Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Is there any Benefit for the Brain Metastasis with Cystic Components Rather than Sold Component only in Non-Small Cell Lung Cancer in terms of Clinical Outcome?
*Corresponding author: Young Zoon Kim, Division of Neurooncology and Department of Neurosurgery, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Korea.
Received: February 24, 2020; Published: March 04, 2020
DOI: 10.34297/AJBSR.2020.07.001207
Abstract
Background: Brain metastases (BM) is an important factor in shortening the lives of patients with lung cancer. It is not rare that the physician encounters the brain metastases with cystic components. We analyzed the efficacy and prognosis of different therapeutic schedules in BM of NSCLC patients with cystic component.
Methods: A retrospective study of patients with pathologically confirmed stage IV non-small cell lung cancer was conducted. All patients were with cystic BMs. We analyzed the clinical characteristics of these patients and the efficacy of targeted drugs and chemotherapy regimens.
Results: A total of 43 patients (43/519, 8.3%) developed cystic BMs. We evaluated these 43 NSCLC patients, including 30 adenocarcinomas and 13 squamous cell carcinomas. The incidence of gene mutation including EGFR mutation and ALK fusion was 75.9% (22/29) of patients who undergoing genetic detecting. Then, 33 patients received treatment and had been evaluable efficacy. There was significant difference between targeted regimens and chemotherapy treatment for progression-free survival (PFS) (12.2 months vs. 6.2 months, P=0.001) and overall survival (OS) (47.4 months vs. 15.0 months, P=0.001). The PFS between patients who were brain lesions progression firstly and extra-cerebral lesions firstly was significant difference (6.2 months vs 13.0 months, P=0.017). In addition, there were no significance difference of patients with or without radiotherapy in patients with cystic BMs for OS (P=0.085).
Conclusion: The BM with cystic component may be more likely to occur in genetic mutated NSCLC patients. There is difference prognosis of cystic BM patients who underwent targeted treatment and chemotherapy.
Keywords: Brain metastases, Cyst, Tyrosine kinase inhibitor, Chemotherapy, Radiotherapy, Lung cancer
Introduction
Lung cancer is the most common causes of cancer death throughout the world [1] . Brain metastasis (BM) is the most common intracranial tumor and an important factor in shortening the lives of patients with lung cancer [2] . BM are presented in 10%- 20% of non-small cell lung cancer (NSCLC) patients at diagnosis [3] . Approximately 30–50% of NSCLC patients will develop BM during their disease course [4] . Patients with BMs commonly have poor prognosis and patients have only a median survival of just 1-2 months without treatment due to mainly impaired performance status [5] .
Whole brain radiotherapy (WBRT) improves median survival to 4–6 month [6] . With the development of treatment, for BM, including surgery, WBRT, stereotactic radiotherapy, chemotherapy, and targeted therapy which have greatly extended the survival time of patients with BMs [7] . The stereotactic radiosurgery (SRS) is a new treatment method, applied in selected conditions of relatively oligometastasis (less than or equal to five BMs) [8] .
As we known, cystic BMs are uncommon and cystic component of BMs are often deemed not suitable for radiation, because of cystic lesion insensitivity to radiotherapy and large volume. Therefore, appropriate treatment strategies for cystic BMs are desperately needed.
However, the efficacy and prognosis according to different treatment options in NSCLC patients with cystic BMs have been seldom reported. Therefore, we retrospectively evaluated the response to different drugs treatment and investigate factors related to local control and overall survival after BMs with cystic components.
Patients and Methods
Patient characteristics
We reviewed 519 NSCLC patients with BMs at our institute from January 2012 to June 2019. We retrospectively reviewed the clinical records, treatment regimens and survival of those patients who was with cystic BMs.
The inclusion criteria were as follows:
1) definite histopathological diagnosis for NSCLC
2) newly diagnosed brain metastases with cystic component or during treatment,
3) without meningeal metastases and
4) candidate with good general condition for active treatment including cytotoxic chemotherapy and tolerable against the adverse effects.
