Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Laboratory Mice Euthanasia: Speed Death and Animal Welfare
*Corresponding author: Gabriel Melo de Oliveira, Laboratório de Biologia Celular/Instituto Oswaldo Cruz (IOC/FIOCRUZ), Rio de Janeiro – RJ, Brasil.
Received: April 04, 2020; Published: April 21, 2020
DOI: 10.34297/AJBSR.2020.08.001300
Abstract
The euthanasia is one of the most studied, debated and controversial topics in the laboratory animal science area. All over the world, the ideal way to perform euthanasia in laboratory animals is sought, seeking, in summary, three main points, animal welfare during the procedure; minimal influence on the animals biological system, not to interfere with test results and accessibility and low cost. In Brazil, after the validity of Law No 11.794-2008, that regulated the use of animals for didactic and scientific purposes, structuring the Animal Experimentation Control Council (CONCEA). In its resolutions, CONCEA standardized the application of accepted euthanasia methods, mainly overdose of injectable anesthetics and restricted use of carbon dioxide (CO2). The aim of our study was to evaluate within the rules of CONCEA and using non-invasive and invasive parameters which would be the best euthanasia promoter in adult Swiss Webster male mice. We compared the use of CO2 (suggested a substitution in the in sufflation valve) Isoflurane saturation, a combination of induction in an environment saturated with isoflurane and progressive CO2 in sufflation and application of the association Ketamin and Xilazin, in overdose. Our results, clearly, demonstrate that the most humane method of inducing the death of the animal is that which is the fastest (Speed Death); does not promote pain, discomfort or distress in the animal; minimally influences the biological systems of the animals and has a low acquisition cost. From our results it was possible to suggest that the combination of isoflurane (5%) and CO2 (1 L/min) is the most efficient methodology for performing euthanasia in mice. Because it is extremely fast, mice does not show discomfort, does not influence the biological systems of the animal after death (except the Central Nervous System) and has a low cost with relative ease of access. Thus, we believe that with this study, we can demonstrate that through projects, studies and tests we can suggest new and reliable methodologies for the application of the welfare of laboratory animals
Keywords: Euthansia, Welfare, Mouse, Inhalation Agents and Injectable Anesthetics.
Introduction
The laboratory animal science, an important scientific and technological development area in all countries, act, in summary, five sub-areas: Education, bioethics, legislation and biosafety in laboratory animals; Animal welfare and bio-models behavior; Technology for breeding animals in the house facilities; Manipulation and experimentation in biomodels and Building and management of the lab animal house facilities. A difficult and widely debated question is how to euthanize laboratory animals and maintain animal welfare during the respective procedure. Projects, studies and meetings clearly demonstrate the advantages and disadvantages of each methodology or procedure and each substance administered. However, despite all efforts, so far, due to the complexity of the topic, there is still no consensus in the scientific community of the ideal euthanasia method for the humanitarian finalization of the laboratory animals [1-3].
In Brazil, the important milestone in laboratory animal science was reached, after 12 years in the National Congress, by the approval of the Law No 11.794, October 2008 (called Arouca Law) (BRASIL (a), 2008; CONCEA, 2020), regulated by Decree No 6.899 in the 2009 (BRASIL (b), 2008). Through this legal regulation, the National Council for Animal Experimentation Control (CONCEA, in Portuguese) was structured and standardizing of the Ethics Commissions on the Use of Animals (CEUAs) implantation to the each institution. CONCEA normative resolutions (RNs) specifically described in the RN No 37, 15 February, 2018 describes the basic guidelines for performing the euthanasia procedure on animals used for educational and scientific purposes (RN 37 - CONCEA, 2020) [4-8].
The CONCEA guidelines to the Rodentia Orders, considers it recommendable, in use order: i) Barbiturics; ii) Intravenous anesthetics; iii) Inhaled anesthetics; iv) Ketamine and Xylazine overdose. The methods accepted with restriction are: i) Carbon Dioxide; ii) Cervical dislocation; iii) Decapitation; iv) Microwave and v) Fast freezing. In 2016, at the II Euthanasia Meeting (Newcastle)showed that the prevalence among the participants of the euthanasia method more used in mouse lab was: 1) Carbon dioxide and cervical dislocation; 2) Injectable Anesthetics; 3) Inhaled anesthetics and 4) Decapitation [9-20].
Through a detailed biometric survey and bibliographic review in relation of the impact of the number of manuscripts published and indexed site PubMed (National Center for Biotechnology Information, US National Library of Medicine - https://www.ncbi. nlm.nih.gov/pubmed - in January, 2020), showed that mouse as the most biomodel used in biomedical preclinical assays. We found a total of the 1.723,604 published manuscripts. When associated (Boolean Term – AND) terms between Mouse AND Euthanasia, only 756 studies are related to this important subject. Then, the investigation and study about euthanasia mouse lab methodologies and your welfare is restricted to only 0.04% of the published manuscripts [16].
Among the most used methods, carbon dioxide (CO2) is the one that most raises questions, debates and studies when compared with the other methods, 45 manuscripts related to the use of CO2; 20 for cervical dislocation; 10 for the use of injectable anesthetics; 15 for inhaled anesthetics and only 7 when the method is decapitation (National Center for Biotechnology Information, U.S. National Library of Medicine - https://www.ncbi.nlm.nih.gov/pubmed/ - January 2020). According to Boivin et al. [3] despite the greater use and study on CO2, this author considers that an alternative and more efficient method for euthanasia in small rodents in animal house facilities must still be identified [3,14]. The AVMA (American Veterinary Medical Association) Panel on Euthanasia recommends the use of CO2 with 10% to 30% exchange of chamber gas [19]. The Canadian Council for the Care of Laboratory Animals in its guidelines on the euthanasia of animals recommended the use of anesthetics before euthanasia with CO2 (CCAC, 2017). Notwithstanding the detailed review by Boivin et al., demonstrate adverse effects of the use of CO2 in mouse and to disagree scientifically with several methodologies suggested by societies related to laboratory animals, also, by the Scientific Meeting in Newcastle (Hawkins et al. 2006)at two points we come together with the critical analysis of this author: i) It must be considered that all euthanasia procedures currently available can be considered as stressors and promote greater or lesser degree of distress and suffering to animals and ii) Whichever method is used, including CO2, one should try to reduce the time of the euthanasia process as much as possible, thus avoiding the adverse effects of euthanasia methods to a minimum and elevated welfare [3].
Propose to add more data to this controversial subject and in accordance with CONCEA Euthanasia Normative (associated with biometric analysis and the worldwide incidence of most used methodologies) we elaborated this study suggesting new procedure for the Swiss Webster Outbred Stock mice (male and adult) euthanasia. Based on this premise, the objectives of our work were: i) Investigation of the use of inhalation agents in the process of euthanasia of mice based on the calculation and evaluation of flow, noise and saturation of the chamber with different types of injector valves [20-27]; ii) Comparison of the effects and death dynamics through etological, cardiological, neurological, immunological, biochemical and behavioral systems between inhaled and injectable methods [28-31]; iii) The use of the combination of anesthetic induction with Isoflurane and CO2 flow; iv) Financial cost to the each substance used in the respective processes.
Then our main objectives will be to investigate the best methodology to be used in euthanasia procedure, that is, contemplate the respective requirements: a) Fast death dynamics; b) Minimal effects on the animal biological systems; c) Low acquisition cost.
