Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Justifying the Antidiabetic Ethnomedicinal Claim of Massularia acuminata Through Its Antihyperglycaemic Activity
*Corresponding author: Marcus Durojaye Ayoola, Department of Pharmacognosy, Obafemi Awolowo University, Ile-Ife, Nigeria.
Received: February 29, 2020; Published: March 13, 2020
DOI: 10.34297/AJBSR.2020.08.001245
Abstract
Diabetes mellitus is becoming the third ‘killer’ of mankind, after cancer, cardiovascular and cerebrovascular diseases, because of its high prevalence, morbidity and mortality. The antihyperglycaemic activity of the leaf of Massularia acuminata that is used ethno medicinally in Nigeria in the management of diabetes mellitus was investigated is this study to justify its antidiabetic folkloric claim. Ethanol leaf extract of the plant at 100,200 and 400mg/kg was investigated for its antihyperglycaemic effect in glucose-induced hyperglycaemic rats using glibenclamide (5mg/kg) and 1% Tween 80 in normal saline as positive and negative controls, respectively. Anti-hyperglycaemic activity-directed purification of the ethanol leaf extract of the plant in glucose-loaded rats, led to the isolation and characterisation of acuminatoside by comparing its spectral data with those in the literature.
The results showed that the median lethal dose, LD50 of the ethanol leaf extract of M. acuminata was greater than 5000mg/kg while its 400mg/ kg was the most active dose with comparable activity (p>0.05) to the standard drug. The ethylacetate partition fraction was the most active fraction among the fractions from which acuminatoside was isolated. The isolate at 10mg/kg elicited a comparable (p>0.05) activity to glibenclamide (5mg/ kg) and was significantly more active than the 20mg/kg dose. The results of this study justified the folkloric antidiabetic use of Massularia acuminata leaf and showed acuminatoside as one of its antihyperglycaemic constituents.
Keywords:Diabetes mellitus; Massularia acuminata; Anti-hyperglycaemic activity; Column chromatography; Acuminatoside; Glucometer; Toxicity signs; Geometric; Glibenclamide
Introduction
Humans have continually depended on plants for their basic needs including food, clothing, shelter and medicine for ages [1] . Many drugs of importance of today such as, digoxin, tubocurarine, vincristine, vinblastine, reserpine, artemisinin, etc were obtained from plants [2] and the use of these drugs in orthodox medicine is related to their use in ethnomedicine [3] . Plants represent a largely untapped reservoir of molecules with diverse chemical structures and a unique source of potentially new drug leads [4-6]. Massularia acuminata commonly called “Pako Ijebu” in South Western Nige riais known to be widespread from Guinea to Western Cameroun and extending into Congo [7,8]. The plant has been implicated in Nigerian ethnomedicine for the management of mouth infections, rheumatism, pain, cancer and diabetes [9,10].
Its leaf extracts and ethylacetate fraction have been reported for antioxidant, antimicrobial, anticancer [11-14] and androgenic potential as well as aphrodisiac activities [15] . Thiophenolic glycoside and oleanolic acid glycoside have been reportedly isolated from the plant [12,14]. The antioxidant and hypoglycaemic activities of the stem of M. acuminata have been reported [16] , but the antihyperglycaemic activity of the leaf has not been established; hence, this study.
Materials and Methods
Chemicals, Equipment and Instrumentation
CareSensTMN Glucometer (model PGA 1E3028 REV3, i-SENS, Inc., Korea) with CareSensTM test strips (i-SENS, Inc., Korea), column chromatographic (dimension: 60×4cm, silica gel mesh 70-230) apparatuses were used. Others were aluminium plated thin-layer chromatographic (silica gel 60F254, 0.25 mm) and glass plated preparative thin-layer chromatographic (silica gel 60F254, 0.25, 0.5, 1, 2mm, Whatman Inc., U.S.A.), silica gel (70-230 mesh, Merck & Co., Inc., U.S.A.). Nuclear magnetic resonance (NMR) spectra (400MHz) were obtained with Bruker AMX400 instruments. All solvents used were of analytical grade.