At last, a total of 43 eligible patients were included and analyzed in our study. Performance status was estimated by use of The Eastern Cooperative Oncology Group (ECOG) score; 0, asymptomatic (Fully active, able to carry on all pre-disease activities without restriction): 1, symptomatic but completely ambulatory (restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature): 2, symptomatic, <50% in bed during the day (ambulatory and capable of all self-care but unable to carry out any work activities and up and about more than 50% of waking hours)
The study was approved by institutional review board of our hospital and was conducted in strict accordance with the principles of the Declaration of Helsinki. Informed consent was waived due to its retrospective nature.
Radiological data
In our study, we defined cystic BM as the volume of the cystic lesions greater than 50% of the total volume. Contrast-enhanced magnetic resonance image (MRI) is necessary for diagnosing cystic BMs. The cystic components revealed hypointense on T1-weighted images, hyperintense on T2-weighted images, and no enhancement. Brain lesion parameters examined included number of enhanced mass in the brain and time of diagnosis of BM. The number of BM was counted as the mass with enhancement with gadolinium in T1 weighted MRI. In terms of the interval between the time of BM and NSCLC, BM diagnosed ≤2 months from NSCLC diagnosis was considered synchronous and that diagnosed >2 months from the time of NSCLC diagnosis was defined as metachronous. Enhanced MRI was performed every 2 months or 2 cycles after the initial treatment.
Treatment and response assessment
Collecting all patients with systemic treatment and efficacy, as well as brain treatment options and efficacy. The systemic treatment included targeted therapy (who did gene detection) and chemotherapy (chemotherapy regimens and doses were based on international standards). Brain treatment options had WBRT, SRS and surgery. All patients underwent imaging examinations after two courses of chemotherapy or every 4 ±1 week for the first 2 months of epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) treatment. Response to systemic therapies was assessed locally by each investigator using Response Evaluation Criteria in Solid Tumors (RECIST v1.1), and included complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) [9] . The ORR was defined as the sum of CR and PR. The disease control rate (DCR) was defined as the sum of the objective response and stabilization rates (CR + PR + SD).
Follow-up and Statistical analysis
Progression-free survival (PFS) was defined as the period from the initial date of drugs treatment to the date of confirmation of disease progression evaluated by RECIST v1.1 or death. Intracranial progression-free survival (iPFS) was defined as the time from using drugs treatment until intracranial progression. OS was measured from the date of confirmed stage IV NSCLC to death or last followup evaluation. The last follow-up date was December 11, 2019.
Statistical analysis was performed using SPSS version 25.0 (IBM Corp. Armonk, NY, USA). The survival estimates were analyzed by the Kaplan–Meier method. Multivariate survival analysis for prognostic factors and cystic BMs was performed by using Cox regression and the forward likelihood ratio method. A two-sided test that resulted in P<0.05 was considered to be statistically significant.
Results
Clinicopathologic characteristics
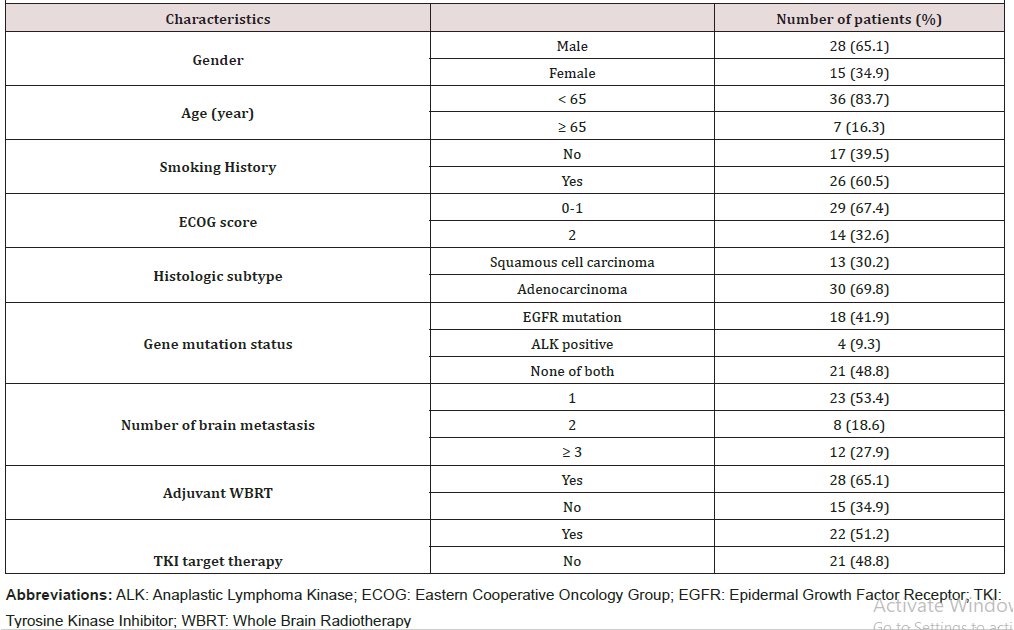
In our study, total of 43 patients (43/519, 8.3%) developed cystic BMs. The patients comprised of 28 men and 15 women. The mean age of patients was 58 (range, 33-75) years. Twelve patients had ≥3 cystic brain metastases lesions, 8 patients had two lesions and 23 patients had a single lesion (Table 1).
