBI complex.
Our data (Table 1) showed that the complex has high DPPH scavenging potential. The IC50 was found to be 29 μg/mL. The highest data was recorded at 50 μg/mL, where 85 % inhibition was noted. Vit C was used as standard, which showed the highest potential i.e. 98% at 50 μg/mL. Its IC50 was 31 μg/mL. However, the complex, presented modest hydroxyl radical scavenging potential. The highest i.e. 61% activity was recorded at 50 μg/mL, while the standard at same concentration shows 65.76 % scavenging activity as shown in (Figure 2). The IC50 for sample and standard (Vit C) were recorded at 40.63μg/mL and 43.97μg/mL respectively.
Discussion
There is considerable literature which confirms the presence of free iron in cytosol and mitochondria, which can initiate oxidative damage by increasing free radical production [13,14]. The Fenton reaction (given below) has been reported to be involved in the process.
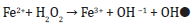
The oxidation of Fe (II) to Fe (III) cannot be neglected which in the presence of oxygen molecule can generate superoxide radical as shown below.
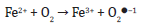
The generation of free radicals in above reactions may trigger the lipid peroxidation, protein oxidation or carbonylation and DNA/RNA damage. Similarly, the iron toxicity has been reported to be involved in the cellular processes. The implication of iron chelation therapy for the management of Fe(II) associated oxidative stress, damage or toxicity cannot be neglected [13]. This compelled us to design the present study and we evaluated the iron chelation potential of the stated complex. The classical chelating agent i.e. EDTA was used as a primary standard. As shown in (Table 1), the complex has a higher capacity to chelate Fe(II). The values for the Fe2+ chelating ranged from 15% to 95% at the concentration of 10 to 50 ug/mL Divalent metals catalyzed oxidative damage has been implicated in the development of various neurological and infact a series of pathological disorders. Thus, the deleterious effects of Fe2+ or divalent atoms can be attenuated by simple chelation. As stated above that hydroxyl radical is one of the products of Fenton reactions. Scavengers may help to remove it from the system and may inhibit degradation of biomolecules (14). For the purpose, we performed the stated assay. The data i.e. hydroxyl radical (OH●) scavenging potential of Co (II) complex is presented in (Figure 2). The highest i.e. (61%) potential was recorded at 50 μg/mL, while IC50 was approximately 40 μg/mL. Furthermore, we confirmed the OH radical scavenging potential against DPPH radical.
The DPPH method is a direct and reliable method for determining radical scavenging potential. The scavenging potential of the complex was decoded by estimating its electron donating ability to DPPH, which was experimentally confirmed by changes in absorbance at 517 nm. The efficacy of complex to scavenge DPPH, at all tested concentrations has been provided in the Table 1. The highest scavenging was observed at 50 ug/mL, where the complex scavenged 85 % radicals. The IC50 or 50% inhibition of DPPH radical was found to be 31 ug/mL. Vitamin C was used as the reference standard for this assay. This was also performed to supplement the hydroxyl radical potential of the Co (II) complex. DPPH and hydroxyl radical scavenging potential suggests that the complex has interesting potential for scavenging free radicals which may be able to prevent the initiation of free radical-mediated chain reactions. However further studies are needed to confirm this assumption. The DPPH and OH radical scavenging activity of the complex increased with an increase in concentration. This was expressed in IC50.
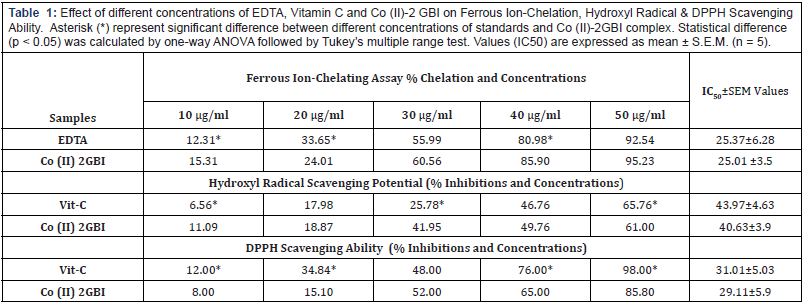
The strong radical quenching potential can be attributed to the presence of amine substituent group in the molecule, which can abstract electron from free radical. Literature revealed that constituent of 2GBI showed promising biological potential. Both guanidine and benzimidazole can be used as antiviral, antihelminthics, antidiabetic, anti-inflammatory agents and as antiparasitic drugs [7,9]. In the present report we are provided the Fe (II) chelation and radical scavenging potential of the stated complex. By a closer inspection of the 2- Guanidinobenzimidazole structure, it is apparent that it has delocalized π system and five labile N-H groups. It can act as a mono- or bidentate ligand to form strong complexes with transition metals. In other words, the interaction of 2 GBI with metal ions can occur through donor Nitrogen atoms of guanidine and imidazole moiety [4]. It is reported that the cobalt complex is a distorted octahedral assembly in 1:3 where the metal cation occupied the centre position and interact with the three ligands through three N atoms of guanidine and imidazole moiety of the ligand [18]. The interaction of two amine group (NH2) in 2 GBI and/or delocalized π system with Fe (II) cannot be neglected, which may play a role in chelation and/or radical scavenging.
