Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
The spectrum of Thyroid function Abnormalities and associated Biochemical factors in Patients with Chronic Kidney Disease in Cameroon
*Corresponding author: Professor Jules Clement Assob Nguedia, Head of Medical Research and Applied Biochemistry Laboratory, Coordinator for Post-graduate programs, Faculty of Health Sciences, University of Buea, Southwest Region, Cameroon.
Received: April 01, 2020;Published: April 28, 2020
DOI: 10.34297/AJBSR.2020.08.001307
Abstract
Background: Chronic Kidney Disease (CKD) can lead to thyroid function disorders. The extent to which this relationship exists among Cameroonian CKD patients is not known. The aim of this study, was to determine the spectrum of thyroid dysfunction (TD) and their associated factors among CKD patients in Cameroon.
Methods: A cross-sectional study was conducted over a period of 12 months (July 2018 to August 2019) in three referral hospitals (Douala General Hospital, Laquintinie Hospital, Bafoussam Regional Hospital) in Cameroon with patients aged 18years and above diagnosed of CKD stage 1 to 5. Patients with stage 5 dialysis and those on thyroid altering medication were excluded. For each participant, we collected socio-demographic and clinical data. Sera were used to determine thyroid hormone profile, lipid profile, liver test, urea, creatinine, calcium, phosphate and uric acid levels. Albumin creatinine ratio (ACR) was estimated. The diagnosis of CKD was done by a nephrologist and classifies using estimated Glomerular Filtration Rate (eGFR) or urinary albumin creatinine ratio at the time of the study.
Results: A total of 374 participants were enrolled with male forming the majority (233(63.66%)). The mean age was 55.85(±13.72) years with an overall prevalence of TD was 57%. Hypertension, diabetes, and gout were the most common comorbidities. In total, 14 types of TDs were identified and grouped into major and minor types. The major types were subclinical hypothyroidism, primary subclinical hypothyroidism, primary overt hypothyroidism, subclinical hyperthyroidism, and overt hyperthyroidism. Low T3 syndrome, low FT3, combine low T3 and low FT3 were the most common minor types. TD increases with the stages of CKD. After logistic regression, albumin [OR: 0.961(0.93-0.991); p=0.015], phosphate [OR: 1.028 (1.014 - 1.045); p=0.001] and calcium [OR: 0.963 (0.941-0.983); p ˂0.001)] were independently associated to TD.
Conclusion: The Spectrum of TD is vast. Low T3, Low FT3, hypothyroidism, combine Low T3 and Low FT3 are the most common thyroid dysfunction. Altered calcium, Phosphate and albumin, were associated to TD in CKD.
Keywords: Chronic Kidney Disease, Thyroid Dysfunction, Prevalence, Spectrum, Hypothyroidism, Associated Factors
Introduction
Chronic kidney disease affects almost every organ-system in the body, and its common complications include; abnormal levels of metabolic waste such as urea and creatinine, mineral bone disorders like hypocalcemia, and hyperphosphatemia, dyslipidemia and thyroid dysfunction [1].
The kidney plays an important role in the metabolism, degradation, and excretion of thyroid hormones [2-4]. Therefore, long-standing and progressive deterioration of renal structure and function such as in chronic kidney disease (CKD) can alters the synthesis, secretion, metabolism, and degradation of thyroid hormones which then presents with different clinical syndromes of thyroid dysfunction [5-8]. Multiple mechanisms can account for these syndromes: lowering circulating thyroid hormone concentration, alteration of peripheral hormone metabolism, disturbed binding to carrier proteins, possible reduction in tissue thyroid content and increased iodine stores in thyroid glands [9]. Triiodothyronine (T3), the most metabolically active thyroid hormone, for instance, can be reduced in CKD patients even with a normal TSH level. This is termed as ‘Low T3 Syndrome’ [9,10]. Thyroid dysfunction may present in one of the following patterns: thyroid enlargement (diffuse or nodular); thyroid hormone deficiency or excess (hypothyroidism or hyperthyroidism); asymptomatic or symptomatic (the subclinical state or overt) [11].