The mode of onset was synchronous in 32 (74.4%) and metachronous in 11 (25.6%). Four patients had received surgery and 28 had brain radiotherapy. WBRT was given in 23 patients and 7 had SRS. Over half of the patients (24/43, 55.8%) had significant neurologic symptoms, including headaches, dizziness, vomiting, muscle weakness, neuropathy and so on. There were 30 adenocarcinomas and 13 squamous cell carcinomas. Twenty-two patients were with EGFR or anaplastic lymphoma kinase (ALK) gene mutation and 21 patients were gene negative or undetected. For patients undergoing genetic detecting, the incidence of gene mutation is 75.9% (22/29) (Table 1).
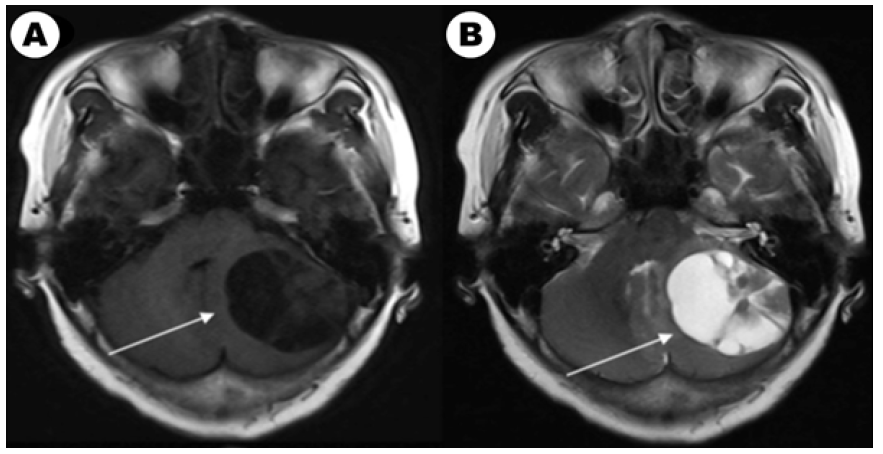
Figure 1A: The brain enhanced MRI of a 61-year-old woman with non-small lung cancer and EGFR 21L858R mutation before and after EGFRTKI treatment. Contrast-enhanced T1-weighted MR image in baseline. Figure 1B: Contrast-enhanced T2-weighted MR image in baseline.
