Opinion 
 Creative Commons, CC-BY
Creative Commons, CC-BY
A Note on Prebiotics Strategy to Cope with Current Health Challenges in Human Beings
*Corresponding author: Nayla Munawar, Department of Chemistry, College of Sciences (COS), United Arab Emirates University (UAEU), Al-Ain, United Arab Emirates.
Received:April 10, 2020;Published: April 20, 2020
DOI: 10.34297/AJBSR.2020.08.001296
Abstract
The current widely used practices of processed food have resulted in elimination of healthy microflora from human gut leading several health issues like chronic constipation, irritable bowel syndrome, colon cancer, obesity, heart diseases and high cholesterol etc. Amongst others, prebiotic approaches have been developed to promote the growth of beneficial bacteria in the gut to treat gut related disorders and improve human health. Importance of prebiotics in maintaining gut microbial diversity, need of novel prebiotics having varied functional properties and their production strategies for commercial applications have been highlighted in this article.
Keywords: Prebiotics, Fructosyltransferase, Probiotics, Oligosaccharides, Non-digestible dietary fiber
Introduction
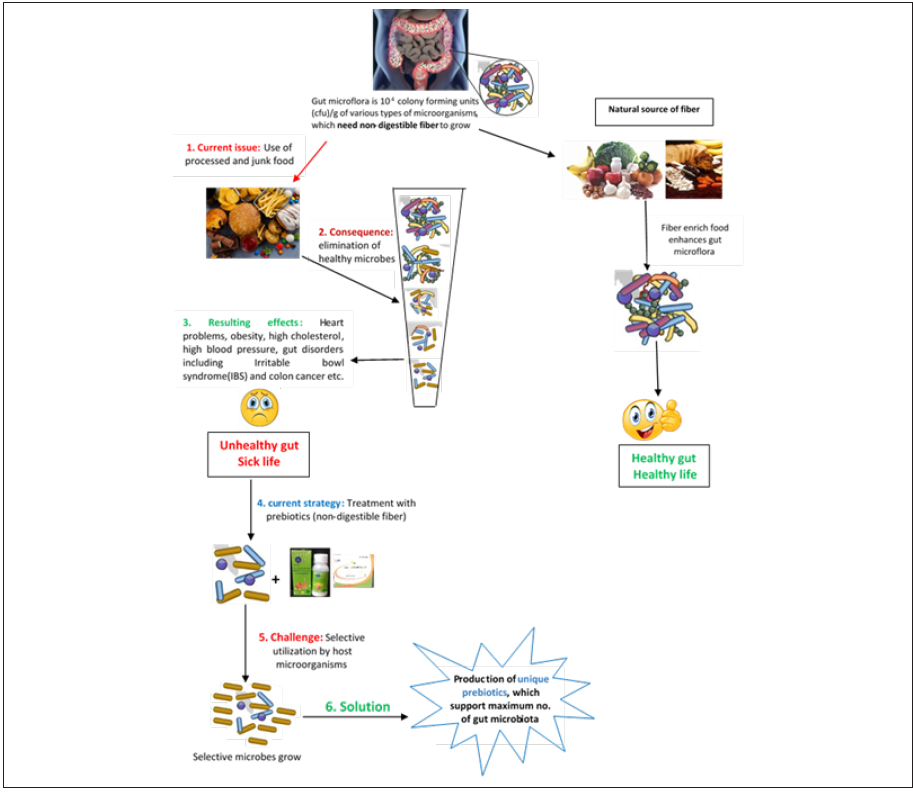
Now a day’s good health and balanced diet are the leading issues among all human beings. Therefore, great enthusiasm has been observed for the development of new variety of food [1]. Different bioactive nutrients including prebiotics (fructooligosaccharides, galactooligosaccharides, xylooligosaccharides, lactulose) are available as supplements or have been added in the variety of commercially available foods like yogurts and juices. Prebiotics are oligosaccharides and are known as non-digestible dietary fibers, which sustain a healthy microbiome by regulating gut microbiota and by modifying microbiota composition as well [2]. They are also used to restore bacterial homeostasis due to any health hazard in terms of disease [3].