Epidemiological studies have shown that the prevalence of thyroid function abnormalities especially hypothyroidism, is substantially higher in persons with chronic kidney disease compared to the general population [10]. In Nepal, South Korea, thyroid dysfunction was found in 38.6 % of patients, with subclinical hypothyroidism (27.2 %), overt hypothyroidism (8.1 %) and subclinical hyperthyroidism (3.3 %) being the most common types encountered [2,3]. In North India (Chennai), 66 % of CKD patients were reported to have thyroid dysfunction. Low T3 syndrome accounted for 58% against 8 % for hypothyroidism [13]. In Nairobi, Kenya, 42% of patients were found to have thyroid dysfunction. 14% had non-thyroidal illness, subclinical and primary hypothyroidism accounting for 15% while different forms of hyperthyroidism accounted for 13 % [7]. Many cases of hypothyroidism may remain latent or undiagnosed in advanced CKD due to symptoms overlapping with uremia and co-existing Comorbidities. The kidney is not only an organ for metabolism and elimination of TH, but also a target organ of some of the iodothyronines’ actions. Thyroid dysfunction causes remarkable changes in glomerulo tubular functions, electrolyte and water homeostasis. Hypothyroidism is accompanied by a decrease in glomerular filtration, hyponatremia, and an alteration of the ability for water excretion. Excessive levels of TH generate an increase in glomerular filtration rate and renal plasma flow. Renal disease, in turn, leads to significant changes in thyroid function. The association of different types of glomerulopathies with both hyperand hypofunction of the thyroid has been reported. Less frequently, tubulointerstitial disease has been associated with functional thyroid disorders. Nephrotic syndrome is accompanied by changes in the concentrations of TH due primarily to loss of protein in the urine. Acute kidney injury and chronic kidney disease are accompanied by notable effects on the hypothalamus–pituitary– thyroid axis. The secretion of pituitary thyrotropin (TSH [6]. These patients with thyroid dysfunction may have clinically important reductions in estimated glomerular filtration rate (eGFR), which can be attenuated by using thyroid hormone replacement therapy [14]. When hypothyroidism becomes severe it can cause reduced cardiac function and lead to progressively worsening of kidney function. Thus thyroid dysfunction may worsen the morbidity in CKD patients and increase cardiovascular mortality [15]. Hypothyroidism can also lead to hyperlipidemia and atherosclerosis in coronary and peripheral vessels. Previous studies have indicated that subclinical and clinical hypothyroidism were the risk factors for all-cause mortality and CVD (Cardiovascular Disease) death. Low T3 syndrome is an independent predictor of cardiovascular mortality in CKD patient [9,10].
Biochemical factors in CKD that affect thyroid function as well as the spectrum of thyroid function abnormalities are not well known, as such we sort to determine the spectrum of thyroid dysfunction and its associated biochemical profile in CKD patients.
Materials and Methods
We carried out a cross-sectional study at the Littoral region (Laquintinie and Douala General Hospitals) and West Region (Bafoussam Regional Hospital) of Cameroon. These health facilities were selected because they provide care to a vast majority of the population in their respective regions and they host a wide range of socio-economic classes. Each of these hospitals is equipped with a nephrology unit that is under the responsibility of at least 1 nephrologist.
Ethical Consideration
The protocol of this study was submitted to and approved by the institutional review board of the Faculty of Health Sciences of the University of Buea (Ref: 2018/753-01B/UB/SG/IRB/FHS). Administrative authorizations were obtained from the Directorate of each institution. Participation was strictly voluntary, after having provided written informed consent.
Inclusion criterion
The target population was made of adult (≥ 18 years) patients diagnosed with CKD by the nephrologists of the study centers.
Exclusion criteria
Patients with known thyroid disorders, those on medications affecting thyroid function (e.g Amiodarone, propranolol), those on maintenance hemodialysis or with nephrotic range proteinuria were not included in the study.
Study procedure
After a detailed explanation of the study and obtaining written consent or assent, a questionnaire was used to collect sociodemographic features (age and sex) and clinical data from each participant and using their medical records. A blood sample (5 ml) was collected from each the subjects who agreed to participate in the study as well as those who met the inclusion criteria using a vacutainer plain tubes and was left for a short time to allow the blood to clot and then serum samples were obtained by centrifugation at 3000 rotation per minutes for 10 minutes. The serum obtained after centrifugation was used to determine thyroid hormone profile (TSH3, FT4, and FT3) using the immune fluorescent assay (MINI VIDAS, BIOMERIEUX, Marcy Etoile, France), TT4 and TT3 were analyzed using ELIZA (Enzyme-Linked Immunosorbent Assay) method (Biorex Diagnostic, Antrim, United Kingdom). The biochemical test for serum creatinine, urea, calcium, phosphorus lipid profile, transaminase and albuminemia, was done using the COBAS C111 (La Roche Diagnostic System, Swiss, Germany) and the spot urine was tested for albuminuria (Roche Diagnostic GMBH, Mannheim, Germany) and creatinuria using COBAS C111and their ratio (ACR) was determined. eGFR was calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) and stages were classify by the Kidney Disease Improving Global Outcomes (KDIGO):
Calculation of sample size
Using the prevalence of 42 % of a study done in Nairobi thor”:[{“dropping-particle”:””,”family”:”Kaggia”,”given”:”S N N”,”nondropping- particle”:””,”parse-names”:false,”suffix”:””}],”id”:”ITEM- 1”,”issued”:{“date-parts”:[[“2013”]]},”title”:”Thyroid Hormone Profiles in Patients With Chronic Kidney Disease at Kenyatta National Hospital”,”type”:”article-journal”},”uris”:[http://www. mendeley.com/documents/?uuid=9daa3261-a1d0-467d-b567- 65ef368139a5],”mendeley”:{“formattedCitation”:”(6[6], the sample size was calculated using the formula below for a cros.....

Where
n = Sample size is 374 patients with CKD;
Z1-α/2 Standard normal deviate at 5% level of significance (95% CI) is 1.96;
d = Margin of error at 5%.