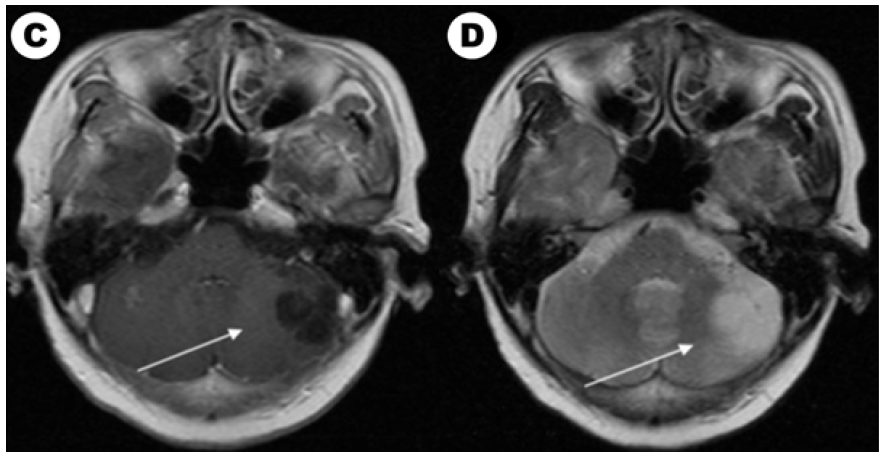
Figure 1C: Contrast-enhanced T1-weighted MR image after EGFR-TKI target therapy and whole brain radiotherapy (the prescription dose and fraction schedule were 30Gy/10F) treatment one month. Figure 1c: Contrast-enhanced T2-weighted MR image after EGFR-TKI target therapy and whole brain radiotherapy treatment one month.
Effect assessment and local control
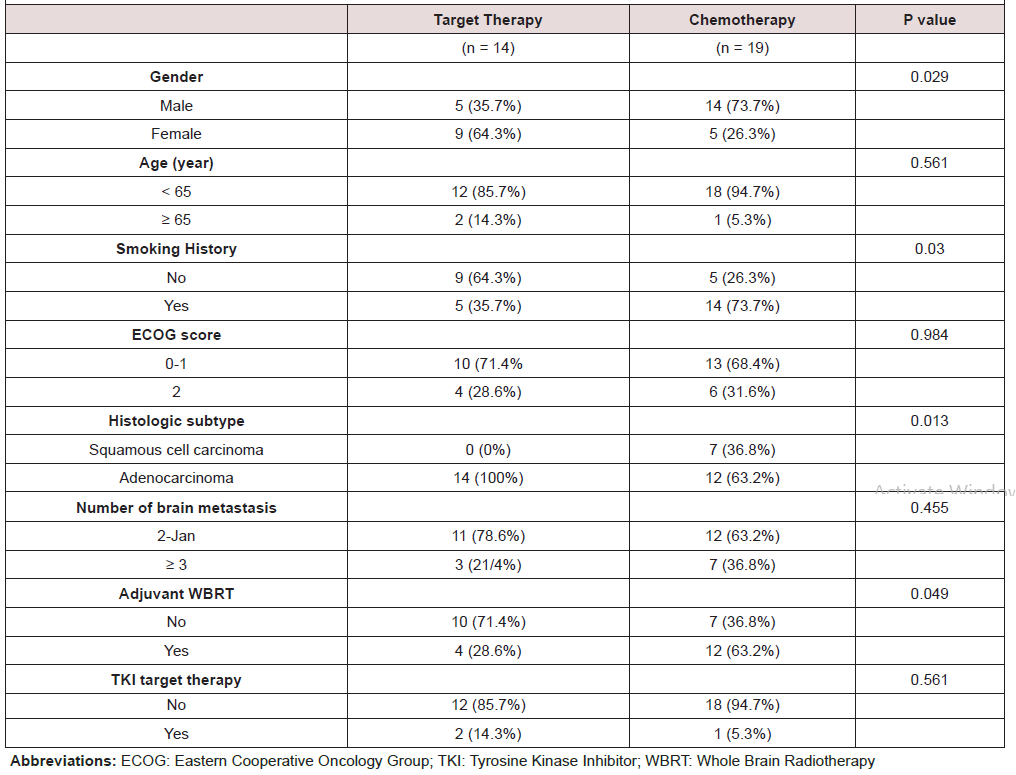
According to our follow-up, 4 patients abandoning therapy and 33 patients which received treatment had evaluable efficacy. Until the last follow-up time, 40 patients had evaluated overall survival and 8 (8/40, 20.0%) patients were still alive. Among them, 14 patients with EGFR/ALK gene mutated had TKI targeted treatment and 19 patients received chemotherapy. For brain radiotherapy, 4 of 14 EGFR/ALK mutated patients received radiotherapy and 12 of 19 patients received chemotherapy had brain radiotherapy. We also compared characteristics between patients received targeted drugs and chemotherapy in (Table 2). We showed a typical case of cystic BM which was EGFR mutated NSCLC patient in (Figure 1A). We showed the baseline brain MRI images in(Figure 1A) (T1-weighted MR image) and(Figure 1C) (T2-weighted MR image). After one month of icotinib-targeted therapy and whole brain radiotherapy, the brain MRI images of the patient presented in (Figure 1C) (T1-weighted MR image) and (Figure 1C) (T2-weighted MR image). The effect+- -+-+-+++-` of this patient showed that the brain lesion had shrunk significantly.