We hope that our results can add effectively to the scientific and technological innovation knowledge laboratory animal science area, specifically, on this very controversial topic, which is euthanasia, but to which everyone is in search of the same purpose, the animal welfare.
Materials and Methods
Animals
Swiss Webster mice Outbred stock, male (weight: 45±2,8 g) and subdivided in 5 individuals per eutanásia methodology in duplicate (ntotal = 70 animals) in according to the reduction present in the 3Rs ethical principle. The experimental procedures were performed in accordance with Brazilian Law 11.794/2008 and the regulations of the National Council of Animal Experimentation Control (CONCEA) [5]. The mice were housed with a maximum of five individuals per group (3 and 2 individual cage) a specific-pathogen-free (SPF) room at 20 to 22ºC under a 12/12 h light/dark cycle with 50 to 60% humidity and provided filtrated water and chow ad libitum. All animal experimental procedures were performed under a license (L-39/2017) approved by the Ethics Committee for Animal Use at the Oswaldo Cruz Institute (CEUA/IOC).
Euthanasia Chamber Elaboration and Calculation of the Flow of Inhalation Agents
Prototype of the Euthanasia Chamber: Made of transparent acrylic with a thickness of
0.5mm, a euthanasia chamber with dimensions of 20 x 20 x 15 cm, making a total area of 7,4 cm3, with a retractable cover and with two specific valves for gas with 3/8”x 1,5 mm silicone hose coupling. The insufflation valves are located on the side and the outlet on the chamber cover (Figure 1).

Figure 1: Study of the flow of insufflation by carbon dioxide in the euthanasia chamber. The validation of the methodologies used came from the design of an euthanasia chamber in which it was possible to carry out all the evaluation parameters. At first we kept the commercial valve for the insufflation of CO2 V (A). Our perception was not satisfactory when using CO2 V, so we developed a system composed of activated carbon, paper filter and metallic protection to minimize odor and noise (B), allowing the use of the euthanasia chamber with CO2 insufflation with the valve modified - CO2 M (C).
Insufflation valves
We initially used the standard insufflation valve for carbon dioxide (CO2 V) (Fig. 1A) and performed the valve modification by applying a flow diffusion and odor retention system composed of: Aluminum cube perforated (used in microisolators racks) associated with a filter paper Ø 18.5 cm and an amount of activated carbon (ac) calculated using the formula: Weightac = Chamber Volume x Insufflation Speed / Insufflation Pressure (Figure 1B) being coupled directly in front valve (CO2 M) (Figure 1C). In the first stage, we conducted the study of the insufflation flow (in the absence of animals) through the ethereal dissipation of “dry ice stones” and the recording and comparison between the methods. We performed the evaluation of all the parameters proposed in the study comparing the two types of valve.
CO2 flow
Regardless of the valve used, we follow the guidelines of CONCEA-RN No 37 and Brazilian Guideline for the Care and Use of Animals in Teaching Activities or Scientific Research (DBCA). The DBCA-CONCEA in section 8.13.5.6.: the restricted use of carbon dioxide in which it is recommended “... the chamber must be filled with a flow of 100% CO2 in the order of 20% of the volume of the chamber per minute and maintain the flow for at least one minute after clinical death.” This flow was calculated using a pressure gauge (0 to 300 KgF/cm2) with flow meter (2 to 32 L / min calibrated at 4 KgF / cm2 - 21 oC) Carbografite® Series 800 (Rio de Janeiro - Brazil). Where the estimated value of 20% was obtained in a flow of 4.0 L / min in 1.5 minutes and 6.5 minutes reaching 100% of the volume of the 7.4 cm3 euthanasia chamber (CONCEA, 2018).
Isoflurane saturation
We compared the efficacy of euthanasia induction in mice by the inhalation agent Isoflurane 100 ml (2L/min) in respective euthanasia chamber conform described in item 1.1. in the guidelines of CONCEA – RN No 17: “... soaked in cotton or made available in the form of steam.” and “... avoid direct contact of the animal with the liquid on the cotton”. We standardize by using 10 grams of hospital gauze pad in the characteristics of 09 threads / cm2; 7.5 x 7.5cm; 5 folds; 8 layers and 100% cotton soaked in 10ml (inside a metal shield) in the case of isolated use of Isoflurane (ISO) as a euthanasia inducer and 5 ml when associated with Carbon Dioxide with the Modified Valve (Figure 1D). The saturation of the environment by isoflurane starts in 5 to 10 seconds after being placed in the euthanasia chamber, as its maximum concentration percentage (at 20oC) is 31.86%, and its stability is only 0.2% this way through of the formula: Consumption = C x F x T / D x Vm x TA + t ºC / PM x 273; where: C = Concentration in volumes (%); F = Flow (ml.min-1); T = Time in minutes; D = density; VM = Molecular volume in liters (22.4); TA = absolute temperature (273º); T ºC = Ambient temperature in degrees centigrade; PM = Molecular weight, we can reach 100% saturation in an area of 7,4 cm3, with 10 ml of saturation maximum Isoflurane in the time of 60 seconds [5, 29-31].
Injectable Anesthetics
Barbiturates and dissociative anesthetics
As described in CONCEA - RN No 37, one of the accepted methods without restriction for use in euthanasia in rodents was: i) Use of Barbiturates (Tiopentax® 1g) in an overdose of 150 mg / kg; and ii) The association between the injectable dissociative agent (10% Ketamine Hydrochloride - Cetamin®) and α2-adrenergic receptor agonists (2% Xylazine Hydrochloride - Xilazin®) that should only be used in overdose. Before assessing the parameters studied, we performed the physical restraint of each animal (in different groups) and intraperitoneally (i.p.) administered Barbituricor associated dosage of 300 mg / kg of ketamine (CET) + 50 mg / kg of xylazine (XIL), in a maximum volume of 0.3 ml of the solution.
Animal Welfare: Evaluation of Efficiency by Non-Invasive Parameters
Death dynamics
Using a camera (Canon PowerShot SX20 IS®) in the frontal position, the euthanasia chamber performs the behavioral recording and euthanasia induction dynamics of each agent used. This ethological method allowed the determination of the following parameters: i) Facial and body expression consistent with distress and suffering; ii) Number of respiratory movements and presence of transient apne; iii) Death time estimated by immobility and later confirmation by cardiological parameters in absence of respiratory movements.
Electrocardiography
The mice were immobilized in the supine position using adhesive tape on a rubber plate and the transducers were attached under the skin in the position according to the electrocardiographic derivation used (DII). The traces were obtained continuously through the digital system (Power Lab 2/20®) connected to a Bio-Amplifier in the equalization of 2mV/second (Pan Lab Instruments®) and the software capturing signals in the frequency of 0.1 to 100 Hz. We determined the death at the moment of absence of respiratory movements, heart rate (heart rate per minute) and the presence of sinus and ventricular arrhythmias and/or systoles absencethrought the Scope software for Windows V3.6.10 program (PanLab Instruments®) each 15 to 30 seconds until the moment of death.