Prebiotics/non-digestible fiber could usually be obtained from several natural foods like cereals, legumes, nuts, many fruits and vegetables [4]. However, because of frequent use of processed and junk food in the whole world, non-digestible fiber has been eliminated from our daily life food. Consequently, gut microflora altered in the gut, which results number of gut disorders in every age group of the humans. Growing research literature shows that dietary fiber not only prevents gut diseases but also reduces the risk of heart disease, diabetes, colon cancer and involved in lowering cholesterol [5,6]. The recommended dose of dietary fiber is 25-30g/day; however, a careful estimation is that dietary fiber consumption by an American adult is 15g/day, which is less than half of the recommended dose. Regardless of using different methods to encourage the use of healthy food, the young generation seems trapped in the taste of junk food, which leads obesity, high cholesterol, heart problems and gut issues like constipation, irritable bowel syndrome and colon cancer in early age [7]. These health issues could be improved by adding non-digestible fiber in the food. In this article we are highlighting the importance, challenges and potential solutions of production of novel prebiotics at commercial level (Figure 1).
Market of Prebiotics
As the importance of prebiotics has been highlighted by growing scientific literature, several researchers around the globe studied the source of natural non-digestible fibers and methods for their production in large quantity to add in the food. Prebiotics have grabbed the attention of pharmaceutical companies and became an important candidate in nutraceuticals to improve human health. Prebiotics market is expected to reach US $ 5.485 billion by 2024, from US $ 3.727 billion in 2018 according to global prebiotics market survey. Great health impact and intense research and innovation in the field are two major factors, which will enhance the market of prebiotics further in the near future.
Enzymes for Prebiotic Synthesis
Two groups of prebiotics/fructans, fructans/fructooligo saccharides (FOS) and glactooligo saccharides (GOS) are well known for health benefits in humans [8-10]. Because of low amount of these oligosaccharides in natural food, it is required to synthesize these compounds in the laboratory for large scale applications. The pre-requisites for commercial production of oligosaccharides/prebiotics at industrial level requires an efficient method and economical raw material to make product cost effective. Biosynthesis of FOS is a preferred method over chemical methods to avoid the production of hazardous by products and low product during acid-catalyzed hydrolysis synthesis of FOS [11,12]. A wide variety of microorganisms and plants synthesize fructans. Several bacterial and fungal species are good sources of FOS producing enzymes, which have been divided into two classes: 1. β-Dfructofuranosidase and 2. Fructosyl transferase according to their enzymatic activity and the product they produced [13] (Figure 2).
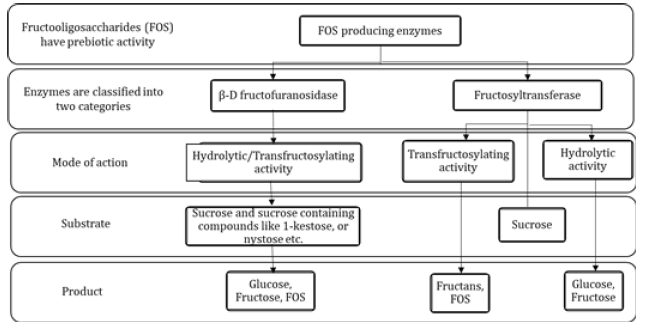
Figure 2: Mechanism of action of prebiotic producing enzymes: β-D fructofuranosidase has more hydrolytic activity than trans-fructosylating activity as compared to fructotransferases. Fructotransferases can produce variety of fructans using sucrose as substrate.