Progress free survival and overall survival
The median PFS of these 33 patients was 8.9 months and there was significant difference between targeted therapy and chemotherapy treatment (12.2 months vs. 6.2 months, P=0.001) (Figure 2A). Among them, 22 patients had a development of brain lesion firstly or simultaneous progression of brain and extracerebral lesions. Ten patients were extra-cerebral lesions firstly progression. The PFS between brain lesions’ progression firstly and extra-cerebral lesions firstly was significant difference (6.2 months vs 13.0 months, P=0.017). Then we explored intracerebral progress free survival (iPFS) in patients who had brain lesions development firstly or simultaneous progression of brain and extra-cerebral lesions. The median iPFS of these 22 patients was 6.2 months and there was also significant difference between targeted therapy and chemotherapy treatment (8.4 months vs. 6.0 months, P=0.042) (Figure 2B). The iPFS was not significantly different of patients with brain radiotherapy (6.5 months) or without radiotherapy (6.2 months; P=0.516).
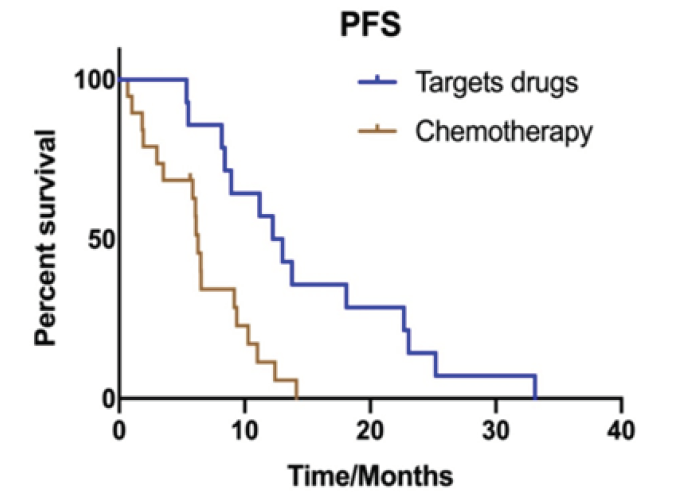
Figure 2A: Kaplan Meier estimates of progression-free survival according to different treatments: (A) targeted drugs and chemotherapy treatment (12.2 months vs. 6.2 months, P=0.001).
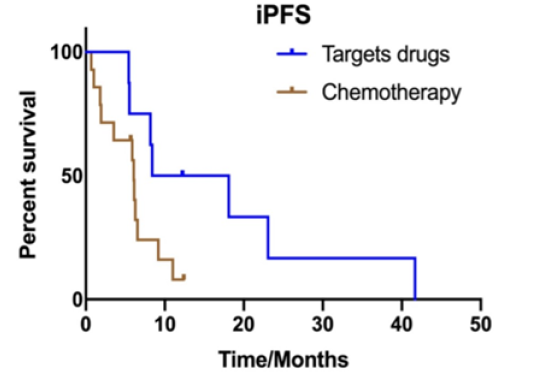
Figure 2B:The median iPFS between targeted regimens and chemotherapy treatment (8.4 months vs. 6.0 months, P=0.042).
In a multivariate analysis, treatment regimens (P=0.002) and brain lesions progression firstly (P=0.022) were independent prognostic factors for PFS. There was no significant association of PFS with gender (P=0.617), age (P=0.239), smoking history (P=0.316), pathological type (P=0.324), ECOG score (P=0.930), number of brain metastases lesions (P=0.325), if received anti-vascular drugs (P=0.126) and if with brain radiotherapy (P=0.806).