Open Field Test
This parameter was used to enable the behavioral assessment of distress and suffering that each euthanasia method could promote to the animal. Our chamber prototype was designed to have dimensions of 20 x 20 x 15 cm. Thus, we divided its area of 40 cm2 into 12 quadrants of 3,5 cm2 to evaluate the animal’s displacement and the height of 15 cm to determine the exploratory activity. We determined from the moment the mouse was placed inside until the moment of death confirmation, the number of horizontal quadrants crossed (motor activity), the number of vertical surveys (Rearing) and the presence/absence of urination and defecation.
Animal Welfare and Influence on Biological Systems: Invasive Parameters
Hematology, Biochemistry and Immunophenotypic Analysis: After death confirmation, we performed the mouse cardiac puncture, obtaining 0,8 to 1,0 ml of blood for processing using EDTA K2 (Vacuplast®) for the hematological evaluation a from the following parameters: i) RBC: Red blood cell count (millions per mm3); ii) Hemoglobin measurement (gram/deciliter); iii) Hematocrit assessment (percentage) and iv) WBC: Leukocyte count (millions per mm3) and the mean leukocyte value between the methodologies (± VM). Biochemical assessment was performed using serum using the following parameters: i) Liver injury markers, aspartate aminotransferase (AST - U/L) and Alanineaminotransferase (ALT - U/L) and ii) Function marker and kidney damage, through the measurement of Nitrogenated Urea (BUN - mg/dl) and creatinine (mg/dl), respectively. All parameters were measured according to the Kit’s instructions and by an automated system, based on colorimetry, kinetics and potentiometry. The leucocyte blood isolated were centrifuged (400g/10 min) and immediately transferred to ice-cold Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% foetal calf serum (Sigma-Aldrich), and the cells were maintained on ice until use. Alternatively, mouse hepatocytes and splenocytes were obtained by mechanical dissociation, and the erythrocytes were lysed by hypotonic shock in RPMI 1640 culture medium (Gibco, Paisley, Great Britain) diluted 1:10 in water for 10s. Hepatocytes and splenocytes were washed in cold RPMI and quantified using a Neubauer chamber. For phenotypical labeling, the cells were incubated for 30 min at 4ºC in RPMI 1640 medium supplemented with 10% foetal calf serum and 10% inactivated normal sheep serum to block FcgR. All samples were incubated for 30 min at 4ºC with anti-CD3 PerCP, anti-CD19 FICT, anti-CD4 FITC, anti-CD8 PECy-7, washed twice in RPMI 1640 medium and acquired with a Cyan ADP flow cytometer (Beckman Coulter, Houston, USA). Data analysis was performed using Summit software version 4.3 (Beckman Coulter).
Detection of the release of reactive oxygen species (ROS)
The ROS detection by the cerebral cortex region was performed by the DHE (Dihydroetide) assay. This technique is based on the chemical reaction between DHE and the superoxide present in the tissue. The sections of the cerebral cortex were delimited with a hydrophobic pen (Sigma-Aldrich), followed by the addition of the DHE solution in the 5μM concentration, diluted in DMEM medium. They were incubated at 37ºC for 30 minutes, with shaking at 50 rpm. Washed three times with PBS, 5 minutes each wash, to finish the reaction, at room temperature; fixed with 4% paraforaldehyde (PFA) for 10 minutes, washed and mounted on Prolong Gold with DAPI. The slides were photographed using a laser confocal microscope, model LSM 710 (Zeiss, GER), in 40X magnification. The measurement of the release of superoxide in the samples was performed by means of the average fluorescence intensity (IMF) of the compound 2-hydroxy ethidium, using the program Image J.
Financial and Comparative Planning of Euthanasia Methodologies
Barbiturates and dissociative anesthetics
Each euthanasia methodology was compared by financial cost (mainly between Inhaled and injectable anesthetics). In relation to Inhaled anesthetics, our calculation started from the area and the total saturation time of the area according to the guidelines of CONCEA - RN No 37, in addition, the costs were based on each animal, showing the time of death, that is, the volume of each agent used for a euthanasia event. In the case of injectable anesthetics, we also followed the guidelines of CONCEA - RN No 37 using overdose, as previously described and we calculated the costs due to the volume used for each animal, showing the dosage of milligrams per kilogram of each anesthetic.
Statisticalanalysis
Statistical analyzes were performed using the Graph Pad Prism program, version 5.0 (Graph-Pad Software Inc.), calculating the group’s mean value and ± SD. In addition, we applied the One Way ANOVA test (between different groups of animals). The statistical significance was confirmed by the post-test Turkey, considering a significant difference when p ≤ 0.05.
Results
Barbiturates and dissociative anesthetics
Our experimental design was based on the use of male and adult Swiss Webster mouse. We performed the non-invasive parameters before and after the start of the euthanasia procedure. Only after animal death we collect biological material to evaluated invasive parameters. Our longest period of evolution to death was 6 minutes and 30 seconds, however we evaluated the animal every 15 to 30 seconds (Figure 2).
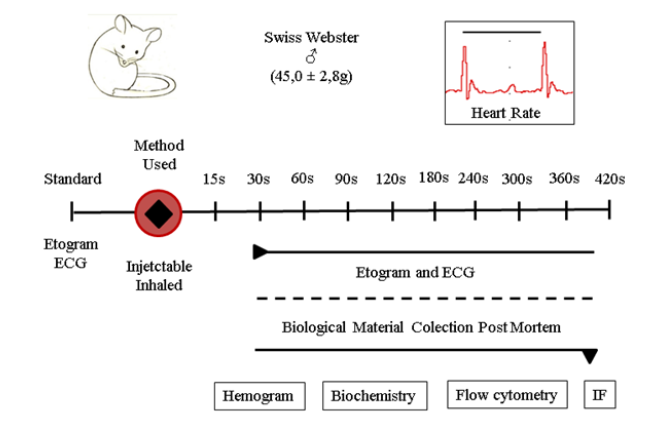
Figure 2: Experimental design: Our scheme was based on the assessment of the presence of pain and suffering during euthanasia induction, the time of death confirmation and the interference in the biological systems of each methodology used through the collection of blood and organs. We used adult Swiss Webster (male) mice to evaluate injectable and inhaled agents. Immediately after the induction of euthanasia, inside the euthanasia chamber, we performed the evaluation by the non-invasive parameters of the ethogram and electrocardiography (ECG) for the presence of distress in the animal and after confirming the death, we collected biological material for carrying out the hemogram, biochemical markers of tissue injury, flow cytometry and brain tissue immunofluorescence (IF).
The first results were related to efficiency and knowledge of the insufflation/saturation and flow dynamics of anesthetics inhalation in our euthanasia chamber (Figure 3). Through continuous recording we perform the insufflation of CO2 and the evaluation of the dissipation flow of dry ice, both when using the standard valve (CO2 V) and for the modified valve (CO2 M). The results showed that, CO2 V, even if the chamber saturation starts within the limit of 20%, still the flow is vigorous. Flow direct impact on the wall of the chamber promoting severe disturbance in the gas distribution inside the euthanasia chamber, as illustrated in the diagram of the Figure 3A. However, when the valve is modified, according to our prototype, there is a significant change in the CO2 insufflation flow. The CO2M, prevents the direct flow force, promoting an initial resistance, thus the gas is blown into the chamber smoothly and tends to descend evenly, we do not observe swirling of the insufflation flow. We suggest that the association between the paper filter and activated carbon also reduce CO2 odor (Figure 3B). The placement of the gauze soaked in Isoflurane inside the chamber does not alter the flow inside the chamber, however there is a uniform saturation of the environment (Figure 3C). The association between the saturation of the environment with isoflurane and subsequently the insufflation of 20% CO2M until the total saturation time (100%) of the chamber by CO2 maintains the same pattern described for both products and methodologies, or in other words, total saturation of the chamber with Isoflurane and CO2 insufflation in a smooth way and without swirling the environment (Figure 3D).
