Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Production of Cyclodextrin Complexes with Biologically Active Milk Peptides
*Corresponding author: TM Halavach, VA Asafov, Belarusian State University (BSU), Nezavisimosti Аv. 4, 220030 Minsk, Belarus.
Received: April 07, 2020;Published: April 14, 2020
DOI: 10.34297/AJBSR.2020.08.001287
Abstract
The inclusion complexes of β cyclodextrin with extensive protein hydrolysates of whey and colostrum were derived. A significant decrease in bitterness of peptides included into clathrates with cyclic oligosaccharide was established in comparison with initial samples of hydrolysates. According to thermo gravimetric analysis, the formation of β cyclodextrin inclusion complexes with milk peptides was confirmed. A 2.1/1.3 fold increase in antioxidant potential of cyclic oligosaccharide clathrates with whey/colostrum hydrolysateswas revealed, when compared with peptide fractions. β cyclodextrin complexing with whey and colostrum peptides resulted in enhanced radical-reducing activity and improved organoleptic properties, making the clathrates promising ingredients of special nutrition formulas.
Keywords: Cyclodextrins, Bitterness of peptides, Clathrates, Colostrum Hydrolysate, Whey hydrolysate
Introduction
Cyclodextrins (CDs), or cyclic oligosaccharides, are distinguished by a cone-shaped spatial structure with hydrophobic cavity, which accounts forthe ability to form inclusion complexes with various compounds [1]. Increased solubility, resistance to physical and chemical factors, tolerance and bioavailability were shown for clathrates in contrast to the initial biologically active substances [2]. CD complexing with peptides and amino acids known to possess a pronounced bitter taste leads to upgraded flavor [3-5]. Introduction of α CD softened bitterness of pure amino acid solutions (phenylalanine, tryptophan, proline, isoleucine, tyrosine, histidine) and soy peptides [4]. 5 % β CD supply (as complexing agent) eliminated bitterness of 5 % soy bean hydrolysate solution by 90 % [5]. It appears natural therefore that β CD was recommended as a preferential component of functional nutrition. Supra molecular complex (insulin/R8 carboxymethyl-β CD) tested on diabetic rut line showed a considerable rise in insulin permeability and excellent biological effect [6]. β CD complexing demonstrated increased resistance of antimicrobial peptides ABP-CM4 [7] and nisin [8] to proteolysis accompanied by stable biological activity.
Antioxidant activity (AOA) of proteins and peptides is associated with reducing properties of amino acid radicals of methionine, histidine, tryptophan, and tyrosine [9-10]. Effect of enzymatic hydrolysis and fermentation on AOA of whey and colostrum protein component was evaluated [11-13], organoleptic and antioxidant properties of β CD clathrates with commercial whey hydrolysate were characterizedin the previous studies [14]. This research was aimed at production of cyclic oligosaccharide complexes with milk peptides (extensive hydrolysates of colostrum and whey).
Materials and Methods
Production of β cyclodextrin clathrates with milk peptides
β CD manufactured by Roquette (France) and extensive whey/ colostrum hydrolysates (produced atlaboratory of applied biology, Faculty of Biology, BSU, Belarus) were engaged for clathrate complexing. Solutions containing β CD and hydrolysates in mass ratio 2:1 (calculated as solids) were prepared. The resulting solutions of cyclic oligosaccharide and peptides were incubated during 4h at temperature 50 °C with constant stirring (200 rpm). Organoleptic properties of liquid samples were evaluated according to the procedure described in [5]. Samples of whey and colostrum hydrolysates were used as the control. Clathrate and hydrolysate samples were freeze-dried at temperature-53 ºС and pressure 0.1atm during 24-48h for subsequent experiments.
Thermo-gravimetric analysis of clathrates and hydrolysates
Thermal degradation parameters of clathrate and hydrolysate samples were determined by thermo-gravimetric analysis (TGA) and differential scanning calorimetry (DSC) on TGA/DSCI instrument (Mettler Toledo, Switzerland). The sample weight was 20mg, resolution of analysis equalled 1μg. TGA/DSC was carried out in the range 30-600 °С, the rate of temperature rise reached 5 °С/min, the accuracy of temperature control was ±2 °С. Effective activation energy (Еа) was calculated according to Broydo method using TGA curves [15]. Pure substances (peptides and β CD) and their mixtures in mass ratio 2:1 were chosenas the control samples.