The median OS of the 33 patients was 18.3 months and there was significant difference between targeted regimen and chemotherapy treatment (47.4 months vs. 15.0 months, P=0.001) (Figure 3). The OS in brain lesions progression firstly was 17.2 months and in extra-cerebral lesions firstly was 33.1 months, P=0.481). The OS was not significantly different between patients with or without radiotherapy (15.0 vs. 22.8 months; P=0.085).
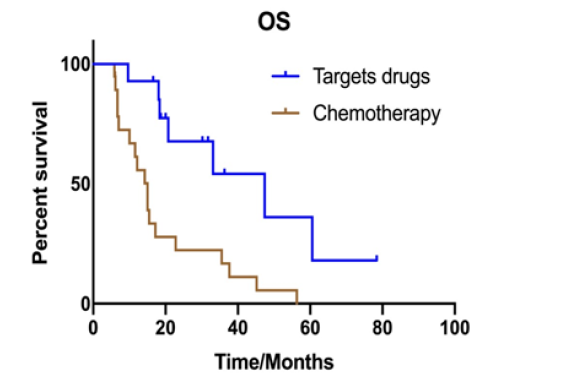
Figure 3:Kaplan Meier estimates of overall survival according to different treatments: targeted drugs and chemotherapy treatment (47.4 months vs. 15.0 months, P=0.001).
In a multivariate analysis, treatment regimen (P=0.019) and pathological type (P<0.001 were independent prognostic factors for the OS. There was no significant association of OS with gender (P=0.964), age (P=0.247), smoking history (P=0.511), PS score (P=0.685) number of BMs (P=0.990), if received TKI drugs (P=0.571) and if with brain radiotherapy (P=0.875).
Discussion
The BM with cystic component is regarded as an important sign of poor prognosis in cancer and NSCLC is the primary cancer in these patients [10] . However, there still exists controversy about treatment strategies for BMs with cystic component in NSCLC. Only some retrospective studies associated with cystic BMs demonstrates that treatment results with brain radiotherapy. At present, there is a lack of large sample studies to explore the effect of chemotherapy or targeted therapy on BMs with cystic component. In our study, we found that BMs with cystic component were more likely to occur in genetically mutation lung cancer patients, including EGFR mutation and ALK positive NSCLC. This is the first report to evaluate the efficacy of different treatment strategies for NSCLC patients with cystic cerebral metastases.
The mechanism that leads to the formation of cystic BMs remains poorly known. Studies suggest that the cause of cystic masses may include the breakdown of the blood–brain barrier or patients with poor histological grade had higher risk of developing cystic BMs [11, 1]. Reports demonstrated that patients with activating EGFR gene had higher risk than EGFR wild type to occur BM in NSCLC patients [13, 14]. In addition, for ALK-positive NSCLC, initial metastases to the brain occur in approximately 30% [15]. We found that the incidence of BMs with cystic component in EGFR and ALK gene positive NSCLC was higher than gene negative patients. This might mean that patients with genes positive were more malignant than negative patients and may easily occur cystic BMs. Rapid growth could be another possible cause for generating cystic components. We found some studies reported BMs with cystic component should more happened in NSCLC patients with ALK rearrangement [16, 17, 18, 19, 20, 21]. However, there was a lack of data in the Korea. Then in our research, we found the incidence of cystic BMs in EGFR mutated NSCLC was 62% (18/29) and ALK positive was 13.8% (4/29) for adenocarcinoma patients with doing gene detection. Nowadays, only a case involved EGFR mutated NSCLC patients who happened fatal cystic change of BM21. In the future, this is worth further exploration and confirmation.
According to our results, systemic treatment strategies were the main factor affecting the efficacy and prognosis. In genetic mutation NSCLC patients who received corresponding targeted drugs therapy could improve brain efficacy and prolong survival. Some reports showed ALK positive NSCLC patients with cystic BMs had response to ALK-TKI [15, 16, 17, 18, 19, 20, 21, 22]. It is thus possible that a good response to agents targeted to corresponding driver oncogenes may result in cystic degeneration of BMs. Our research also showed that the efficacy of patients who received targeted drugs was significantly better than those who received chemotherapy. We also demonstrated targeted drugs could prolong survival.