β-D-fructofuranosidase (FFase, EC 3.2.1.26) belong to GH32 family of glycoside hydrolases and involve in hydrolysis of 𝛼-1,4- glycosidic bonds of sucrose to produce equimolar mixture of monosaccharide D-glucose and D-fructose [14,15]. When subjected with sucrose containing substrates, these enzymes can be utilized for the production of plasticizing agents, fructooligosaccharides (FOS) and artificial honey [16]. On the other hand, fructosyltransferase (FTase, EC 2.4.1.9 and EC 2.4.1.10), which are grouped in family 68 of Glycoside Hydrolyzing enzymes (www.cazy.org), not only hydrolyze sucrose molecule to yield fructose and glucose but also transfer fructosyl group to sucrose accepter and produce oligosaccharides/fructans. The fructans synthesized from sucrose contain a terminal glucose molecule [17] and are designated as GFn, where G refers to the terminal glucose unit, F refers to fructose units and n is the number of fructosyl units per fructan molecule, also designated degree of polymerization [DP]. The products obtained by FTase enzymes have high biotechnological potential and have large number of applications in food and pharmaceutical industry [18].
FTase can be produced by different plants and microorganisms [15,19]. Because of easy handling, low cost and production of large quantity of enzyme, microbial FTase are preferred over plant FTase for prebiotics synthesis [20]. Therefore, fructosyltransferases have been isolated from different fungal and bacterial strains for commercial production of fructooligosaccharides [FOS] [21- 25]. The type of FOS produced by different fructosyl transferases depend of the origin of the enzyme. Different enzymes are specific for the types of linkages that are synthesized in their respective FOS and polymer products.
Challenge in Prebiotic and Probiotic Relationship
Every FOS produced by plants, microorganisms or in the laboratory cannot be prebiotic. FOS should have certain properties to declare as prebiotic like
1. They should be resistant to stomach acidic pH and nondigestible by intestinal enzymes,
2. They must be hydrolyzed by gut microorganisms [probiotics],

Figure 3: Health benefits of fructooligosaccharides (FOS)/prebiotics: prebiotics can not only be beneficial in gut disorders but also effective against common health problem, like obesity and diabetes, in young generation.
3. They should have positive health impact on host. Health benefits of prebiotics have been investigated and reviewed y several scientists around the globe [26-29] (Figure 3) Most of the prebiotics take part in health benefits by enhancing the growth of microbial species present in the gut. The normal human gut microflora is very diverse and dominated by anaerobic bacterial species like Fusobacterium spp., Bacteroides spp., Clostridium spp, additionally, biofido bacterium spp. and lactobacillus spp. [30]. All these microorganisms are important for healthy gut. However, gut microflora could be altered by changing life style and eating habits. Change in food from healthy, fiber rich [fruits and vegetables] to junk is one of the major reasons of alteration in gut microflora, which results in number of diseases in young age.
Diversity of gut microflora can be maintained by two ways; 1. By taking beneficial bacteria with food as probiotics however, all gut bacterial species are not culturable in the lab. 2. by continuous supply of variety of fiber [prebiotics] to enhance the growth of already existing bacterial species in the gut. Prebiotics produced from different plant, and bacterial sources are being added in yoghurts, juices and even baby formulas by many food companies these days [31]. Nevertheless, the gastrointestinal microflora consists of approximately 1014 colony forming units [cfu]/g of various types of both harmful and beneficial bacteria. It is not clear, which fructans are the most suitable substrates for the selective growth of specific beneficial species or strains. Therefore, there is huge need of understanding the relationship between prebiotics and probiotics to maintain the diversity of beneficial microbial species in the gut to get maximum health benefits from these microorganisms. Moreover, it is direly needed to explore diverse range of living systems to acquire variety of unique prebiotic compounds.
Novel Prebiotics
It has been observed that one type of fiber helps in growth of few microorganisms, which are not enough to maintain the diversity of gut microflora. Scientists are continuously looking for novel fructosyl transferases from plant and microbial species to produce variety of prebiotics for commercial applications. However, production of novel prebiotics which, could support the growth of large number of gut microbial species is need of the time. To this end we suggest to explore the fructosyltransferases from third domain of life (archaea). Several archaeal strains are annotated to harbourfructan synthesis genes in their genome sequences (www.cazy.org). So far, a few studies have been conducted to determine the role of archaeal or their enzymes in the synthesis of novel prebiotic compounds. These include a levansucrase from a halophilic microorganism Halomonassmyrnensis produced novel saccharides from raffinose, cellobiose, lactose and L-arabinose substrates [32]. In the other study, the GH68 genes from Halorubrumsaccharovorum and Natronococcusamylolyticus were expressed in the heterologous host E.coli, and the two recombinant enzymes displayed levan-forming activity [33]. Our research group is also involved in characterizing archaeal FTase genes that may code for novel prebiotic synthesizing enzymes. Thus, in addition to other sources of fructosyltransferases, archaea may also serve a source of novel fructosyltransferase enzymes for the production of unique prebiotics for nutraceutical market.