Animals
Male and female healthy Wistar albino rats with the average weight of 170g were used for the experiments. They were bred under standard conditions (temp. 27±3°C, relative humidity 65%, natural 12h day-night) and housed in different cages in the animal house, Department of Pharmacology, Faculty of Pharmacy, O.A.U., Ile-Ife, Nigeria. They were acclimatized for at least 5 days before commencement of the experiments and fed on a standard pellet diet (Bendel Feeds, Benin, Nigeria). The rats had access to water freely throughout the experiment. They were divided into groups of five rats each and fasted for 24h before administration of either glucose, extract, fractions, isolate, drugs or vehicle [17,18]. The Guide for the Care and Use of Laboratory Animals published by the National Academies Press [19] was strictly adhered to in carrying out all the animal experiments in this study.
Plant Material, Extraction and Solvent Partitioning of the Extract
The leaf of M. acuminata was collected at the ‘‘Shasha’’ Forest Reserve, Area J4, Osun State, Nigeria and authenticated at the Ife Herbarium, Department of Botany, Faculty of Science, Obafemi Awolowo University, Ile-Ife, Nigeria, with Voucher number; IFE16337. It was air dried, powdered and 1.0kg of the powered leaf was exhaustively extracted with 80% ethanol by maceration, filtered and concentrated using rotary evaporator which gave 12.0% w/w yield and the extract obtained was coded, MAE (155.4g).The ethanol leaf extract (150g) was suspended in 300mL of distilled water and partitioned with n-hexane (3x400mL), ethylacetate (4x400mL) and n-butanol (2x250mL) to obtain their corresponding n-hexane (B1, 7.7g), EtOAc (B2, 18.9g), n-BuOH (B3, 75.7g) and aqueous (B4, 38.8g) fractions.
Isolation of Compound and Structure Elucidation
The EtOAc fraction (B2) was purified using silica gel open column chromatography. A 17.0g of B2 was adsorbed unto 17.0g of Silica gel (200-400 Mesh) and eluted on 170g Silica gel column, using binary solvent systems of increasing polarity from Hex-EtOAc (9:1-1:9), and EtOAc-MeOH (95:5-1:1). The eluates were bulked into six sub-fractions, C1-C6 based on their TLC analysis. Sub-fraction C5(4.5g) was eluted with a solvent system on a Sephadex LH- 20 column, using DCM-MeOH (8:2). The eluates were analysed on TLC and bulked into three sub-fractions C5i-C5iii. Sub-fraction C5iii(400mg) was chromatographed on a Silica gel column, using DCM-MeOH (4:1; 1:1; and 1:4), to isolate a brown semi-solid compound coded, D(30.8mg).
Characterization of Acuminatoside
1H-NMR
(CD3OD)δ: 2.30 (2H, bd, H-2´), 3.20 (1H, m, H-1´), 4.60 (1H, d, J=9.0 Hz, H-3´), 6.60 (1H, dd, H-2, H-6), 7.50 (1H, dd, H-3, H-5).
Sugar Moiety: 4.20 (1H, m, 2-H, 3-H, 4-H, 5-H, 6-H of glucose), 5.60 (1H, d, J=6.0, 1-H of glucose)
13C-NMR
(CD3OD)δ-of aglycone: 35.00 (C-1′), 39.70 (C-2′), 96.40 (C-3′), 115.40 (C-2, C-6), 131.90 (C-3, C-5), 142.10 (C-4), 154.60 (C-1).
Sugar Moiety (Glucose): 62.75 (C-6′′), 71.20 (C-4′′), 74.90 (C- 3′′), 77.45 (C-2′′), 78.10 (C-5′′), 100.15 (C-1′′).
The spectra data of the isolated compound was compared with those reported in literature and was characterized as 4-(3’,3’-dihydroxy- 1-mercaptopropyl) phenyl glucosylpyranoside, known as acuminatoside [12,14] (Figure 1).