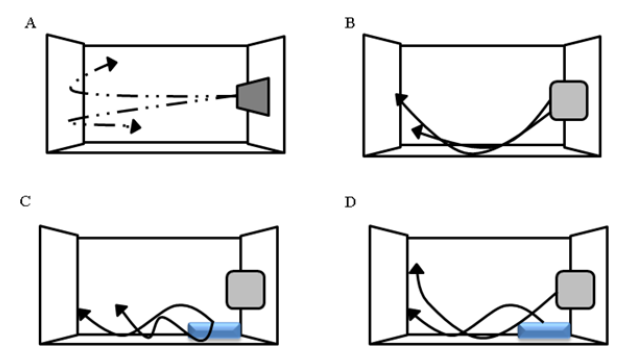
Figure 3: Evaluation of the saturation flow and insufflation of inhalation agents. In our euthanasia chamber, we insert dry ice stones, which, at room temperature, begins to give off a smoke which during continuous filming we could observe that during the insufflation of CO2 V (A) the gas flow enters the chamber in the opposite direction. Straight and crashes against the wall of the chamber forming a whirlwind of air. However, when we modify the valve (B) the insufflation of CO2 M demonstrates that the resistance promoted by the valve prevents the swirling, forcing the flow to the floor of the chamber. In the case of the insertion of Isoflurane, there is no insufflation, there is only saturation of the environment which does not modify the air flow (C), so the ideal was the association between Isoflurane saturation and CO2 insufflation with the modified valve (D).
After the insufflation and saturation mechanical tests, we performed the non-invasive parameters comparing the animal welfare and the effectiveness of the different euthanasia methodologies. Etological parameters, it was possible to evaluate the animal behavior death dynamics of each euthanasia procedure. During CO2 V insufflation, the animal shows irritability and tries to “clean its nose” as if there is a strong odor bothering. Despite the relatively rapid dynamics of death, the mouse shows a marked sensation of discomfort and distress. CO2 M use demonstrated the same characteristics as the CO2 V, but with significantly lower intensity and faster evolution to death. ISO saturation demonstrates that its action takes a little longer than CO2 flow, but this period is not marked by the presence of behavioral signs of discomfort or suffering. Only the sensation of odor is very striking in this situation. The association between saturation (anesthetics induction) of the environment with ISO and subsequently insufflation with CO2 M promotes a very fast death. The animal does not show signs of discomfort or distress, in 15 to 20 seconds it is already unconscious, being confirmed by urination and defection involuntary. Between 50 and 60 seconds we can confirm the animal’s death. Regarding injectable agents, the use of barbiturate showed a significant burning sensation at the time of application and at the injected site (i.p.). The animal staggers and tries to move for a relatively long time, it is not clear the feeling of discomfort and suffering like CO2 V, but it is also not possible to say that the animal is completely unconscious. In addition, it presents a period of marked transient apnea and a long period for the evolution to death. The use of the combination of Ketamine and Xylazine, in its administration does not show burning in the place, but in the same way there is a strong respiratory depression, not reaching apnea and the animal performs an uncoordinated displacement for about 3 to 4 minutes, which is known to be under the action of the euthanasia-inducing agent, however it does not make it possible to state how much discomfort and how much unconsciousness is being expressed by the animal (Data no showed).
Through the continuous evaluation of the cardiac electrical conduction system and the possible changes in the cardiac rhythm, it was possible to obtain two essentials information. The first was that the presence of pain, suffering or distress is directly captured by electrocardiographic tracings in the form of changes in the coordination of cardiac electrical traces, reflecting in heart rate and heartbeat, mainly due to the presence of arrhythmias. The second information is death confirmation, characterized by the stopping of the respiratory rate and the number of heartbeats ≤ 100 bpm (Figure 4). ECG traces showed that the use of CO2 V to induce euthanasia with the standard valve confirms death in 120 seconds. However, after 30 seconds of exposure to this methodology, the electrical conduction system demonstrated significant disturbances in the cardiac electric conduction, with the presence of sinus arrhythmia in 67% of the animals used and sinus tachycardia characterized by 961±104 heart beats per minute (bpm). Within 45 seconds, it was not possible to calculate the heart rate by severe sinus arrhythmia in 100% of the animals. After 60 seconds, there is a dizzying drop in heart rate and asystole and death within 120 seconds (Figure 4A). Through the modification of the valve (CO2 M) we observed that the death of the animal occurs in about 120 seconds, however, the euthanasia induction begins with 30 seconds (553±25 bpm) with a smooth and progressive sinus bradycardia , with only 33% of the animals showing mild sinus arrhythmia (Figure 4B).
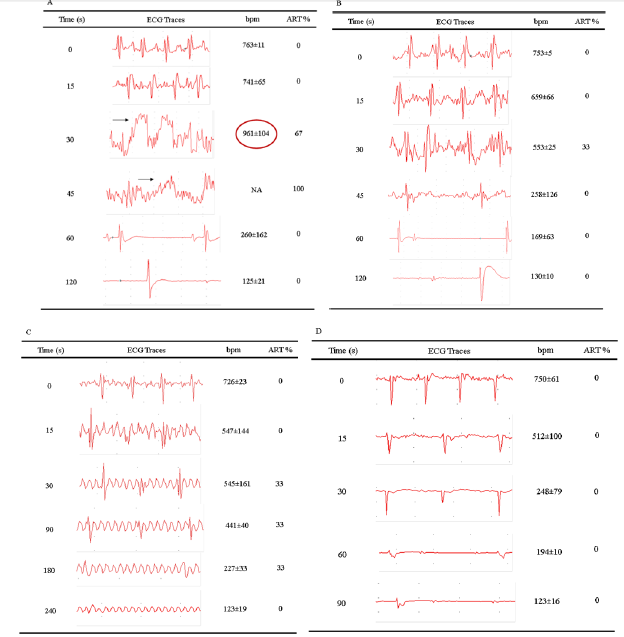
Figure 4: Cardiac monitoring for the presence of suffering during euthanasia induction and the time to confirm death. Initially, we performed through cardiac monitoring using the ECG the influence on the cardiac electrical conduction system of pain or suffering, to the percentage of the arrhythmias (ART%) during the induction of euthanasia and also the confirmation of death. The ECG tracings demonstrated that the use of CO2 V between 30 and 45 seconds promotes intense discomfort in the animal due to the presence of cardiac arrhythmias (A) and increased of the Heart Rate (bpm), which only in 33% of the mice was perceived by the animals within 30 seconds when using the CO2 M (B). The use of the Isoflurane saturated euthanasia chamber (ISO) did not demonstrate the presence of discomfort / arrhythmias, only after 30 seconds of exposure to the presence of cardiac fibrillation and the confirmation of death with 240 seconds (C). However, the association between Isoflurane and CO2 M did not, at any time, show changes in the electrocardiographic tracing compatible with discomfort, and confirmation of death can be indicated within 90 seconds (D).