Determination of CKD Parameters
Chronic Kidney Disease: eGFR< 60 ml/min per 1.73 m2 for more than 3 months with or without evidence of kidney damage or albuminuria (≥ 30 mg/g) with or without decreased GFR for ≥ 3 months, as diagnosed by a nephrologist [17-19].
Estimated glomerular filtration rate (eGFR) was computed from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [20].
Chronic kidney disease stages were defined as described by the Kidney Disease Improving Global Outcomes (KDIGO) and classify using estimated eGFR or urinary albumin creatinine ratio at the time of the study as:
- CKD1: eGFR> 90 ml/min and albuminuria,
- CKD 2:60-89 mil/min and albuminuria,
- CKD 3a: 45-59ml/min,
- CKD 3b: 44-30ml/min,
- CKD 4: 29 - 15 ml/min and
- CKD 5:<15ml/min or dialysis [21].
Albuminuria was used to describe albumin creatinine ratio(ACR) between 30 and 299 mg/g and 300 mg/g or over, respectively [22,23].
Nephrotic range Proteinuria was defined as proteinuria of 3+ to 4 or as albuminuria of > 2.2g/g [24].
Definition and classification of categories of thyroid dysfunction
Thyroid dysfunction was considered if patients’ thyroid hormones fall outside the reference range. The categories of thyroid dysfunction were classified based on the reference intervals for the hormones and pattern of derangement in the thyroid hormones profile.
The abnormal thyroid function tests result was classified into any of the following:
Subclinical hypothyroidism: TSH elevation between ˃4.7 mIU/L in patients with normal serum TT3 or FT3 and TT4 or FT4.
Primary subclinical hypothyroidism: TSH ˃4.7 mIU/L and suppressed serum TT3 or FT3 and TT4 or FT4T4.
Primary overt hypothyroidism: TSH (>20 μIU/dl) with low serum FT4 and low FT3 [25].
Subclinical hyperthyroidism: suppressed TSH (<0.27) mIU/L and normal TT3 or FT3 and TT4 or FT4 serum concentration.
Overt hyperthyroidism: suppressed TSH (<0.27) mIU/L and elevated serum TT3 or FT3 and TT4 or FT4 concentration.
Non-thyroidal illness or low T3 syndrome: Low TT3 or FT3 in the presence of normal TSH, TT4 and FT4 levels.
Euthyroid hyperthyroxinaemia: isolated elevation of FT4 or TT4 in the presence of TSH, FT3 and TT3 within reference limits [26].