Wang et al compared the survival between cystic and solid brain metastases and assess OS in patients who underwent radiosurgery treatment [23]. They found there is no difference in survival time between cystic and solid brain tumor and radiosurgery could be a suitable treatment option for BMs with cystic component. However, in this study, it includes different primary cancers and the gene status in 54 lung cancer patients were not informed. It was not clear what treatments other than radiotherapy were available, which might also affect the overall prognosis. Therefore, in our study, we explored the value of radiotherapy in the treatment process. We found there was no difference in whether received brain radiotherapy or not. Of course, we needed to consider whether the patients had symptoms originated from BMs and the role of brain radiotherapy. Our results showed targeted therapy was the key treatment for the patients with cystic BMs to get better prognosis, which means that cystic BMs could benefit from targeted therapy like solid BM. Moving forward, additional prospective data is needed to explore the radiotherapy mode and treatment timing. Therefore, in the era of individualized treatment, patients should be genetically tested as much as possible to find more suitable therapeutic drugs.
In addition, we further to explore the correlation between progression pattern. The results demonstrated the PFS in brain lesions progression firstly was significantly poorer than extracerebral lesions progression firstly. Therefore, we thought brain lesions progression was one of the signs of poor prognosis.
Although this is the first study to evaluate the efficacy of different treatment strategy for NSCLC patients with cystic cerebral metastases, the limitations of our study must be discussed. The retrospective nature of this study might influence some results, including heterogeneous patients and line of therapy. In the future, it needs further to explore the relationship between target drugs and radiotherapy mode or timing. The sample size were also relatively small. Therefore, prospective studies with a larger patient population are needed for validation. Additionally, the diagnosis of cystic BM was just performed in the radiological analysis with MRI without histological examination. In fact, we tried to reduce the bias on the diagnosis of cystic BMs by the individual 2 radiologists who did not have any information of the patients. In order to give a general practice in the clinic, the further comprehensive study such as randomized clinical trial is essential.
Conclusion
The presenting study demonstrated NSCLC patients with genetic mutations might be more likely to occur cystic BM. These patients should be genetically tested as much as possible to find more suitable therapeutic drugs and have good prognosis who could receive targeted drugs.
Acknowledgements
We thank the following individuals: Seok-Hyun Kim, M.D., (Department of Internal Medicine, Samsung Changwon Hospital) for the effort to manage the patients; Tae Gyu Kim, M.D., (Department of Radiation Oncology, Samsung Changwon Hospital) for administering the radiotherapy detailed in this work; Young Min Kim, M.D., and Mi-Ok Sunwoo, M.D., (Department of Radiology, Samsung Changwon Hospital) for their review of the neuroradiological images: Eun Hee Lee, M.D., and Mee-Seon Kim, M.D., (Department of Pathology, Samsung Changwon Hospital) for their pathological review; Young Wook Kim, M.D., (Department of Biostatistics, Samsung Changwon Hospital) for assistance with the statistical analysis.
Disclosure
The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A (2017) Cancer statistics. CA Cancer J Clin 67(1): 7-30.
- Nussbaum ES, Djalilian HR, Cho KH, Hall WA (1996) Brain metastases. Histology, multiplicity, surgery, and survival. Cancer 78(8): 1781-1788.
- Shi Y, Sun Y, Yu J, Ding C, Ma Z, et al. (2017) China experts’ consensus on the diagnosis and treatment of brain metastases of lung cancer. Zhongguo Fei Ai Za Zhi 20(1): 1-13.
- Lombardi G, Di Stefano AL, Farina P, Zagonel V, Tabouret E (2014) Systemic treatments for brain metastases from breast cancer, non-small cell lung cancer, melanoma and renal cell carcinoma an overview of the literature. Cancer Treat Rev 40(8): 951-959.
- Owonikoko TK, Arbiser J, Zelnak A, Shu HK, Shim H, et al. (2014) Current approaches to the treatment of metastatic brain tumours. Nat Rev Clin Oncol 11(4): 203-222.