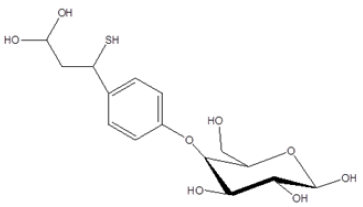
Figure 1: 4-(3′,3′-dihydroxy-1-mercaptopropyl) phenyl glucosylpyranoside isolated from the Ethylacetate fraction of M. acuminata leaf.
Acute Toxicity Study of the Extract
The median lethal dose, LD50 of Massularia acuminata ethanol leaf was determined in rats by using the 1983 Lorke’s method [20]. Rats of average weight of 150g were fasted for 18h after which the extract at dose range of 10-5000mg/kg were given orally. The experiment was carried out in two phases. The first phase constituted of nine (9) rats that were divided into 3 groups of 3 ra each to which three geometrically increasing doses of 10, 100 and 1000mg/kg of the extract were administered. Each group of the animals were observed for death and or toxicity signs for more than at least 24-hours. The second phase of the toxicity study was carried out with eight (8) rats that were divided into 4 groups of 2 rats each. Four doses of the extract, 1000, 1600, 2900 and 5000mg/kg were administered to each group of rats, respectively. The animals were also observed for death and other signs of toxicity for 24 hours. The LD50 was calculated as the geometric mean of the dose that resulted in 100% lethality and that which caused no lethality at all [21].
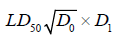
Where, D₀=highest dose that gave no mortality: D1=lowest dose that produced mortality
Antihyperglycaemic Assay of Extract, Fractions and Isolates
Set of normoglycemic rats were fasted for 24h and orally given 10g/kg of glucose and those with blood glucose levels ≥7.0mMol/L or 126mg/dL after 0.5h (T0) were considered hyperglycaemic. They were selected and divided into groups of five and administered with extract at 100,200,400mg/kg, or 1% Tween 80 in normal saline (negative control), or glibenclamide, (5mg/kg, positive control). The tip of the tail of each rat was pricked with lancet and a drop of blood was obtained and dropped onto a glucometer strip and the blood glucose level was read off directly from the glucometer display. The blood glucose levels of the rats were determined and recorded at 0.0, 0.5, 1.0, 2.0, 4.0h after administration of the extract and drug. The blood glucose levels at 0.0h (T0) were taken as 100%, while those at other times were expressed as percentages of these values [21-23]. Also, glucose lowering effects of the partitioned, bulked column fractions and isolate were similarly determined.
Statistical Analysis
The data obtained from the study were expressed as the mean ± SEM for the number (n=5) of animals in the groups. One Way Analysis of Variance (ANOVA) was used followed by Bonferroni t-test or Student Newman-Keuls post hoc tests to determine the source of significant differences. P<0.05 was statistically significant.
Results and Discussion
Glibenclamide, a sulphonyl urea, has been established to elicit its antihyperglycaemic effect through early extra-pancreatic and late insulin stimulation [24,25]. Using glucose-induced hyperglycaemic rats’ model, activity profile of plant extracts/fractions/isolates like that of glibenclamide or other insulin stimulatory drugs as standards could be ascribed to extra-pancreatic and insulin stimulating mechanisms of action [21-23]
Safety Profile of M. acuminata
In the acute toxicity experiment, no death nor any serious changes in behaviours of the rats related to breathing, cutaneous effect, sensory and nervous system responses or gastrointestinal effects were observed when 5000mg/kg of M. acuminata ethanol leaf extract was administered to the rats. This result confirmed the safety of the extract as well as the doses of the extract used in this work. After the establishment of the safety of M. acuminata ethanol leaf extract, it was further investigated for antihyperglycaemic activity in order to justify or otherwise its folkloric antidiabetic usage.