Reference ranges for the thyroid hormones were: TSH: 0,27 - 4,7 mIU/L, FT4: 10,6 - 19,4 pmol/L, FT3: 2,6 - 5,4pmol/LTT4: 5,0 - 13,0 ug/mL TT3: 0.52–1.85 ηg/dL. Creatinine: 0.6–1.2 mg/dL for women and 0.7-1.4mg/dL for men according to the manufacturer.
Statistical analysis
Data were analyzed using SPSS version 20.0. Nominal variables were summarized using counts and percentages while continuous variables such as age, creatinine, and serum albumin levels were summarized using means, standard deviations. Group comparisons for categorical variables were done using the Chisquared test (or Fisher’s exact test where appropriate) while the independent samples t-test (or ANOVA where appropriate) was used for comparing group means for continuous variables. Logistic regression was used to assess the association between predictor variables and thyroid dysfunction. The p-values below 5% were considered statistically significant.
Results and Discussions
Results
Biochemical characteristics
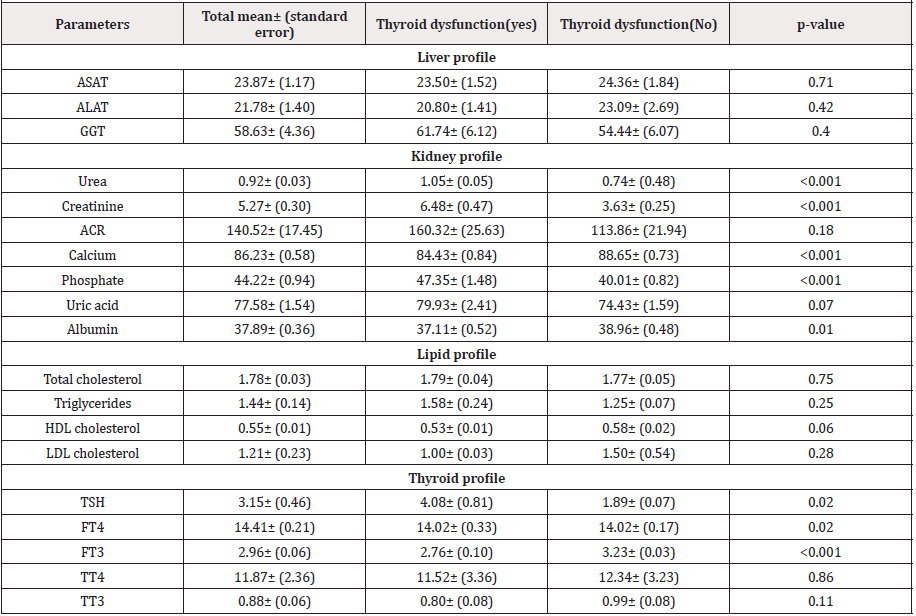
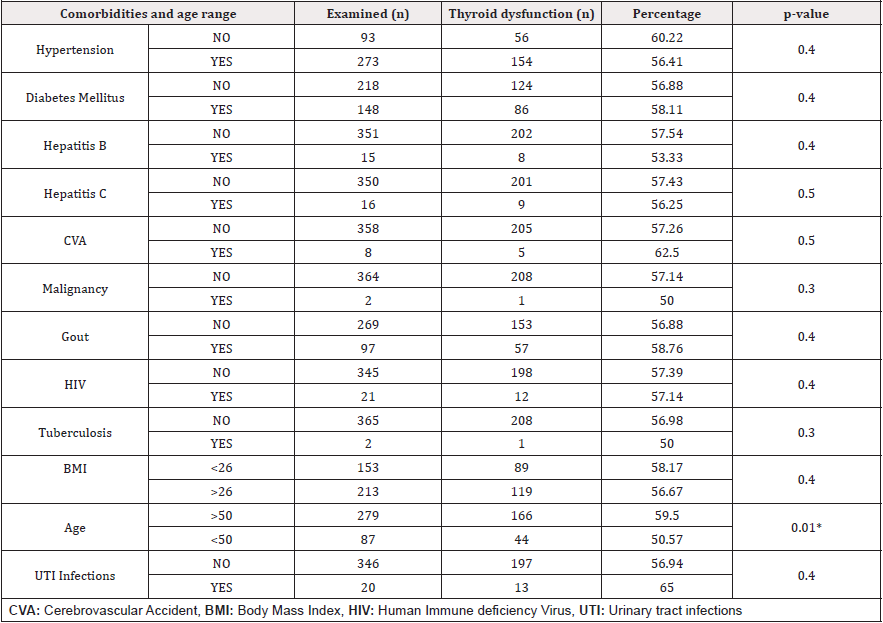
In a total of 374 participants enrolled for this study, 233(63.66%) males. The mean age was 55.85(±13.72) years. 210 patients had thyroid dysfunction. Serum urea, creatinine, phosphate and TSHwere significantly high in patients with thyroid dysfunction while calcium, albumin, FT4 and FT3 where significantly low in these patients (Table I).
Prevalence by stages of CKD
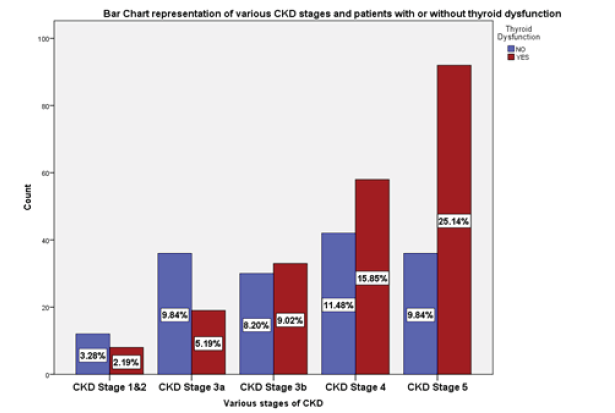
The prevalence of thyroid dysfunction was 57, 38% and was found to increase with the severity of CKD, with stage 5 having the highest prevalence of 25.1 % (Figure 1)..
The spectrum of thyroid function abnormalities
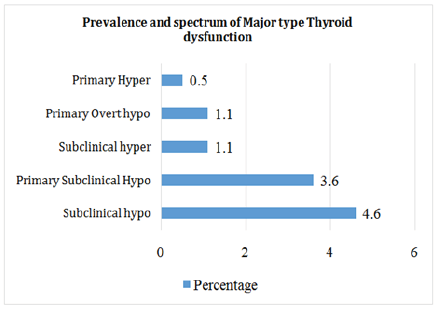
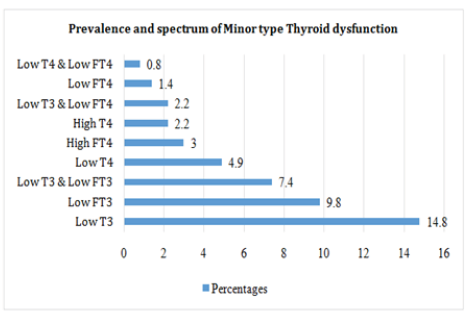
In all, we identified 14 types of thyroid dysfunction which were grouped into major and minor types. The major types were Subclinical Hypothyroidism (4.6%), primary subclinical hypothyroidism (3.6%), primary overt Hypothyroidism (1.1%), subclinical Hyperthyroidism (1.1%), and overt hyperthyroidism (0.5%) (Figure 2A). While Low T3 syndrome (14.8%), low FT3 (9.8%), combine low T3 and low FT3 (7.4%) were the most common minor types (Figure 2B).
Comorbidities associated with thyroid dysfunction
Age above 50years was the only factor significantly related with thyroid dysfunction(p<0.05) (Table 2).