Estimation of antioxidant activity
AOA of experimental samples was evaluated by fluorimetric method (Oxygen Radical Absorbance Capacity, ORAC). It is based on the suppression of fluorescein (Fl) fluorescence as a result of its oxidation by oxygen radicals and inhibition of this process by antioxidants. The technique presented in EI Tarun’s paper (2014) [16] was applied in this research. The results of 3 independent experiments were expressed as the mean value ± confidence interval.
Results and Discussion
Organoleptic properties of extensive dairy hydrolysates and the derived clathrates
β CD inclusion complexes with whey and colostrum peptides were producedin this studyunder optimized conditions. Solutions containing hydrolysates and β CD in mass ratio (1:2) were prepared and further incubated for 4h at temperature 50 ºС. Samples of hydrolysates and corresponding clathrates were subjected to organoleptic test, and freeze-dried for thermo-gravimetric analysis. The obtained extensive hydrolysates of whey and colostrum displayed evident bitter taste. The bitterness of colostrum hydrolysate hit the maximum on 10 point scale, while the sample of cleaved whey proteins scored only 8 points. Incubation of peptides with cyclic oligosaccharide altered the taste to moderately bitter (5 points) when compared with control samples of hydrolysates. Thus, β CD complexing drastically upgraded flavor of the resulting clathrates.
Thermal degradation parameters of inclusion complexes and hydrolysates
TGA/DSC analysis was performed to corroboratethe formation of β CD clathrates with whey and colostrum peptides. TGA results were presented as the curve of sample weight loss (thermogravimetry, TG/TG) and the curve correlating sample weight alteration with temperature of the system (differential thermogravimetry, DTG/DTG). The stages of thermal decomposition of samples under controlled heating regime from 30 °C to 600 °C at therate 5 °C/min were determined.
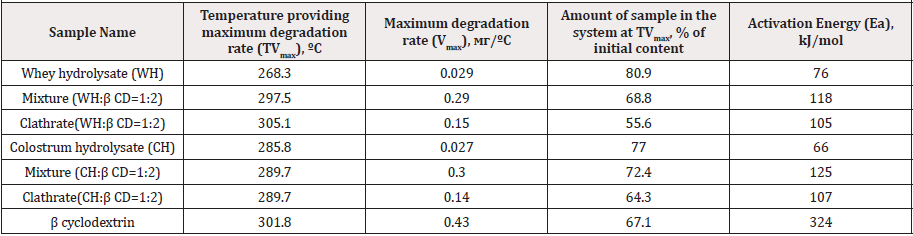
Table 1: Comparative analysis of thermal decomposition parameters of control hydrolysate samples and their clathrates according to data of DTG/ TG profiles (in the region of dominant peak corresponding to thermal degradation of β CD).
Thermal decomposition parameters of pure substances (hydrolysates of whey and colostrum, β CD), mixtures thereof and clathrates according to DTG/TG profiles in the region of dominant peak corresponding to β CD thermal degradation were reflected in Table 1.
DTG profiles indicated the maximum weight loss of β CD sampleat 301.8 °С, with the highestthermal decomposition rate reaching 0.43mg/°С. In case of whey protein hydrolysate decomposition peaks with maximal values of mass decay were recorded at 159.6, 203.9, 268.3 and 541.3 C (0.006, 0.014, 0.29 and 0.40mg/°C, respectively). Upon thermal break-down of colostrum protein hydrolysate the peaks of weight decline were detected at 158.8, 285.8 and 531.3 °C (0.016, 0.27 and 0.55 mg/°C, respectively).
DTG profiles of hydrolysates mixtures with β CD represent an overlap of mass loss peaks of separate compounds (Figure 1). The shift of thermal degradation peak of cyclic oligosaccharide from 301.8 to 297.5/289.7 °C was revealed during analysis of mixtures containing whey/colostrum hydrolysates, respectively.
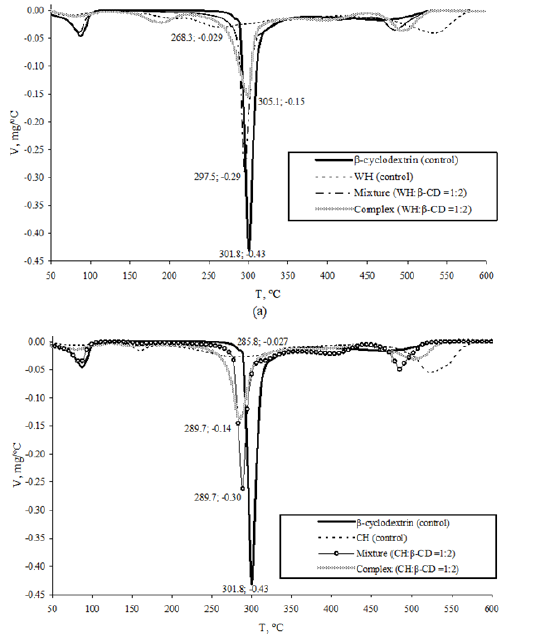
Figure 1: DTG profiles of control and test samples of whey (a) and colostrum (b) β CD – β cyclodextrin, WH – Whey Hydrolysate, CH – Colostrum hydrolysate.