Still in relation to inhalation anesthetics, we performed the ECG evaluation of euthanasia induction by saturation of the chamber with ISO. The results demonstrate that the death confirmation occurs in 240 seconds, however the induction process starts from 15 seconds, with an evident sinus bradycardia (547 ± 144 bpm), however 33% of the animals show cardiac fibrillation in the period between 30 to 180 seconds (Figure 4C). The association between ISO and CO2 M, interestingly, demonstrates that euthanasia induction occurs quickly, progressively and smoothly. After 30 seconds of exposure to the respective association, the animals demonstrate a significant decrease in heart rate (512 ± 100 bpm) that will confirm the death with asystole in 90 seconds. We emphasize that during this period, as shown in Figure 4D, the presence of arrhythmias or any other alteration in the cardiac electrical conduction system was not observed in any animal.
The evaluation of injectable anesthetics, we did not observe at any time, after the administration of the drugs, the presence of cardiac arrhythmias or any other alteration in the cardiac electrical system that was not characterized by the progressive decrease in heart rate (sinus bradycardia). Confirmation of death occurred around 420 seconds for both Thiopental and for the association between Ketamine and Xylazine. However, after a period of 180 seconds, we can observe the presence of severe respiratory depression, transitory apnea, which only characterized death with the complete absence of respiratory movements after only 360 seconds (Table 1).Thus, we chose, for the welfare of the animals, to continue using CO2 M and Thiopental excluded in the rest of the study.
As indicated, behavioral tests are important to evaluated discomfort and suffering in lab animals (Figure 5). Our euthanasia chamber prototype, was designed to allow the realization of the Open Field Test during the induction of death of the animals through their motor and exploratory activity (Figure 5A). Our results demonstrate that the mice induced to death by injectable anesthetics, as Ketamine and Xylazine, presented a significantly increased horizontal displacement (33,3 ± 10,7 quadrants) when compared with the other methodologies: CO2 M: 6,7 ± 2,1; ISO: 12,7 ± 1,5; ISO + CO2 M: 3,0 ± 1,7 quadrants crossed in an interval of 5 minutes (Figure 5B). Regarding the exploratory activity, or the realization of rearing behavior, in all the methodologies used we can consider the low value (CO2 M: 1,7 ± 1,2; ISO: 1,3 ± 1,5; CET + XIL: 0,3 ± 0,6 vertical liftiting / 5 minutes), however the association of ISO + CO2 demonstrated that during the euthanasia dynamics not observed motor activity or rearing until death confirmation (Figure 5C).
Regarding the invasive parameters, constituted by the influence of euthanasia-inducing agents on the biological systems of the mouse, we can start our results with the hematological evaluation. In relation to the red blood series, the main requirements, i) Number of red blood cells per mm3; ii) Hemoglobin concentration and iii) Hematocrit percentage; none of the euthanasia methodologies demonstrated to induce any change in these values when related to the average values of normality (VM) (Table 2). However, in relation to the white blood series, the leukocyte count, the use of ketamine and xylazine promotes a marked leukopenia immediately after the animal’s death (3,8 ± 0,1 million per mm3). For the other methodologies, CO2 M: 8,5 ± 3,0; ISO: 11,3 ± 4,1 and ISO + CO2 M: 12,8 ± 3,6 million leukocytes per mm3, which values are similar to VM (9,1 ± 1,8 million/mm3) (Figure 6). We investigated the leukopenia profile, by marking leukocyte populations by flow cytometry. This methodology made it possible to elucidate the quantification of populations of B and T cells in different organs (Figure 7). In the thymus and spleen, no changes were observed in the percentage of the leukocyte population in any of the methodologies used (data not shown). However, in the liver, we observed statistically significant changes through the drop of 20% and 10% of the populations of CD3+ (Figure 7A) and CD19+ (Figure 7B) cells, respectively by the use of the combination of Ketamine and Xylazine. This association also promoted, after death, a 20% decrease in CD4+ cells (Figure 7C) and a 2% increase in CD8 cells positively marked in the liver tissue (Figure 7D).
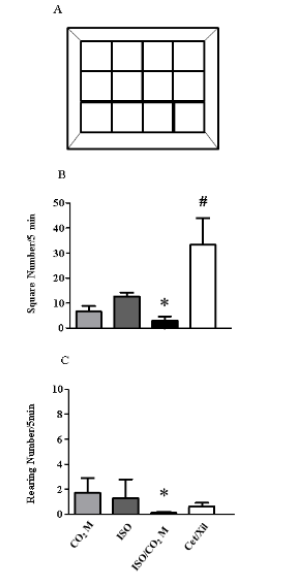
Figure 5: Behavioral assessment through the Open Field Test during euthanasia induction. The prototype developed for our euthanasia chamber allowed the Open Field Test to be carried out, by dividing its floor with lateral and central quadrants (A) in the different euthanasia methods Carbon Dioxide (CO2 M), Isoflurane (ISO), Isoflurane and Carbon Dioxide (ISO+CO2 M) and Cetamine and Xilazine (Cet + Xil). From this, we carry out the evaluation of the behavior of each animal during the induction of euthanasia through motor activity, by the number of quadrants crossed in its horizontal displacement (B) and its exploratory activity, through its vertical survey or Rearing (C) in 5 minutes. *indicates the statistical significance p ≤ 0, 05 between ISO+CO2 M and other categories. # indicates the statistical significance p ≤ 0,05 between CET + XIL and other categories
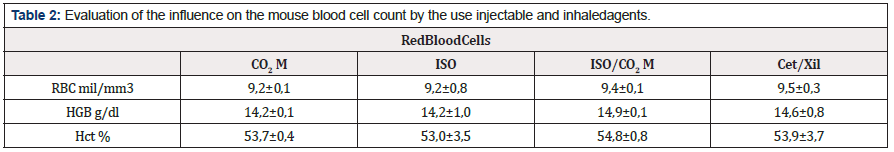
Table 2: Evaluation of the influence on the mouse blood cell count by the use injectable and inhaledagents.
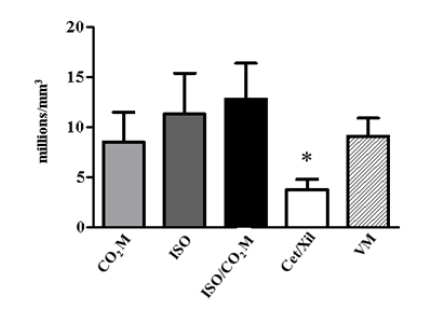
Figure 6: Influence of the euthanasia induction method on the total leukocyte count. The values show the count of the total number of leukocytes in the mice submitted to different euthanasia methodologies, through the use of carbon dioxide with the modified valve (CO2 M - light gray bar); use of isoflurane (ISO - dark gray bar); combination of ISO and CO2 M (black bar) and the use of injectable anesthetics by the combination of Ketamine and Xylazine (Cet + Xil - white bar) compared to the normalized mean values of the white blood cell (VM - hatched bar). *Indicates the statistical significance p ≤ 0,05 between CET + XIL and other categories.