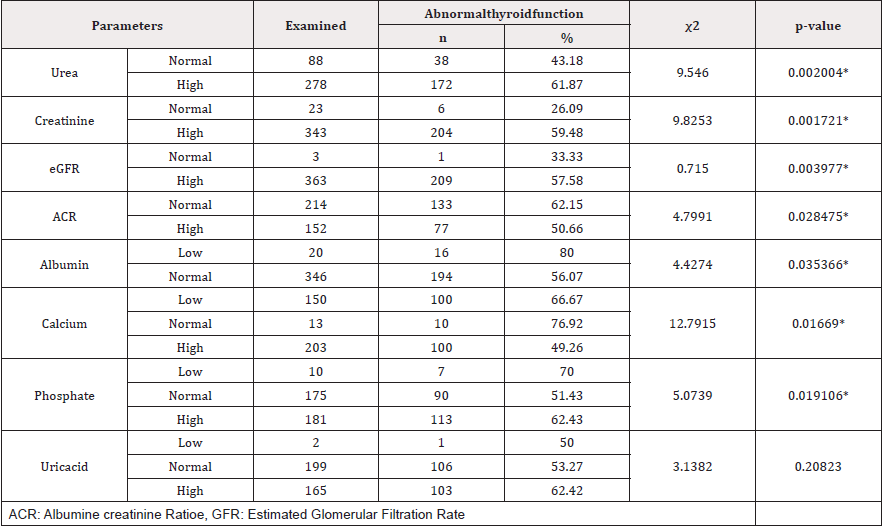
Association between biomarkers of CKD and thyroid dysfunction
Serum urea, creatinine, albumin, ACR, calcium and phosphate were significantly associated with all forms of thyroid dysfunction (p<0.05) (Table 3).
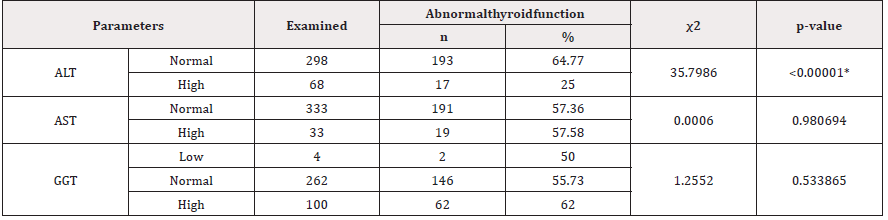
Association of Thyroid Function Abnormalities with Liver Biomarkers
Only ALT was significantly associated with all forms of thyroid dysfunction (p<0.05) (Table 4).
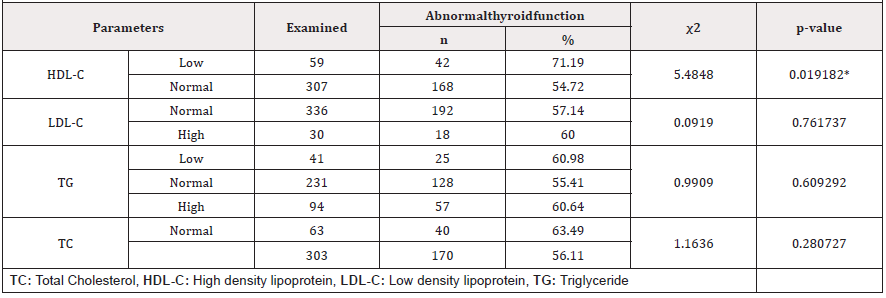
Association of Thyroid Function Abnormalities with Lipid Profile
Only HDL was significantly associated with all forms of thyroid dysfunction (p<0.05) (Table 5).
Associated factors to thyroid dysfunction in chronic kidney disease
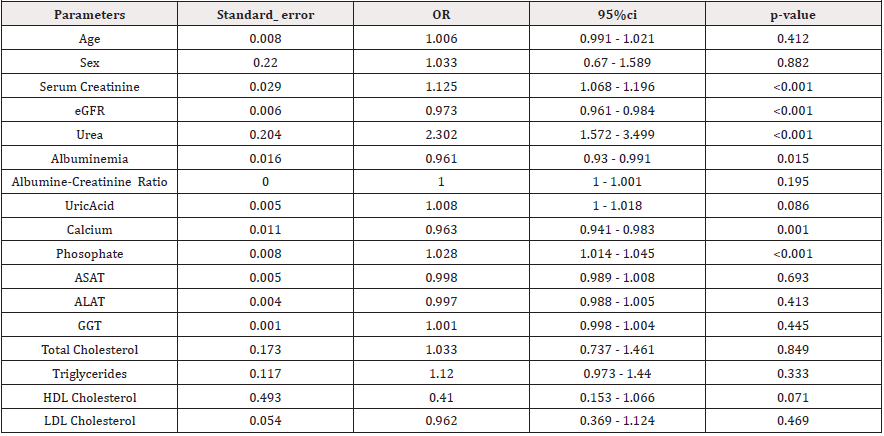
After logistic regression analysis only albumin, calcium and phosphate were independently associated to thyroid dysfunction. Albumin [OR: 0.961 (0.93 - 0.991); p=0.015], Phosphate [OR:1.028 (1.014 - 1.045); p=0.001]and calcium[OR: 0.963(0.941 (Table 6).