Clathrate samples retained the prevalent peak of β CD thermal decomposition somewhat shifted in temperature and shape whereas degradation peaks typical for peptide mixture were not observed, evidencing generation of inclusion complexes. A shift of β CD thermal degradation peak from 297.5 to 305.1 °C and a decrease in mass loss rate of the sample from 0.29 to 0.15mg/ºС was shown for clathrate derived from cyclic oligosaccharide and whey hydrolysate as compared with peptide mixture (Figure 1a). This indicated an increased resistance of clathrate sample to thermal degradation. A decrease in the rate of thermal degradation from 0.30 to 0.14mg/ºС was found for inclusion complex containing colostrum hydrolysate at the same melting temperature peak of 289.7 ºС (Figure 1b). In general, enhanced thermal stability of peptides as components of clathrates with cyclic oligosaccharide was confirmed.
TG profiles demonstrated the larger weight loss of whey-based complex at the temperature of maximal β CD degradation (13.2 %) than that of clathrate with colostrum hydrolysate (8.1 %) in comparison with peptide mixtures (Table 1). The data clearly prove superior thermal stability of colostrum-containing clathrate. Eaparameter of whey and colostrum hydrolysates as constituents of mixtures and clathrates was estimated to rise by 1.4-1.6 times and 1.6-1.9 times, respectively (Table 1). It may be deduced that dairy peptides originating from whey and colostrum tend to be more stable upon mixing and complexing with β CD.
Antioxidant activity of β cyclodextrin clathrates with milk peptides
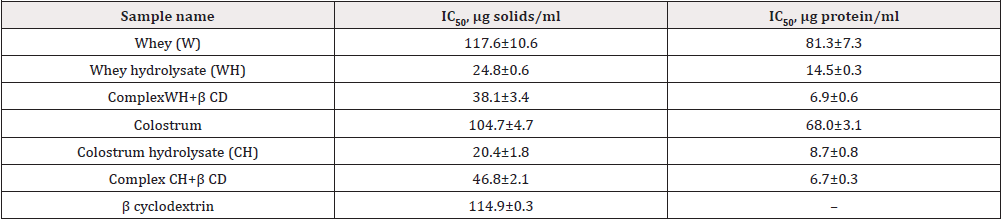
Antioxidant activity of β CD clathrates with whey and colostrum peptides was determined. Correlations of FL fluorescence intensity with concentrations of hydrolysates, cyclic oligosaccharide and inclusion complexes were established. The studies were conducted in wide concentration range of tested compounds (0.002-0.8 mg solids/ml). Experimental samples restored the FL fluorescence to 67-96 %. The IC50 values were graphically presented(the concentration of samples accounting for 50 % inhibition of reactive oxygen species).
According to literature reports, AOA of peptides is determined by reducing capacityof amino acid radicals, while antiradical properties of β CD are due to additional hydroxyl groups in cyclic oligosaccharide molecule [1,2,9]. Accordingly the IC50 was calculated relative to solids content and protein ratioin composition of hydrolysates and clathrates as reflected in Table 2. IC50 values computed per solids concentration were comparable for initial substrates (whey, colostrum) and β CD. Hydrolysis of wheyand first milk with alcalase and subsequent ultra-filtration promoted radical-reducing potential of peptide fractions by 4.7 and 5.1 times, respectively. Comparison of clathrates and hydrolysates pointed out reduction of antioxidant activity with shrinking of peptide fraction. Evaluation of IC50 parameters per content of protein component showed augmentation of AOA in hydrolysed and fractionated whey and colostrum by 5.6 and 7.8 times, respectively. It should be noted that radical-reducing activity of whey peptides was 1.7 times higher than that of cleaved colostrum. Complexing with β CD caused an increase in antiradical properties of whey/ colostrum hydrolysates by 2.1/1.3 times when compared with the respective peptide fractions. However, no significant distinctions were stated for both clathrate samples in recovery of fluorescence level. According to literature sources [3-5], production of clathrates with β CD improved organoleptic properties of amino acids and peptides. The previous paper [13] was focused on growing antioxidant ability of inclusion complexes exceeding 1.3-1.6 times AOA level of commercial whey protein hydrolysate.