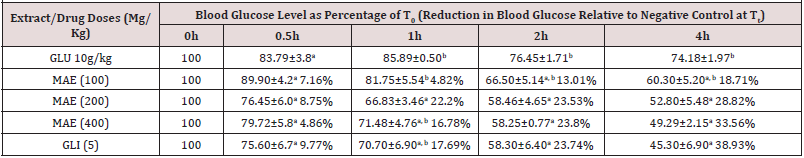
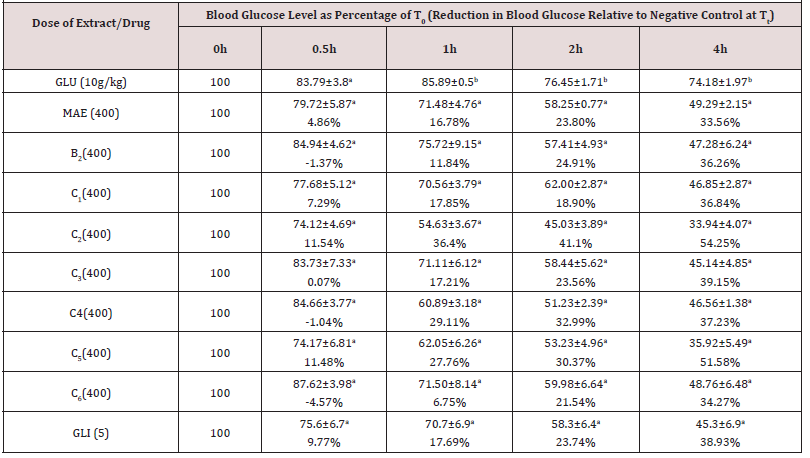
Antihyperglycaemic Effects of The Extract of M. acuminata (Table 1)
Data show the mean ± SEM blood glucose levels at the different time points expressed as percentages of levels at 0h (T0), n=5. Values in parentheses represent the percentage reductions in blood glucose levels relative to negative control for each time point. Values with different superscripts within columns are significantly different (p< 0.05). GLU (10g/kg): Glucose in < 1% of Tween 80 in normal saline administered at 10g/kg; MAE: Massularia acuminata Extract; GLI (5mg/kg): Glibenclamide (5mg/kg).
There was a significant time dependent reduction in blood glucose levels up to the fourth hour (Table 1) of glucose-induced hyperglycaemic rats administered with normal saline (negative control) that was due to the homeostatic regulatory mechanism in the normal animals [26]. The reductions explained the healthy state of pancreases of the experimental rats. The M. acuminata extract at 100mg/kg demonstrated a non-time dependent anti-hyperglycaemic activity that was comparable (p>0.05) to the negative control at 0.5-4h while its 200 and 400mg/kg gave a comparable and time dependent effect (Table 1). An activity profile of the extract at 200 and 400kg/kg that was similar and comparable (p>0.05) to that of glibenclamide (Table 1), may indicate similar stimulation of insulin release as its main mechanism of action [21-24]. Furthermore, based on similar profile of anti-hyperglycaemic activity as glibenclamide, extracts of Carica papaya, Xylopia aethiopica, Parquetina nigrescence and Terminalia superba [22,23,27,28] have been reported to have insulin stimulation as their main mechanism of action.
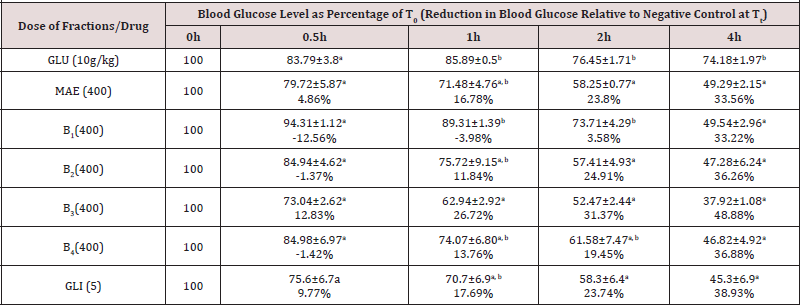
Antihyperglycaemic Effects of the Partition Fractions of M. acuminata (Table 2)
Data show the mean ± SEM blood glucose levels at the different time points expressed as percentages of levels at 0h (T0), n=5. Values in parentheses represent the percentage reductions in blood glucose levels relative to negative control for each time point. Values with different superscripts within columns are significantly different (p< 0.05). GLU (10g/kg): Glucose in < 1% of Tween 80 in normal saline administered at 10g/kg (hyperglycaemic negative control); MAE: Massularia acuminata Extract; GLI (5mg/kg): Glibenclamide (5mg/kg); B1=n-Hexane fraction; B2=Ethylacetate fraction; B3=Butanol fraction and B4=Aqueous fraction.