Discussion
In this study, of which our objectives were to determine the prevalence, describe the spectrum and biochemical factors associated with thyroid function abnormalities in patients with CKD, we found a high prevalence of thyroid dysfunction and an impressive array of abnormalities. We, however, proceeded to classify them as either major or minor based on data reported in recent literature; the most frequently identified and cited as well as those of proven direct clinical significance were classed as major and vice.
The prevalence of thyroid function abnormalities was 57.4%. This is similar to that in India, estimated at 58% [25]. It is, however, higher than that in Nepal (38.6 %) and Nairobi (42%) [3,7]. Given that thyroid function deteriorates as CKD worsens, it was most likely to find patients with thyroid dysfunction. The high prevalence of thyroid dysfunction can be explained by several factors;
a. Metabolism of thyroid hormones occurs mostly in the kidney, as a consequence deterioration in kidney function leads to altered thyroid physiology [27]. Our population consist principally of persons with altered kidney function as such a high prevalence of thyroid dysfunction.
b. Age; most participants were elderly with a mean age of 55years and the 61-70years age group being the most frequent. The D1-785T variant of the D1 receptor involved in the conversion of T4 to T3 can undergo polymorphism and results in a decreased activity of D1 [28]. Although the D1- 785T variant is not associated with serum rT3 levels in the general population, its association with lower levels of T3 in an elderly population can supports the hypothesis of lower activity of D1 in carriers of this polymorphism [28]. In young subjects, a decreased T3 production by D1 may be masked by the production of serum T3 by skeletal muscle D2. Throughout adult life, skeletal muscle size and strength gradually decline, resulting in a decrease in D2-expressing skeletal muscle. Furthermore, rT3 levels increase with age, and degradation of the D2 protein is accelerated when it is exposed to its substrates T4 and rT3 [28,29]. This results in the increased possibility of developing thyroid dysfunction in advanced age (Table 2). Swaminathan observed the same trend [30].
c. Other factors such as comorbidities or hereditary disorders, severe illness and nutrition may also have increased the prevalence especially in non-thyroidal illness [31].
These factors put together can result in an amalgam of thyroid function abnormalities.
In all, we identified 14 types of thyroid function abnormalities in patients with chronic kidney disease. To make sense of this data, we decided to group them into major and minor disorders. The major types of thyroid dysfunction were subclinical hypothyroidism (4.6%), primary subclinical hypothyroidism (3.6%), primary overt hypothyroidism (1.1%) and Hyperthyroidism (1.1%) (Figure 2A). Only primary subclinical hypothyroidism was statistically significantly associated with the stages of CKD (p<0.05).However, in our study, the overall hypothyroidism was 9.3%. Similar study was done by Tewari in India who had 10.9% of hypothyroidism affecting the study population [32] but different from Ayree in Ghana who had 2% of hypothyroidism [33].
Among the minor type of thyroid dysfunction, the majority of patients had Low T3(14.8 %). Followed by Low FT3 (9.8 %), combine Low T3& LowFT3 (7.4%), Low T4 (4.9%), High FT4(3.0%), High T4(2.2%), LowT3 & LowT4 (2.2%), Low FT4(1.4%), and Low T4 & Low FT4 (0.8%) (Figure 2B).
Low T3 has been reported in recent literature to be the most frequent type of thyroid dysfunction in patients with CKD [31]. Free and total T3 and T4 concentrations are usually normal or low in patients with CKD [33]. This reduction in T3 concentration has been linked to a decrease in the peripheral conversion of T4 to T3 [6]. Low T3 levels have been reported by Gowda in 28% patients with CKD [25]. Zoccali et al. had similar results in 2006 [34]. The high prevalence of LowT3 can be explained by the fact that there is free inorganic iodide accumulates in CKD. This then results in inhibition of iodine tapping and consequent decrease in T4 to T3 production. The drop in the levels of T3 stimulates the thyroid–pituitary feedback loop, leading to excessive secretion of TSH [35,36]. In some cases the levels of T3 and T4 are normalized. Failure of this coping mechanism will lead to overt hypothyroidism. Furthermore, low T3 syndrome is closely associated with both malnutrition-inflammation complex syndrome (MICS) and anemia, conditions common in CKD [37]. A Decrease in D1 (de-iodinase) activity caused by the accumulation of uremic toxins, metabolic acidosis, and markers of inflammation such as TNFα, IL-1 results in decreased peripheral conversion of T4 to T3 leading to Low T3 as well as Low FT3, accumulation of T4, consequently High T4 and High FT4, thus producing the spectrum of minor thyroid dysfunctions seen above [10].