The present study investigated effect of complexing process on flavor and antiradical activity of dairy hydrolysate samples. Clathrates of β CD with peptides of first milk and whey exhibited increased antioxidant potential surpassing by 1.3 and 2.1 times the similar values of unbound peptide fraction. The difference in AOA levels of native substrates and hydrolysates may be interpreted by peculiarities of protein-peptide composition (the ratio of casein to whey proteins) and the content of non-protein constituent.
Conclusion
A comparative examination of β CD inclusion complexes with whey and colostrum peptides was carried out. Organoleptic and antioxidant properties of the resulting clathrates were evaluated. The taste of the obtained products was characterized by considerable loss of bitterness in comparison with initial dairy hydrolysates. Thermo-gravimetric analysis confirmed formation of β CD complexes with whey and colostrum peptides. Stabilization of hydrolysate samples was found to occur in mixtures and clathrates with cyclic oligosaccharide. Fluorimetric investigations have shown that β CD complexing with whey and colostrum hydrolysates raised antioxidant potential of milk peptides by 2.1 and 1.3 times, respectively. Application of β CD clathrates with dairy peptides possessing acceptable flavor parameters and confirmed antioxidant capacity as additives in specialized foodstuffs (for infant, sport, dietetic nutrition) appears an extremely attractive option. Further research efforts should be focused on the effect of β CD complexing on the bioactive spectrum of milk peptides (antimutagenic, antimicrobial, antigenic properties).
References
- Szente L, Szejtli J (2004)Cyclodextrinsas Food Ingredients. Trends Food Sci Technol 15(3-4): 137-142.
- Martin Del Valle EM (2004) Cyclodextrinsand their uses: A Review. Process Biochem. 39(9): 1033–1046.
- Nishijo J, Tsuchitani M (2001) Pharm J Sci 90(2): 134-140.
- Linde GA, Junior AL, de Faria EV, Colauto NB, de Moraes FF, et al. (2009) Taste modification of amino acids and protein hydrolysate by α-cyclodextrin. Food Res Int 42: 814–818.
- Linde GA, Junior AL, de Faria EV, Colauto NB, de Moraes FF, et al. (2010) The use of 2D NMR to study β-cyclodextrin complexation and debittering of amino acids and peptides. Food Res Int 43: 187–192.
- Yang, M. Li, Y. Sun, and L. Zhang, (2018) A cell-penetratingPeptide Conjugated Carboxymethyl-β-cyclodextrin to Improve Intestinal Absorption of insulin.InJ Biol Macromol. 111: P. 685–695.
- Li JF, Zhang JX, Wang ZG, Yao YJ, Han X (2017) Food Chem 221: 296-301.
- Li J, Pan D, Yi J, Hao L, Kang Q, et al. (2019) Protective Effect of β-Cyclodextrin on Stability of Nisin and Corresponding Interactions Involved. Carbohyd Polym 115115.
- Hernández-Ledesma B, Dávalos A, Bartolomé B, Amigo L, Agric J (2005) Preparation of Antioxidant Enzymatic Hydrolysates From Alpha-Lactalbumin and Beta-Lactoglobulin. Identification of Active Peptides by HPLC-MS/MS. Food Chem. 53(3): 588-593.
- Zulueta, Maurizi A, Frígola A, Esteve MJ, Coli R, et al. (2009) Int Dairy J 19(6-7): 380-385.
- Halavach TN, Kurchenko VP, Zhygankov VG, Evdokimov IA (2015) DETERMINATION OF PHYSICOCHEMICAL, IMMUNOCHEMICAL AND ANTIOXIDANT PROPERTIES, TOXICOLOGICAL AND HYGIENIC ASSESSMENT OF WHEY PROTEIN COMCENTRATE AND ITS HYDROLYSATE. Foods and Raw Materials 3(2): 105–114.
- Golovach TN, Dudchik NV, Veremeenko EG, Tsygankou VG, Bondaruk AM, et al. (2016) Foods and Raw Materials 4(2): 38-47.
- Halavach TM, Dudchik NV, Tarun EI, Zhygankov VG, Kurchenko VP, et al. (2020) Biotechnol Food Sci 9(4): 714-720.
- Halavach TM, Tarun EI, Tsygankou VG, Butina AD, Kurchenko VP (2018) BSU Journal (Biology) 3: 3-13.
- Broido, Polym J (1969) Sc Part B: Polymer Physics. 7(10): 1761–1773.
- Tarun EI (2014) Proceedings of BSU. 9(1): 186-191.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.