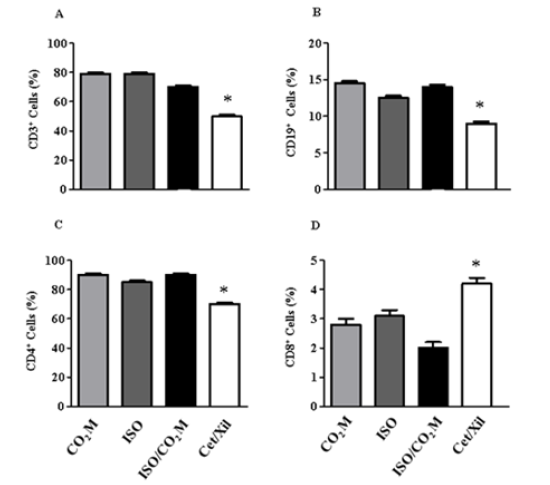
Figure 7:Phenotypic analysis of the leukocyte population in liver tissue. Through the dissociation of liver tissue and the identification of the lymphocyte population, it was possible to quantify in percentage (%), the total of each type of population, T - CD3+ (A) and B - CD19+ (B) lymphocytes and the sub-populations of T cells - CD4+ (C) and CD8+ (D) after euthanasia in mice in the different methodologies applied. * indicates the statistical significance p ≤ 0,05 between Cet + Xil and other categories.
These results are in accordance with the serum dosage for injury markers in hepatocytes, AST and ALT (Figure 8). The combination of Ketamine and Xylazine promotes a significant increase in AST (1572 ± 394 U/L) and ALT (1997 ± 219 U/L) when compared with the other methodologies for AST (CO2 M: 191 ± 30; ISO: 422 ± 67; ISO + CO2 M: 835 ± 106 U/L), for ALT (CO2 M: 169 ± 96; ISO: 362 ± 135; CO2 M + ISO: 811 ± 132 U/L) and the standard enzyme value for the healthy liver tissue of mice was AST: 755 ± 165 U / L (Figure 8A) and ALT: 835±145 U/L (Figure 8B). The serum dosage of markers of renal dysfunction and injury, such as urea and creatinine, did not show significant changes between the values in the methodologies used (data not shown).
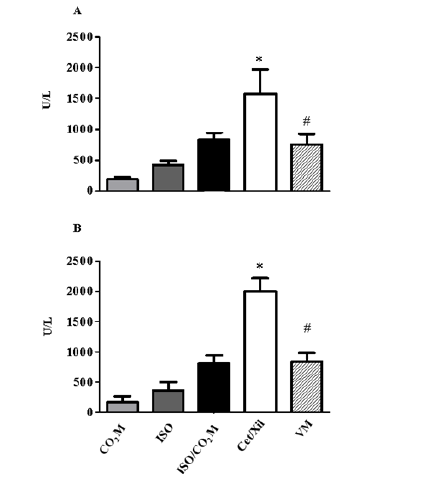
Figure 8: Effect of inhaled and injectable agents on hepatic tissue injury markers after euthanasia. The measurement of kidney and liver injury markers in the animals’ serum after death confirmed that the liver enzymes, Aspartate alaninotransferase - ALT (A) and Aspartate amino transferase - AST (B) demonstrate high values when related to the use of agents injectable anesthetics. * indicates the statistical significance p ≤ 0,05 between Cet + Xil and other categories. # indicates the statistical significance p ≤ 0,05 between Cet+ Xil and VM.
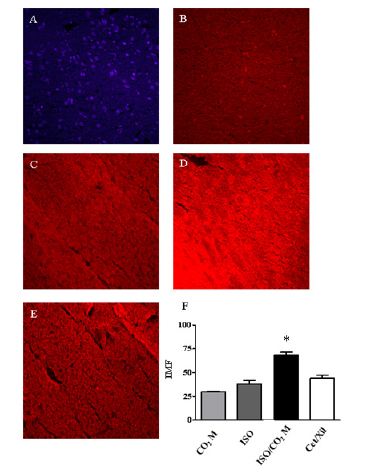
Figure 9: Expression of Reactive Oxygen Species in the brain tissue of mice subjected to different types of euthanasia promoters. Through the use of the Immunofluorescence technique it was possible to perform the estimated measurement of ROS release in the animals’ brain tissue after each type of euthanasia. The test’s reliability was demonstrated by the negative standard (A) compared to the average fluorescence intensity (IMF) marking in the CO2 M (B), ISO (C), ISO and CO2 M (D) and Cet + Xil (E) groups. The graph (F) quantifies by the Image J software the intensity of marking, that is, the amount of ROS release in the brain tissue for each euthanasia category. * indicates the statistical significance p ≤ 0,05 between ISO and CO2 M and other categories.
Finally, we performed the neurobiological evaluation of animals induced by euthanasia through the expression of ROS by the average intensity of immune fluorescence (IMF) staining (Figure 9). Through parasagital cuts, we performed our negative control, due to the absence of the primary antibody, demonstrating the functionality of the assay (Figure 9A-9C). The use of the system with ISO induction and CO2 M flow demonstrated a significant and high expression of ROS in brain tissue, 68,1 ± 3,0 IMF (Figure 9D), when compared with the other methodologies: CO2 M: 29, 9 ± 0,9 IMF (Figure 9B); ISO: 38,3 ± 3,6 IMF (Figure 9C); Ketamine and Xylazine: 44,4 ± 3,1 IMF (Figure 9E) as described in graph 9F.
Regarding the costs of acquisition and use of the respective inhaled and injectable anesthetics used in this study, we performed a calculation basis for the euthanasia induction an event with a number of 10 animals, starting from the animal’s weight and estimating for younger ages, that is, lighter weights. Initially, we describe the main injectable and used agents such as Thiopental, Ketamine and Xylazine (Table 3). Euthanasia due to overdose of Thiopental (150 mg/kg) in mice weighing 45 grams determines a cost for each event R$ 21,00 and the association in ketamine and xylazine overdose (300 + 50 mg/kg) defines a cost of R $ 7,80 for each event. When we estimated the cost of inhalation agents (Table 4), we determined that for mice weighing 45g, the costs were R$ 0,10 for CO2 M (2,0 L/min ); R$ 0,55 for ISO (10%) at a saturation of 2 L/min and R$ 0,35 for a saturation of 2,0 L/min for ISO (5%) and CO2 M (1 L/min).
Discussion
The objective of our study was in accordance with national legislation, mainly through CONCEA -RN 37 and DBCA of the Ministry of Science and Technology (MICT - Brazil) to evaluate the effectiveness of the proposed methods and which one would be most adequate for use, under our conditions, in mouse, to induce euthanasia in order to reduce the discomfort and suffering of the animal during the process, preserving animal welfare and the ease of handling and access of employees to perform the respective methodology [6,8,28].
The euthanasia practice guideline - CONCEA (2018) in its Table 1 for Mammalia Class (Mammals); Order Rodentia - Rodents and small mammals - mice, rats, hamsters, gerbil, guinea pigs determines the use of Barbiturates and overdosage of the association of dissociative anesthetics (e.g. Ketamine) and alpha-2 adreno receptor agonists (e.g. Xylazine)and Isoflurane as an inhalation anesthetic as recommended methodologies. Regarding the use of carbon dioxide (CO2) the respective guideline determines this accepted methodology with the restriction of insufflation control and the characteristics of the euthanasia chamber. However, in all methodologies we can see that there are advantages and disadvantages in each method and that despite the use of bibliographic references there is also a lack of studies evaluating together the efficiency of euthanasia induction of the respective agents, revealing structural conditions, resilience/susceptibility the handler, perception of suffering and interpersonal distress, biological markers of pain and distress of the animal and also the financial costs of acquiring and using each agent recommended or accepted with restriction [17,12].