The most active antihyperglycaemic dose of the extract (400mg/kg) with 34% blood glucose level reduction at 4h (Table 1) was used in testing this effect in its partitioned fractions. Generally, all the partitioned fractions (B1-B4) of the leaf extract and glibenlamide (5mg/kg) demonstrated a comparable activity (p>0.05) at 4h indicating stimulation of insulin release as their major mechanism of action (Table 2).The n-hexane fraction (B1) was devoid of activity at 0.5-2h indicating lack of extra pancreatic effect while B2, B3 and B4 gave antihyperglycaemic activity at 0.5-4h that was comparable to glibenclamide (5mg/kg) at all-time points showing both extra pancreatic and insulin stimulation mechanisms of action [24]. The 27 and 31% glucose level reduction of B3 at 1-2h indicated higher extra pancreatic effect of this fraction. None of the partitioned fractions had better antihyperglycaemic activity than the extract suggesting that the antihyperglycaemic constituents of the extract were working in synergism (Table 2).
Antihyperglycaemic Effects of the Column Fractions of M. acuminata (Table 3)
Data show the mean ± SEM blood glucose levels at the different time points expressed as percentages of levels at 0h (T0), n=5. Values in parentheses represent the percentage reductions in blood glucose levels relative to negative control for each time point. Values with different superscripts within columns are significantly different (p<0.05). GLU (10g/kg): Glucose in <1% of Tween 80 in normal saline; MAE: M. acuminata Extract; B2: Ethylacetate partition fraction of M. acuminata; C1-C6: Bulked column fractions of M. acuminata; GLI (5mg/kg): Glibenclamide (5mg/kg).
The ethylacetate fraction, B2 with comparable (p>0.05) and similar profile of antihyperglycaemic activity with glibenclamide (5mg/kg) (Table 2) and fewer number of spots on TLC plate than other fractions was chosen for further purification. Like B2, all the bulked column fractions (C1-C6) elicited comparable blood glucose level reduction effect with the standard drug indicating similar mechanism of action [26]. Furthermore, comparable activity of C1-C6 with B2 and the extract further confirmed synergism of the antihyperglycaemic constituents of the plant. Similar synergistic activity of antihyperglycaemic constituents has been reported for Eugenia uniflora [17] . Sub fractions C2 and C5 with 12, 36, 41 and 12, 28 and 30% blood glucose levels reduction activity at 0.5-2h, respectively indicated additional extra pancreatic effect of these sub fractions while the 54 and 52% activity of C2 and C5, respectively at 4h showed them as the most promising insulinotropic sub fractions. Therefore, C5 with good weight and fewer TLC spots was chosen for further purification in order to isolate its insulin stimulating constituent(s).
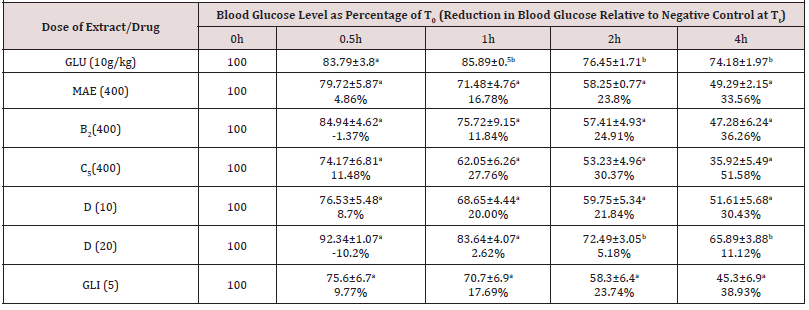
Antihyperglycaemic Effects of the Isolated Compound from M. acuminata (Table 4)
Data show the mean ± SEM blood glucose levels at the different time points expressed as percentages of levels at 0h (T0), n=5. Values in parentheses represent the percentage reductions in blood glucose levels relative to negative control for each time point. Values with different superscripts within columns are significantly different (p<0.05). GLU (10g/kg): Glucose in <1% of Tween 80 in normal saline; MAE: M. acuminata Extract; B2: Ethyl acetate partition fraction of M. acuminata; C5: Bulked column fraction of M. acuminata; D: acuminatoside from M. acuminata; GLI: Glibenclamide (5mg/kg).