CKD populations had lower albumin and calcium but higher phosphate using linear regression (Table 6) in our study. This is similar to Singh’s study in 2010 who found significant association between thyroid dysfunction, calcium and phosphate in CKD patients (p<0.05) using Kruskal-Wallis test [37]. Thyroid hormones play an important role in homeostasis of Calcium and Phosphorous levels by their direct action on bone turnover. Thyroid hormones Stimulate bone resorption directly there by increasing the serum calcium and phosphorous levels and suppressing PTH [38]. In CKD, patients usually have symptoms of nausea, vomiting, bad appetite because of accumulation of toxin, and anemia. Therefore, it is common to observe malnutrition in these patients which can result in various mechanisms: spontaneous reduction of dietary proteincaloric intake, all the more pronounced as the renal function is more impaired, alteration of the metabolism of the main nutrients, exaggerated protein catabolism linked to metabolic acidosis, insulin resistance and hyperparathyroidism due to undercurrent infectious or inflammatory pathologies [38]. This can result in a malnutrition-inflammation complex syndrome which is a major cause of non-thyroidal illness, characterized by the minor thyroid function abnormalities seen above. Thus, hypoalbuminemia is an independent predictor of thyroid dysfunction. Pan et al observed similar association [10].
Metabolic toxin such as urea and creatinine inhibit peripheral conversion of T4 to T3 as such leading to thyroid function abnormalities. Following linear regression analysis, phosphate, calcium and albumin were identified as factors associated with thyroid dysfunction. Albuminaemia [OR: 0.961 (0.93 - 0.991); p=0.015], phosphatemia [OR: 1.028 (1.014 - 1.045); p=0.001]and calcium[OR: 0.963 (0.941 - 0.983); p ˂0.001)] (Table 6).
Conclusion
The prevalence of thyroid dysfunction in CKD is very high, with Low T3 being the most common type. The spectrum of TD is vast. Low T3, Low FT3, Hypothyroidism, combine LowT3 and Low FT3 are the most common thyroid dysfunction. This study supported the contribution of chronic kidney disease in thyroid dysfunction. Increased phosphataemia, as well as decreased calcaemia and albuminaemia were identified as predictors of TD in CKD patients.
Acknowledgment
We heartily thank the management and staff of the Nephrology Units of; The Douala General Hospital, Laquintinie Hospital Douala and the Bafoussam Regional Hospital.
Authors’ Contributions
PK: Participated in the conception and study design, collected, and interpreted data, reviewed literature and drafted the manuscript.
MPH: Conceived and supervised the work, oversaw data collection, participated in data analysis and interpretation, drafted the manuscript. A critical review of the manuscript
NAJ: Over saw data collection, participated in data analysis, drafted the manuscript, Critical review of the manuscript
JCAN: Contributed in the conception and supervised the work, oversaw data collection, participated in data analysis and interpretation, drafted the manuscript. A critical review of the manuscript
TJF: Participated in the study design and reviewed literature and data interpretation
MNN: Study design and supervised the work, oversaw literature search and revised the manuscript.
All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that they have no competing interest.
References
- Singh S, Verma A, Aryal G, Thapa S, Khakurel S, Shrestha K (2016) Thyroid hormone profile in patients with chronic kidney disease: a single centre study. Hypertension 24(3): 23-30.
- Khatiwada S, Rajendra KC, Gautam S, Lamsal M, Baral N (2015) Thyroid dysfunction and dyslipidemia in chronic kidney disease patients. BMC Endocr Disord 15(1): 1-7.
- Tripathy SK, Dhal N, Kanungo M, Das S, Mishra SK, et al. (2018) Study of thyroid dysfunction and dyslipidemia in chronic kidney diseases 6(1): 110-116.
- Ibrahim IA, Ramadan YK, Hassan EA (2016) Abnormalities in Thyroid Function and Morphology in Chronic Hemodialysis Patients. 84 (1): 143-148.
- Madariaga AG, Santos Palacios S, Guillén-Grima F, Galofré JC (2014) The incidence and prevalence of thyroid dysfunction in Europe: A meta-analysis. J Clin Endocrinol Metab 99(3): 923-931.
- Iglesias P, Díez JJ (2009) Thyroid dysfunction and kidney disease. Eur J Endocrinol 160(4): 503-515.
- Kaggia SNN (2013) Thyroid Hormone Profiles in Patients With Chronic Kidney Disease at Kenyatta National Hospital.
- Iglesias P, Bajo MA, Selgas R, Díez JJ (2017) Thyroid dysfunction and kidney disease: An update. Rev Endocr Metab Disord. 18(1): 131-144.
- Farag SES (2013) Functional and Morphological Thyroid Disorders in Hemodialysis Patients. J Thyroid Disord Ther 02(01): 2-5.
- Rhee CM, Brent GA, Kovesdy CP, Soldin P, Nguyen D, et al. (2018) Thyroid functional disease : an under-recognized cardiovascular risk factor in kidney disease patients. (February 2014): 724-737.
- Pan B, Du X, Zhang H, Hua X, Wan X, Cao C (2019) Relationships of Chronic Kidney Disease and Thyroid Dysfunction in Non-Dialysis Patients: A Pilot Study. Kidney Blood Press Res 44(2): 170-178.
- Chandra A (2016) Prevalence of hypothyroidism in patients with chronic kidney disease: a cross-sectional study from North India. Kidney Res Clin Pract 35(3): 165-168.
- Shin DH, Lee MJ, Lee HS, Oh HJ, Ko K Il, et al. (2013) Thyroid Hormone Replacement Therapy Attenuates the Decline of Renal Function in Chronic Kidney Disease Patients with Subclinical Hypothyroidism. Thyroid 23(6): 654-661.
