Opinion 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Advanced Laboratory Investigations Conducted at Medical Research Unit Laboratory, MHWT, Uttara Model Town, Dhaka, Bangladesh
*Corresponding author: ASM Giasuddin, Professor of Biochemistry & Immunology & Director, Medical Research Unit (MRU), Medical College For Women & Hospital (MCW&H) Building, The Medical & health Welfare Trust (MHWT), Plot-4 Road-9 Sector-1, Uttara Model Town, Dhaka-1230, Bangladesh
Received: June 09, 2020;Published: July 15, 2020
DOI: 10.34297/AJBSR.2020.09.001416
Abstract
Among the medical sciences, Diagnostic Immunology has become a versatile branch due to availability of monoclonal antibody (MCA)-based advanced techniques such as radioimmunoassay (RIA), enzyme immunoassay (EIA), fluorescence activated cell sorter (FACS), Nephelometry, Immunochromatographic technique (ICT), Immunofluorescence technique (IFT), etc. Laboratory diagnosis and follow-up of immunologically -mediated as well as other diseases are made now-a-days with high precision and accuracy with the application of these advanced techniques. Diagnostic immunology was reorganized and more versatile investigations were introduced at the Medical College for Women & Hospital (MCW&H) laboratory, Uttara, Dhaka in August 2002. The idea was to provide high quality laboratory services for specialized diagnostic immunology tests in a cost effective way. In 2005 Medical Research Unit (MRU) had been set up in at MCW&H building to conduct high quality clinical and biomedical research and provide further specialized biochemical and immunological laboratory tests at affordable costs. Many diagnostic tests were introduced and carried out regularly at MRU (Diagnostic) Laboratory to confirm and pin-point the clinical (provisional) diagnosis of human diseases. The analysis of the results of some advanced laboratory investigations and also the immunodiagnostic tests in various antibody classes for TORCH/ ToRCH infections conducted at MRU Laboratory have been presented in the present article and discussed accordingly.
Keywords: Diagnostic immunology, Hepatitis marker, Autoantibody, Tumour marker, Immunoglobulin E, Complement, Hormone, ToRCH, TORCH
Background
Since the establishment of radioimmunoassay (RIA), enzyme immunoassay (EIA), discovery of monoclonal antibody (MCA) and fluorescence activated cell sorter (FACS), tremendous progress have been made in the field of diagnostic immunology. MCA based advanced techniques such as RIA, EIA, FACS, nephelometry, immunofluorescence technique, immunochromatographic technique (ICT), etc are presently used in modern diagnostic immunology. With the application of these powerful techniques, laboratory diagnosis and follow-up of immunologically-mediated as well as other diseases are made now-a-days with high precision and accuracy [1-6].
Immunology laboratory were reorganized and more versatile tests were introduced at the Medical College for Women & Hospital (MCW&H) in August 2002. The idea was to provide high quality laboratory service for specialized diagnostic immunology tests in a cost effective way. In 2005 Medical Research Unit (MRU) had been set up in at MCW&H building to conduct high quality clinical and biomedical research and provide further specialized biochemical and immunological laboratory tests at affordable costs. Many diagnostic tests were introduced and carried out regularly at MRU (Diagnostic) Laboratory to confirm and pinpoint the clinical (provisional) diagnosis of human diseases. The analysis of the results of some advanced laboratory investigations and also the immunodiagnostic tests in various antibody classes for TORCH/ToRCH infections carried out at MRU (Diagnostic) Laboratory have been presented in the present article. Some of the other immunological test results produced at MRU (Diagnostic) Laboratory such as antinuclear antibody (ANA), anti-dsDNA antibody (Anti-dsDNA), complements (C3, C4), immunoglobulin E (IgE), etc had been published in 2005 previously [6].
Materials and Methods
Immunodiagnostic Tests
The diagnostic tests introduced and routinely carried out at MCW&H since August 2002 were the following: Antinuclear antibody (ANA), Anti- dsDNA antibody (Anti-dsDNA), Complement3 (C3), Complement 4 (C4), Immunoglobulin MCW&H since August 2002 were the following: ANA, anti-dsDNA, C3, C4, IgE, PSA, CEA, AFP, T3, T4, TSH, PRL, TEST, HBsAg, HBc IgM Ab, HBeAg, HBsAb, HCVAb, HAV-IgM Ab and HEV-IgM Ab [6]. The laboratory procedures adopted were the following.
Autoantibody Profile
The diagnostic Kits for autoantibodies were based on indirect solid phase enzyme immunometric assay (EIA) [7-9]. C3, C4 and IgE: Serum complement components (C3, C4) were quantitatively determined using diagnostic kits based on liquid phase immunoprecipitation assay with nephelometric end point detection [1-4]. The quantitative estimation of total IgE in human serum was made using assay kit based on EIA [6,10].