The antihyperglycaemic activity demonstrated by 10mg/kg of the isolated acuminatoside, D was comparable with those of the standard drug (glibenclamide, 5mg/kg), 400mg/kg of the extract, B2 and C5 (Table 4). This therefore identified acuminatoside as one of the antihyperglycaemic constituents of M. acuminata. However, its 20mg/kg dose gave a significantly lower antihyperglycaemic activity than the 10mg/kg dose and those of glibenclamide and the fraction as well as the ethanol extract indicating that the activity of acuminatoside is not dose dependent (Table 4). The similar profile of antihyperglycaemic effect of acuminatoside to that of glibenclamide indicated that it had minor extra pancreatic and major insulin.
Conclusion
Lack of death and other signs of toxicity in the animals at high dose of the ethanol leaf extract of Massularia acuminata in this study confirmed its safety. Also, the antihyperglycaemic activities of the extract, partitioned fractions and the isolated acuminatoside at low doses that were comparable to glibenclamide, justified its antidiabetic ethno-medicinal claims.
Acknowledgements
The authors appreciated Mr. T. Oladele of the Department of Pharmacognosy, Faculty of Pharmacy, Obafemi Awolowo University for his assistance in collecting the plant. Also, the effort of Dr Frank Schweizer of Department of Chemistry, University of Manitoba, Canada in providing the NMR spectra was highly appreciated.
References
- Hostettman K, Marston A (2002) Twenty years of research into medicinal plants: Results and perspectives. Phytochemistry Review 1: 275-285.
- Trease GE, Evan WC (2002) Pharmacognosy. 15th (edn), Edinburgh, London, UK p.1-2.
- Sofowora A (2008) Medicinal Plants and Traditional Medicine in Africa, 3rd (edn), Spectrum Books Ltd, Ibadan, Nigeria pp.117-133.
- Amin A, Gali Muhtasib H, Ocker M, Schneider SR (2009) Overview of major classes of plant-derived anticancer drugs. Int J Biom Sci 5(1): 1-11.
- Pandey KB and Rizvi SI (2009) Plant polyphenols as dietary antioxidants in human health and diseases. Oxid Med Cell Longev 2(5): 270-278.
- Prakash O, Kumar A, Kumar P, Ajit (2013) Anticancer potential of plants and natural products: A review. Amer J Pharm Sci 1(6): 104-115.
- Burkill HM (1997) Useful Plants of West Tropical Africa. Royal Botanic Garden, UK 4: 969.
- Kenfack D (2010) Vascular Plants of Korup National Park: Conservation, Description, Ecology and Distribution of Massularia acuminata (G. Don) Bullock ex Hoyle. Ann Missouri Bot Gard 3(1): 64-65.
- Gill LS (1992) Ethnomedical Uses of Plants in Nigeria. UniBenin Press, Nigeria pp. 209.
- Ajibesin KK, Ekpo BA, Bala DN, Essien EE, Adesanya SA (2008) Ethnobotanical survey of AkwaIbom State of Nigeria. Journal of Ethnopharmacology 115: 387-408.
- Aladesanmi AJ, Iwalewa EO, Akinkunmi EO, Adebajo AC, Taiwo BJ, et al. (2006) Antimicrobial and Antioxidant Activities of some Nigerian Medicinal Plants. Afr J Trad Comp Alter Medi 4(2): 173-184.