- Carrero J, Qureshi A (2015) Clinical and biochemical implications of low thyroid hormone levels (total and free forms) in euthyroid patients with chronic kidney disease. Journal of internal …. 2007. p. 2015 30(2): 282-287.
- Chonchol M, Lippi G, Salvagno G, Zoppini G, Muggeo M, et al. (2008) Prevalence of subclinical hypothyroidism in patients with chronic kidney disease. Clinical Journal of the American Society of Nephrology 3: 1296-1300.
- Lesley A (2012) Inker; Brad C. Astor; Chester H. Fox. KDOQI US Commentary on the 2012 KDIGO Clinical Practice Guideline for the Evaluation and Management of CKD. Ajkd. p. 1-23.
- Mohamedali M, Maddika SR, Vyas A, Iyer V, Cheriyath P (2014) Thyroid Disorders and Chronic Kidney Disease 2014: 1-7.
- Miulescu RD, Cristian M, Ma D, Poiană C, Păun DL (2014) Associations between Thyroid Dysfunction and Chronic Kidney Diseaseb21(1): 37-42.
- Levey AS, Coresh J (2012) Chronic kidney disease. Lancet 379(9811): 165-180.
- Levin A, Stevens PE (2014) Summary of KDIGO 2012 CKD Guideline: Behind the scenes, need for guidance, and a framework for moving forward. Kidney Int 85(1): 49-61.
- Rhee CM, Chen Y, You AS, Brunelli SM, Kovesdy CP, Budoff MJ, et al. (2017) Thyroid status, quality of life, and mental health in patients on hemodialysis. Clin J Am Soc Nephrol 12(8): 1274-1283.
- Chen W, Liu Q, Wang H, Chen W, Johnson RJ, Dong X, et al. (2011) Prevalence and risk factors of chronic kidney disease: A population study in the Tibetan population. Nephrol Dial Transplant 26(5): 1592-1599.
- Cassia MA, Pozzi FE, Bascape S, Saggiante L, Daminelli G, et al. (2016) Proteinuria and albuminuria at point of care. Nephrol @ Point Care 2(1): E8-E16.
- Gowda MAS (2016) Evaluation of Thyroid Function Status in Patients with Chronic Kidney Disease. J Med Sci Clin Res 4(11): 14235-14241.
- Okpara HC (2017) Spectrum of Thyroid Dysfunction among Patients Evaluated by Thyroid Function Tests at a Tertiary Clinical Laboratory in Calabar, Nigeria 411-417.
- Massolt ET, Salih M, Beukhof CM, Kam BLR, Burger JW, Visser WE, et al. (2017) Effects of Thyroid Hormone on Urinary Concentrating Ability. Eur Thyroid J 6(5): 238-242.
- Peeters RP, Deure WM Van Der, Visser TJ (2006) Genetic variation in thyroid hormone pathway genes ; polymorphisms in the TSH receptor and the iodothyronine deiodinases p. 655-662.
- Peeters RP, Van Den Beld AW, Van Toor H, Uitterlinden AG, Janssen JAMJL, et al.(2005) A polymorphism in type I deiodinase is associated with circulating free insulin-like growth factor I levels and body composition in humans. J Clin Endocrinol Metab 90(1): 256-263.
- Swaminathan DK, Rajesh DS, Avudaiappan DS (2016) “A Study of Thyroid Function Abnormalities in Patients with Chronic Kidney Disease.” IOSR J Dent Med Sci 15(08): 07-15.
- Lim VS (2001) Thyroid function in patients with chronic renal failure. Am J Kidney Dis 38: S80-S84.
- Tewari N (2014) Prevalence of hypothyroidism in adults: An epidemiological study in eight cities of India. Indian Journal of Endocrinology and Metabolism 18: 116.
- Aryee NA, Tagoe EA, Anomah V, Arko-Boham B, Adjei DN (2018) Thyroid hormone status in Ghanaian patients with chronic kidney disease. Pan Afr Med J 29: 1-6.
- Zoccali C, Mallamaci F, Tripepi G, Cutrupi S, Pizzini P (2006) Low triiodothyronine and survival in end-stage renal disease. Kidney Int 70(3): 523-528.
- Dousdampanis P, Trigka K, Vagenakis GA, Fourtounas C (2014) The thyroid and the kidney : a complex interplay in health and disease 37: 1-12.
- Rhosni PRMM (2016) Risk factors associated with chronic kidney disease: An overview. Int J Pharm Sci Rev Res 40(2): 255-257.
- Samir Singh (2016) “Status of thyroid hormone profile in patients with chronic kidney disease Under the Supervision of Supervisor Co-Supervisor.”
- Mb Shivaleela, Poornima Rt, Jayaprakash Murthy Ds, J J M Medical College (2012) “Serum Calcium and Phosphorous Levels in Thyroid.” 2(2): 179-83.
- Fan J, Yan P, Wang Y, Shen B, Ding F, Liu Y (2016) Prevalence and Clinical Significance of Low T3 Syndrome in Non-Dialysis Patients with Chronic Kidney Disease. Med Sci Monit 22: 1171-1179.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.