AVMA recently published the Guidelines Panel for the Euthanasia of Animals: 2020 Edition (AVMA, 2020) [1]. Similarly to Brazilian law, guidelines for the application of the different types of euthanasia induction methodologies present benefits and possible compromise to animal welfare. As used in our manuscript, the use of Tiopental has the advantages of the speed action depends on the dose, concentration, route, and rate of the injection, however some animals may go through an excitatory phase that may be distressing to observers. The efficiency of the combination in ketamine and xylazineoverdosage is related to doses that consistently produce rapid death have not been established for most drugs and species. The cost of the higher doses of agents required to cause death may substantially exceed that of an approved euthanasia methodology. The use of Isoflurane can be useful as the sole euthanasia agent or as part of a 2-step process, where animals are first rendered unconscious through exposure to inhaled anesthetic agents and subsequently killed via a secondary method. In those species where aversion or overt escape behaviors have not been noted, exposure to high concentrations resulting in rapid loss of consciousness is preferred. Otherwise, gradual-fill methods can be used, keeping in mind the effect that chamber volume, flow rate, and anesthetic concentration will have on the time constant and rate of rise of anesthetic concentration. CO2 is acceptable with conditions for euthanasia in those species where aversion or distress can be minimized. CO2 exposure using a gradual-fill method is less likely to cause pain due to nociceptor activation by carbonic acid prior to onset of unconsciousness; a displacement rate from 30% to 70% of the chamber volume/min is recommended for rodents. Consideration should be given to the benefits of using a darkened home cage, while also keeping in mind the need to have the animal under observation. Whenever gradual displacement methods are used, CO2 flow should be maintained for at least 1 minute after respiratory arrest [1,9,10,15,33].
Our results showed that the methodology that was most appropriate to the respective requirements was the association between Isoflurane and CO2 M, because through the ethogram the animal did not show any excitation or disorientation behavior, which was confirmed by the Open Field Test. In addition, during the euthanasia induction, she did not show cardiological signs, due to ECG monitoring, of pain, discomfort or suffering. Death was confirmed in 60 to 90 seconds and at no time did we observe the presence of arrhythmias in any animal. Demonstrated marked and rapid action on the Central Nervous System through mitochondrial respiratory chain block and the high expression of the ROS. The respective association did not interfere with biological systems, mainly immune and other vital organs such as heart, liver, spleen, thymus and kidney. Finally, this association has a relatively low cost, compared to other methodologies, but it requires the acquisition of specific equipment for the correct realization of the methodology [32].
On the other hand, the use of injectable anesthetics, both Thiopental and the combination of Ketamine and Xylazine, presented a dynamic of death compatible with signs of discomfort and disorientation, making it impossible to assess the state of unconsciousness and the confirmation of death only occurred after 360 seconds. Although the presence of arrhythmias, consistent with pain, suffering and distress, does not present at any time by ECG monitoring. However, a complicating behavioral factor of this methodology is the motor activity and persistent special disorientation. The evaluation of the respiratory rate, as both agents have transient apnea which can lead to the error of death confirmation. The combination of Ketamine and Xylazine promotes a serious interference in biological systems, mainly in the immune system, due to the decrease in the total number of leukocytes and the leukocyte population B and T lymphocytes and an increase in the TCD8+ lymphocytes population in the liver tissue. These data corroborate the assessment of markers of hepatic tissue damage with significant elevation of AST and ALT immediately after death was confirmed by the respective agent. In addition, both Thiopental and the combination of Ketamine and Xylazine have a high financial cost when compared to other methodologies.
Isolated Isoflurane saturation showed intermediate results. The death dynamics are smooth and do not show any electrocardiographic or behavioral changes, however, their confirmation occurs only in about 120 to 180 seconds. No does it affect biological systems, including immune modulation or vital organs, only a reduced elevation of ROS in brain tissue. Costs are also reduced and similar to CO2 when compared to injectable anesthetics.
Regarding the mechanisms of action of injectable anesthetics (Brabiturics and association of dissociative anesthetics and alpha-2 adrenoreceptor agonists), as well as inhalation agents (Isoflurane and CO2) are well described. However, the innovative character in our work was the association between combination between induction with ISO and CO2 with modified valve. Thus, we are convinced that the Good Death proposal is closely related to the concept of Speed Death [20].
The Speed Death suggest arehighly released ROS. This reactive molecules produced in the cell endoplasmic reticulum, peroxisosomes, cytosol, plasma membrane, extracellular space and mainly in the energy metabolism process, that is, in the mitochondria [13,7,26]. This organelle acts in the redox/oxidative balance, regulating cell death and survival [24,30]. Thus, it assists in the proper functioning of brain cells and other essential organs for life [11,25]. So that we have not yet been able to elucidate the association between ISO and CO2 M, it quickly demonstrates altering mitochondrial functioning leading to redox/oxidative imbalance, and/or change in ATP production, resulting in damage to the mitochondria, causing greater production of ROS, leading the cell to an irreversible oxidative stress state, causing cell death.
Thus, the dynamics of death by this respective association suggests promoting a fast death (by means of necrosis or apoptosis ways) in a rapid and extensive manner, preventing the possibility of disconfort and suffering of the animal due to the irreversible involvement of the cells of the Central Nervous System [23].
Conclusion
Initially, we would like to emphasize that the value of our study was its ethical aspect of developing and investigating new techniques, procedures and knowledge that will directly raise the quality of life of animals used for didactic and scientific purposes, in our case the animal model, the lab mouse.
Our results demonstrate that the best methodology to induce euthanasia in the lab mouse during its use for didactic and scientific purposes is through the euthanasia chamber with an environment saturated with 5% Isoflurane (through a hospital gauze soaked in anesthetic liquid and protected from contact with and the insufflation of Carbon Dioxide 1 L/min, initially in 20% to 100% of the total area of the chamber, with the restriction of the use of a flow meter and modification of the insufflation valve [32,33].
However, other methodologies also have their applicability. We suggest that for neonates or animals from breeding colonies the ideal protocol consists of using a chamber with an environment saturated with 10% Isoflurane, also soaked in hospital gauze and protected from contact with animals. Regarding injectable anesthetics, we suggest not using Thiopental (150 mg/kg) in mice and using the combination of ketamine (300 mg/kg) and xylazine (50 mg/kg) overdose by i.p. only in cases of Humanitarian Finalization of the animals during the preclinical assays. However, do not use it during the tests, mainly due to direct and marked interference in biological systems.
The use of Carbon Dioxide, even with the use of a flow meter and the progression of insufflation from 20 to 100% in the euthanasia chamber, should not be used when the chamber valve is of a commercial standard and there is no modification in relation to the odor and noise.
Finally, we believe that this study can add of the laboratory animal science, demonstrating through relevant scientific methodologies, that the induction of euthanasia must use materials, methodologies and procedures that allow the absence of pain, discomfort and distress to the animal, a speed death dynamics and a relatively low financial cost.