Conclusion
Our results indicated that cobalt complex have significant DPPH and hydroxyl radical scavenging potential and ferrous ion chelating activity. The IC50 values showed by Co (II)-2GBI complex are statistically higher than the standard chelator, EDTA and standard radical scavenger, Vitamin C. However, further investigations are required to explore its probable mechanism of action.
References
- Silvia E, Blum C, Norah B (2000) Coordination chemistry of some biologically active ligands. Coord. Chem. Rev 196(1): 3-30.
- Ali T, Wadud A, Hussain MS (1982) Thermal Properties of some Nitrogen Ligated Complexes of Co (II). J Chem Soc Pak 4(1): 1-5.
- Fialon M, Lopez N, Behrens N, Contre R (1998) Organometallic tin complexes derived from 2-Guanidinobenzimidazole. Hetroatom Chem 9(7): 637-641.
- Lopez A, Rosado CR, Baez E, Contreras R, Tlahuext H, et al. (1998) Boron heterocycles derived from 2- Guanidinobenzimidazole. Heteroatom Chem 9(4): 339-409.
- Aremu JA, Durosinmi LM, Oluyemi EA (2018) Synthesis, Characterization and Chelating Properties of Guanidinobenzimidazole and Guanidophosphonatebenzimidazole. Ife Journal of Science 20(3): 679-704.
- Olmos A, Blum S, Hojer G, Hojer S, Norah B, et al. (1996) Coordination behavior of 2-guanidinobenzimidazole towards cobalt (II), nickel (II), copper (II) and zinc (II). An experimentally and theoretical study. Transit. Met. Chem 21: 31-37.
- Galal SA, Hegab KH, Hashem A (2010) Synthesis and antitumor activity of novel benzimidazole-5-carboxylic acid derivatives and their transition metal complexes as topoisomerease II inhibitors. Eur J Med Chem 45(12): 5685-5691.
- Saczewski F, Balewski L (2009) Biological activities of guanidine compounds. Expert Opin. Ther. Pat 19(10): 1417-1448.
- Saczewski F, Balewski L (2013) Biological activities of guanidine compounds, 2008 – 2012. update Expert Opin Ther Pat 23(8): 965-995.
- Skrzypek S, Mircesk V, Ciesielski W (2011) Volumatric Study of 2-Guanidinobenzimidazole: Electrode Mechanism and Determination at Mercury Electrode. Collect. Czech. Chem. Commun 76(12): 1699-1715.
- Waseem Hassan, Hamsa Noreen, ShakilaRehman, Shehnaz Gul, Mohammad Amjad Kamal, et al. (2017) Oxidative Stress and Antioxidant Potential of One Hundred (100) Medicinal Plants. Current Topics in Medicinal Chemistry 17(12): 1336-1370.
- Waseem Hassan, Hamsa Noreen, Vitor Castro-Gomes, ImdadullahMohammadzai, Landeira-Fernandez, et al. (2016) Association of Oxidative Stress with Psychiatric Disorders. Current Pharmaceutical Design 22(20): 2960-2974.
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M, et al. (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. ChemBiol Interact 160(1): 1-40.
- Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J, et al. (2004) Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem 266(1-2): 37-56.
- Puntel RL, Nogueira CW, Rocha JBT (2005) Krebs cycle intermediates modulate thiobarbituric acid reactive species (TBARS) production in rat brain in vitro. Neurochemical Research 30: 225-235.
- Halliwell B, Gutteridge JMC (1989) Free Radicals in Biology and Medicine 2nd edn. Oxford: Clarendon Press 73-74.
- CW Choi, SC Kim, SS Hwang, BK Choi, HJ Ahn, et al. (2002) Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci 163(6): 1161-1168.
- Noth N, Norah B, Bernes S (2000) Synthesis, X-ray and NMR characterisation of cobalt (III) coordination compounds with 2-guanidinobenzimidazole. Inorganica Chimica Acta 304(2): 230-236.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.