- Oriola AO, Aladesanmi AJ, Idowu TO, Akinkunmi EO, Obuotor EM, et al. (2014) A New Bioactive Thiophenolic Glycoside from the Leaf of Massularia acuminata (G. Don Bullock) Ex Hoyle (Rubiaceae). Afr J Tradit Complemen Alter Medi 11(2): 319-323.
- Aladesanmi AJ, Oriola AO, Arthur G, Schweizer F (2016) A New Anticancer Oleanolic acid Glycoside from the Leaf of Massularia acuminata G. Don (Bullock) ex Hoyle. Patent.
- Oriola AO, Aladesanmi AJ, Arthur G (2016) Acuminatoside: A New Anticancer Compound from the Maiden Breast Plant. Nigerian J Natu Prod Medi 20: 116-120.
- Yakubu MT, Akanji MA, Oladiji AT, Adesokan AA (2008) Androgenic potentials of aqueous extract of Massularia acuminata (G. Don) Bullock ex Hoyle. Stem in male Wistar rats. J Ethnopharmacol 118(3): 508-513.
- Ullmann AJ, Aguado JM, Arikan Akdagli S, Denning DW, Groll AH, et al. (2018) Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS Guideline. Clini Microbiol Infec 24: 1-38.
- Adebajo AC, Ayoola MD, Obagbemi OR, Obuotor EM, Ogunsina MO, et al. (2013) Antihyperglycaemic and antioxidant activities of Eugenia uniflora leaf: evaluation of ethnomedical claims IV. Ife Journal of Science Technology 1: 1-18.
- Adebajo AC, Ayoola MD, Odediran SA, Aladesanmi AJ, Schmidt TJ, et al. (2013) Evaluation of ethnomedical claims III: anti-hyperglycaemic activities of Gongronema latifolium root and stem. J Diabetes 5(3): 336-43.
- Committee for the update of the guide for the care and use of laboratory animals Institute for laboratory animal research, division on earth and life studies, national research council of the national academies (2011) Guide for the Care and Use of Laboratory Animals. 8th (edn), National Academies Press, Washington DC, USA.
- Lorke D (1983) A new approach to practical acute toxicity testing. Arch Toxicol 54(4): 275-287.
- Ayoola MD, Adebajo AC, Obuotor EM, Oladapo TO, Fleischer TC (2017) Anti-hyperglycaemic and anti-oxidant activities of five Nigerian antidiabetic plants. J Sci Technol KNUST 37(2): 71-84.
- Akinwunmi KF, Ayoola MD (2018) Antihyperglycaemic, Anti-inflammatory and Antioxidant activities of Carica papaya and Citrus lanatus seeds. Ife Journal of Science 20(2): 207-217.
- Famuyiwa FG, Ayoola MD, Famuyiwa SO, Aladesanmi AJ (2018) Hyperglycaemia Lowering Effect of Kaurane Diterpenoids from the Fruits of Xylopia aethiopica (A. Dunal) Rich. Inter J Medi Plants Natur Prod 4(3): 11-19.
- Luzi L, Pozza G (1997) Glibenclamide: an old drug with a novel mechanism of action? Acta Diabetologica 34(4): 239-244.
- Murray RK, Granner DK, Rodwell VW (2006) Harper’s Illustrated Biochemistry. 27th (edn), Internat Edition McGraw-Hill Education (Asia), Singapore pp.172-175.
- Kar A, Choudhary BK, Bandyopadhyay NG (1999) Preliminary studies on the inorganic constituents of some indigenous hypoglycaemic herbs on oral glucose tolerance test. J Ethnopharmacol 64(2): 179-184.
- Faloye KO, Ayoola MD, Amos Tautau BM, Famuyiwa SO (2018) Antidiabetic activity of Convallatoxin isolated from the Root bark of Parquetina nigrescens (Afzel.) Bullock Asclepiadaceae. Euro Journal Medic Plant 25(4): 1-9.
- Oluwarotimi CD, Ayoola MD, Olayiwola G, Famuyiwa SO (2019) Methyl gallate from the antihyperglycaemic fraction of the root bark extract of Terminalia superba. Inter J Plant Studies 2(1): 1-6.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.