References
- AVMA (2020) American Veterinary Medical Association. The AVMA Guidelines for the Euthanasia of Animals: 2020 Edition Disponívelem.
- BinsfeldPC, Caminho para a Legalidade (2017) Lapchik VBV, Mattaraia VGM, Ko GM (Orgs.) Cuidados e manejos de animais de laboratório. (2 eds) Rio de Janeiro, Ateneu, p. 11-24.
- Boivin GP, Hickman DL, Creamer-Hente MA, Pritchett-Corning KR, Bratcher NA (2017) Review of CO₂ as a EuthanasiaAgent for Laboratory Rats and Mice. J Am Assoc Lab Anim Sci 56(5): 491-499
- Brazil (a) Lei no 11.794/2008 de 08 de outubro. Estabelece procedimentos para o uso científico de animais. Diário Oficial [da] República Federativa do Brasil, Brasília, DF, 9 out. 2008.
- Brasil (b). Decreto no 6.899/2009 de 15 de julho. Dispõe sobre a composição do Conselho Nacional de Controle de Experimentação Animal-CONCEA, estabelece as normas para o seu funcionamento e de sua Secretaria-Executiva, cria o Cadastro das Instituições de Uso Científico de Animais-CIUCA, mediante a regulamentação da Lei no 11.794, de 8 de outubro de 2008, que dispõe sobre procedimentos para o uso científico de animais, e dá outras providências. Diário Oficial [da] República Federativa do Brasil, Brasília, DF.
- BRASIL (c) Ministério da Ciência, Tecnologia e Inovação. Resolução Normativa no 30, de 02 de fevereiro de 2016a. Baixa a Diretriz Brasileira para o Cuidado e a Utilização de Animais em Atividades de Ensino ou de Pesquisa Científica - DBCA. Diário Oficial [da] República Federativa do Brasil, Brasília, DF, 3 fev.
- Burton GJ, Jauniaux E (2011) Oxidative stress, Best Pract. Res Clin Obstet Gynaecol 25(769): 287-299.
- Campos JDDES (2016) O comportamento do camundongo SwissWebster em biotério de experimentação: observações e reflexõ Revista da Sociedade Brasileira de Ciência em Animais de Laboratório 4(1): 32-43.
- Cao JL, Covington HE, Friedman AK, Wilkinson MB, Walsh JJ, et al.(2010) Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressent action JNeurosci. 30(49): 16453-16458, 2010.
- Clifford DH (1984) Preanesthesia anesthesia analgesia, and euthanasia. In: Fox JG, Cohen BJ, Loew FM, eds. Laboratory animal medicine. Academic Press Inc, New York, p. 528-563.
- Coimbra-Costa D, Alva N, Duran M, Carbonell T, Rama R (2017) Oxidative stress and apoptosis after acute respiratory hypoxia and reoxygenation in rat brain. Redox Biol 12, 216-225.
- CONCEA (2020) Normativas do CONCEA para produção, manutenção ou utilização de animais em atividades de ensino ou pesquisa científica. Brasíl
- Dikalov S, Griendling KK, Harrison DG (2007) Measurement of Reactive Oxygen Species in Cardiovascular Studies. Hypertension 49(4) 1-11.
- FELASA (2020) Position paper on the proposed ban of carbon dioxide for rodent euthanasia - Policy documents - Felasa | Federation for Laboratory Animal Science Associations. Disponível em:
- Forslid A (1987) Transient neocortical, hippocampal, and amygdaloid EEG silence induced by one minute inhalation of high CO2 concentration in swine. Acta PhysiolScand. 130(1):1-10.
- FurtadoAK, OliveiraGM (2018) Análise Biométrica Relacionada A Importância Do Bem-Estar De Camundongos E A Influência Nos Resultados Dos Ensaios Científicos. São 6(2): 111-128.
- Garnett N (2010) PHS policy on humane care and use of laboratory animals clarification regarding use of carbon dioxide for euthanasia of small laboratory animals.
- Guimarães MV, Freire JEDAC, Menezes LMBDE (2016) Utilização de animais em pesquisas: breve revisão da legislação no Brasil. Revista Bioé 24: 217-224.
- Hackbarth H, Kuppers N, Bohnet W (2000) Euthanasia of rats with carbon dioxide--animal welfare aspects. Lab Anim. 34:91-96.
- Hawkins P, Playle L, Golledge H, Leach M, Banzett R, et al. (2006) Newcastle consensus meeting on carbon dioxide euthanasia of laboratory animals. National Centre for the Replacement, Refinement and Reduction of Animals in Science, London.
- Hawkins P, Prescott MJ, Carbone L, Dennison N, Johnson C, et al. (2016) A good death?Report of the Second Newcastle Meeting on Laboratory Animal Euthanasia.Animals (Basel) 6: 50. J Am Assoc Lab Anim Sci 56(5):491-499.
- Leach MC, Bowell VA, Allan TF (2002) Aversion to gaseous euthanasia agents in rats and mice. Comp Med.52:249-257.
- Liu B, Oltvai ZN, Bayır H, Silverman GA, Pak SC, et al. (2017) Quantitative assessment of cell fate decision between autophagy and apoptosis. SciRep 7(1): 17605.
- ManoliI, Alesci S, Blackman MR, Yan AS, Rennert OM, et al. (2007) Mitochondria as key components of the stress response.Trends in Endocrinology and Metabolism. 18(5): 190-198.
- Mendonça APM, Luanda Yanaan Hoppeb, Alessandro Gaviraghi, Tânia Cremonini de Araújo-Jorge, Gabriel Melode Oliveir, et al. (2019) Highly aggressive behavior induced by social stress is asociated to reduced to cytochrome c oxydase activity in mice brain cortex. Neurochemistry International 26: 210-217.
- Picard M, McEwen BS, Epel ES, Sandi C (2018) An energetic view of stress: focus on mitochondria. Front Neuroendocrinol 49: 72-85.
- Powell K, Ethun K, Taylor DK (2016) The effect of light level, CO2 flow rate, and anesthesia on the stress response of mice during CO2 LabAnim (NY) 45: 386-395.
- Rivera EA (2017) B Bem-Estar de Animais de Laboratório. In: Lapchik VBV, Mattaraia VGM, Ko, G M (Orgs.). Cuidados e manejos de animais de laboratório, 2 (Eds.), Rio de Janeiro, Ateneu, p. 35-45.
- Schmid RD, Hodgson DS, McMurphy RM (2008) Comparison of anesthetic induction in cats by use of isoflurane in an anesthetic chamber with a conventional vapor or liquid injection technique. J Am Vet Med Assoc 233(2): 262-266.
- Sena LA, Chandel NS (2012) Physiological roles of mitochondrial reactive oxygen species. Mol 48(2):158-167.
- Smith W, Harrap SB (1997) Behavioural and cardiovascular responses of rats to euthanasia using carbon dioxide gas. Lab Anim 31: 337-346.
- Thomas AA, Flecknell PA, Golledge HDR. (2012) Combining nitrous oxide with carbon dioxide decreases the time to loss of consciousness during euthanasia in mice-refinement of animal welfare? PLoS One 7: e32290
- Voss LJ, Sleigh JW, Barnard JP (2008) The howling cortex: seizures and general anesthetic drugs. AnesthAnalg 107: 1689-1703.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.