- Mehta MP, Rodrigus P, Terhaard CH, Shu HK, Shim H, et al. (2003) Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole brain radiation therapy in brain metastases. J Clin Oncol 21(13): 2529-2536.
- Bulbul A, Forde PM, Murtuza A, Woodward B, Yang H, et al. (2018) Systemic treatment options for brain metastases from non-small-cell lung cancer. Oncology 32(4): 156-163.
- Lim SH, Lee JY, Lee MY, Kim HS, Lee J, et al. (2015) A randomized phase III trial of stereotactic radiosurgery (SRS) versus observation for patients with asymptomatic cerebral oligo-metastases in non-small lung cancer. Ann Oncol 26(4): 762-768.
- Lawrence H Schwartz, Saskia Litiere, Elisabeth de Vries, Robert Ford, Stephen Gwyther, et al. (2016) RECIST 1.1 - Update and Clarification: From the RECIST Committee. Eur J Cancer 62: 132-137.
- Uchino M, Nagao T, Seiki Y, Shibata I, Terao H, et al. (2000) Radiosurgery for cystic metastatic brain tumor. No Shinkei Geka 28(5): 417-421.
- Gardner WJ, Collis JS, Lewis LA (1963) Cystic brain tumors and the blood brain barrier. Comparison of protein fractions in cyst fluids and sera. Arch Neurol 8: 291-298.
- Sun B, Huang Z, Wu S, Ding L, Shen G, et al. (2016) Cystic brain metastasis is associated with poor prognosis in patients with advanced breast cancer. Oncotarget 7(45): 74006-74014.
- Sekine A, Kato T, Hagiwara E, Shinohara T, Komagata T, et al. (2012) Metastatic brain tumors from non-small cell lung cancer with EGFR mutations: distinguishing influence of exon 19 deletion on radiographic features. Lung Cancer 77(1): 64-69.
- Shin DY, Na II, Kim CH, Park S, Baek H, et al. (2014) EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol 9(2): 195-199.
- Rusthoven GC, Doebele RC (2016) Management of brain metastases in ALK-positive non-small-cell lung cancer. J Clin Oncol 34(24): 2814-2819.
- Grubb WR, Machtay M, Dowlati A, Biswas T (2019) Diffuse Atypical Cystic Brain Metastases in ALK+ NSCLC Treated with Whole Brain Radiation and Second-Generation ALK-Targeted Therapy. Pract Radiat Oncol 9(2):129-133.
- Marta GN, da Cunha Colombo Bonadio RR, Martins RE, Zuppani HB, de Castro G Junior (2018) Cystic brain metastases in ALK-rearranged non-small cell lung cancer. E cancer medical science 12(14): 818.
- Kim SH, Hyun JW, Kim HJ, Gwak HS, Lee SH, et al. (2017) De novo cystic brain lesions mimicking neurocysticercosis in ALK-positive lung cancer. Lung Cancer 110: 53-55.
- Saraceni C, Li PM, Gainor JF, Stopyra GA, Friedman EL (2015) Cystic Brain Metastases in NSCLC Harboring the EML4-ALK Translocation after Treatment with Crizotinib. J Thorac Oncol 10(7): 1116-1117.
- Narayanan V, Honce MJ, Mehrotra S, Camidge DR (2016) Cystic Brain Metastases Occurring in Anaplastic Lymphoma Kinase Gene Rearranged Non-Small-Cell Lung Cancer Patients Receiving Crizotinib. Clin Lung Cancer 17(1): 85-90.
- Hayashi H, Okamoto I, Tanizaki J, Tanaka K, Okuda T, et al. (2014) Cystic brain metastasis in non-small-cell lung cancer with ALK rearrangement. J Clin Oncol 32(36): 122-124.
- Zee YK, Chin TM, Wong AS (2009) Fatal cystic change of brain metastasis after response to gefitinib in non-small-cell lung cancer. J Clin Oncol 27(30): 145-146.
- Wang H, Liu X, Jiang X, Song Y, Wang X, et al. (2019) Cystic brain metastases had slower speed of tumor shrinkage but similar prognosis compared with solid tumors that underwent radiosurgery treatment. Cancer Manag Res 11(20): 1753-1763.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.