Conclusion
Prebiotics, also known as non-digestible dietary fiber, are vital for the nourishment of gut microbial species (probiotics) and can be obtained from fiber rich food. However, because of elimination of natural fiberfrom food, by frequent use of junk and processed food, gut microflora has been altered. Abolition of specific microbial species from gut thought to be a potent cause of several diseases in early age. Therefore, addition of prebiotics in food has been emerged as an influential way to maintain growth of gut microflora to contest current health challenges in young generation. All research efforts in the field so far directed at the production of novel oligosaccharides to enhance the growth of diverse population of gut microflora. However, an improved understanding of relation between prebiotics and probiotics is required for the synthesis of better oligosaccharides with varied functional properties. Moreover, sincere research efforts are recommended to explore the usefulness of archaeal fructosyltransferase enzymes for the production of novel multifunctional prebiotics supplements for goodhuman health.
Acknowledgement
Author would like to acknowledge United Arab Emirates University Research Council for supporting her prebiotics research activities in the laboratory.
Conflict of Interest
Authors declare there is no conflict of interests exists.
References
- Bitzios M, Fraser I, Haddock Fraser J (2011) Functional ingredients and food choice: Results from a dual-mode study employing means-end-chain analysis and a choice experiment. Food Policy 36(5): 715-725.
- Radke M, Picaud JC, Loui A, Cambonie G, Faas D, et al. (2017) Starter formula enriched in prebiotics and probiotics ensures normal growth of infants and promotes gut health: a randomized clinical trial. Pediatr Res 81(4): 622-631.
- Quigley EMM (2019) Prebiotics and Probiotics in Digestive Health. Clin Gastroenterol Hepatol. 17(2): 333-344.
- Slavin J (2013) Fiber and prebiotics: mechanisms and health benefits. Nutrients 5 (4): 1417–1435.
- Gibson RG, Hutkins R, Sanders EM, Prescott LS, Reimer AR, et al. (2017) The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nature. 14: 491-502.
- Guinane MC, Cotter DP (2013) Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. TherapAdv in Gastro 6(4): 295-308.
- Sekirov I, Russell LS, Antunes MCL, Finlay BB (2010) Gut microbiota in health and disease. Physiol Rev 90(3): 859–904.
- Lomax AR, Calder PC (2009) Prebiotics, immune function, infection and inflammation: a review of the evidence. British J Nutrition 101(5): 633-658.
- Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, et al. (2010) Prebiotic effects: metabolic and health benefits. British Journal of Nutrition 104: S1-S63.
- Sangwan V, Tomar SK, Singh RRB, Singh AK, Ali B (2011) Galactooligosaccharides: novel components of designer foods. J Food Science 76: R103-R111.
- Prapulla S, Subhaprada V, Karanth N (2000) Microbial production of oligosaccharides: A review. Adv Appl Microbiol 47: 299–343.
- Khandekar DC, Palai T, Agarwal A, Bhattacharya PK, (2014) Kinetics of sucrose conversion to fructo-oligosaccharides using enzyme (invertase) under free condition. Bioprocess and Biosystems Engineering 37 (12): 2529-2537.
- Chien CS, Lee WC, Lin TJ (2001) Immobilization of Aspergillusjaponicus by entrapping cells in gluten for production of fructooligosaccharides. Enzyme and microbial technology 29(4-5): 252-257.
- vanHijum SAFT, Kralj S, Ozimek LK, Dijkhuizen L, Ineke GH, et al (2006) Structure-function relationships of glucansucrase and fructansucrase enzymes from lactic acid bacteria. Microbiol Mol Biol Rev 70(1): 157-176.
- Lammens W, Le Roy K, Schroeven L, Van Laere A, Rabijns A, et al (2009) Structural insights into glycoside hydrolase family 32 and 68enzymes: functional implications. J of Experimental Botany 60(3): 727-740.
- Akardere E, Ozer B,Celem EB, Onal S (2010) Three-phase partitioning of invertase from Baker’s yeast. Separation and Purification Technology. 72(3): 335-339.
- French AD, Waterhouse AL (1993) Chemical structure and characteristics of fructans, in Science and Technology of Fructans, Suzuki M, Chatterton NJ, Eds. CRC Press Boca Raton FL, pp. 41-81.
- Ganaie MA, Gupta US, Kango N (2013) Screening of biocatalysts for transformation of sucrose to fructooligosaccharides. J Molecular Catalysis B Enzymatic 97: 12–17.
- Mussatto SI, Aguilar CN, Rodrigues LR, Teixeira JA (2009) Colonization of Aspergillusjaponicus on synthetic material and application to the production of fructooligosaccharides. Carbohydrates Research 344: 795-800.
- Maiorano AE, Piccoli RM, Da Silva ES, De Andrade Rodrigues MF (2008) Microbial production of fructosyltransferase for synthesis of prebiotics. Biotech Lett. 30: 1867-1877.
- Kralj S, Leeflang C, Sierra EI, Kempiński B, Alkan V, et al. (2018) Synthesis of fructooligosaccharides (FosA) and inulin (InuO) by GH68 fructosyltransferases from Bacillus agaradhaerens strain WDG185. Carbohydrate polymers 179: 350-359.
- Dominguez AL, Rodrigues LR, Lima NM, Teixeira JA (2013) An overview of the recent developments on fructooligosaccharide production and applications. Food Biopro Tech 7(2): 324–337.
- Singh SP, Jadaun JS, Narnoliya LK, Pandey A (2017) Prebiotic oligosaccharides: special focus on fructooligo-saccharides, its biosynthesis and bioactivity. Appl Biochem Biotechnol. 183(2): 613–635.
- Zhang J, Liu C, Xie Y, Li N, Ning Z, et al. (2017) Enhancing fructooligosaccharides production by genetic improvement of the industrial fungus Aspergillusniger ATCC 20611. J Bio tecnol 249: 25-33.
- Castillo BG, Díez-Municio M, de la Cruz JC, Moreno J (2017) Obtaining mutant fungal strains of Aspergillusniger with high production of fructooligosaccharides (FOS) using ultraviolet light irradiation. African J Biotechnology 16(35): 1810-1818.
- Mussatto SI, Teixeira JA (2010) Increase in the fructooligosaccharides yield and productivity by solid-state fermentation with Aspergillusjaponicus using agro-industrial residues as support and nutrient source. Biochemical Engineering J 53: 154-157.
- Charalampopoulos D, Rastall RA (2012) Prebiotics in foods. Curr Opin Biotechnol 23(2): 187-191.
- Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, et al. (2019) Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 8(3): 92-119.
- Dobrange E, Peshev D, LoedolffB, Van den Ende W (2019) Fructans as Immunomodulatory and Antiviral Agents: The Case of Echinacea. Biomolecules 9(10): 615-627.
- Rastall RA (2004) Bacteria in the gut: friends and foes and how to alter the balance. J Nutr 134: 2022S–2026S.
- Davis CD, Milner JA (2009) Gastrointestinal microflora, food components and colon cancer prevention. J Nutr Biochem 20(10): 743-752.
- Kirtel O, Menéndez C, Versluys M, Van den Ende W, Hernández L, et al. (2018) Levansucrase from Halomonassmyrnensis AAD6T: first halophilic GH-J clan enzyme recombinantly expressed, purified, and characterized. Appl Microbiol Biotechnol 102 (21): 9207–9220.
- Hill A, Chen L, Mariage A, Petit JL, de Berardinis V, et al. (2019). Discovery of new levansucrase enzymes with interesting properties and improved catalytic activity to produce levan and fructooligosaccharides. Cat Sci Tech 9(11): 2931-2944.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.