Tumour/Cancer Markers
The tumour markers (PSA, CEA, AFP) were quantitatively analysed using diagnostic kits based on third generation EIA in human serum11,12,13; Hormone Profile: The analysis of hormones (T3, T4, TSH), were carried out using indirect and competitive EIA based assay kits [6,14-17]. Hepatitis Virus Profile: All the hepatitis virus infection tests were done using diagnostic kits based on third generation EIA obtained from different internationally reputed companies [18,19].
TORCH Panel
The immunodiagnostic tests in IgG and IgM class antibodies were done at MRU from January 2017 to Septemder2018 against Toxoplasma (TO), Rubella virus (R), Cytomegalo virus (C) and Herpes simplex viruses 1 & 2 (H) using kits based on solid phase indirect and competitive binding EIA supplied by reputed international companies from USA, UK, Germany, Belgium, China, etc [20-26].
Results
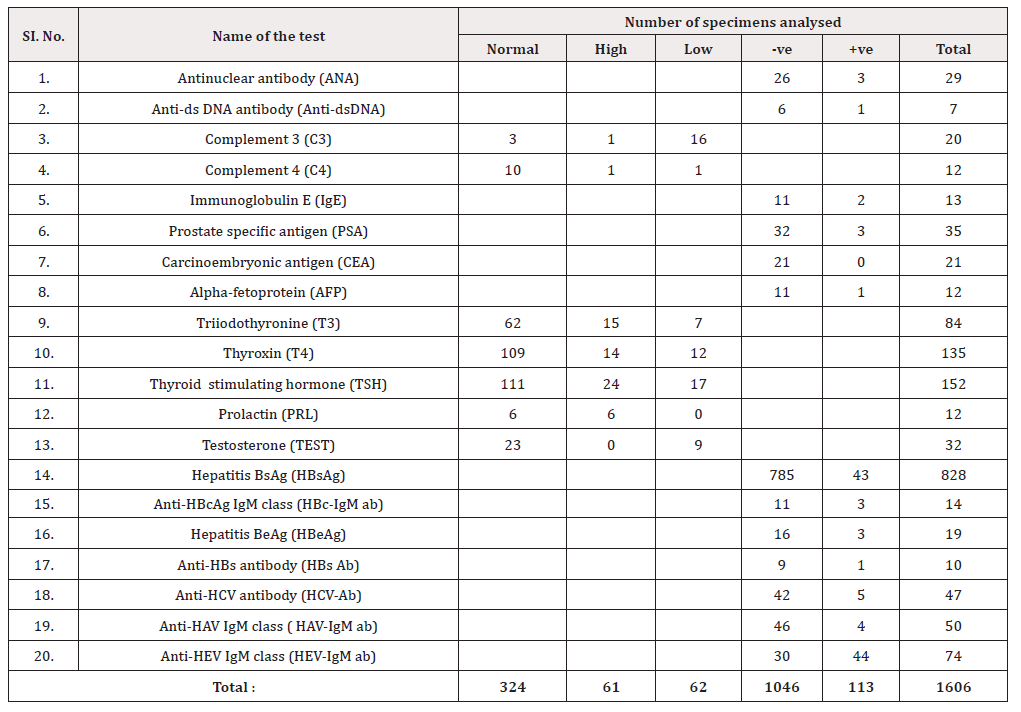
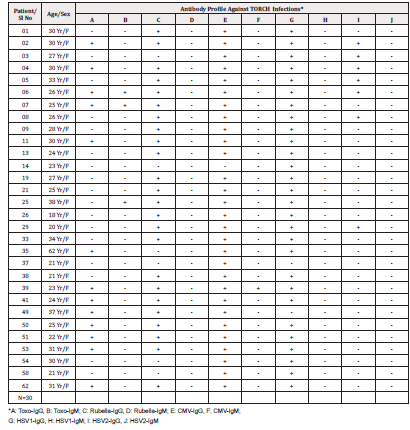
The results of the serum specimens analysed from June 2003- July 2004 for autoantibody profile, C3, C4 and IgE, tumour/cancer markers, hormone profile and hepatitis virus profile are presented in Table 1. The results of the complete TORCH panel are stated in Table 2. The results of tests in serum for one or the other component (organism) of TORCH infections are stated in Table 3. Table 2 shows that all 30 specimens analysed for TORCH infections were females (N: 30, Age range: 18-38 years; Mean age + SD: 28+20 years). It was evident from Table 2 that only 3 specimens (i.e. specimen no 06, 07, 25) and 1 specimen (i.e. specimen no 39) were positive for current/ recent infection with Toxoplasma and CMV respectively. All other specimens were positive for past exposure to either all components or negative for one or more components of TORCH infections.
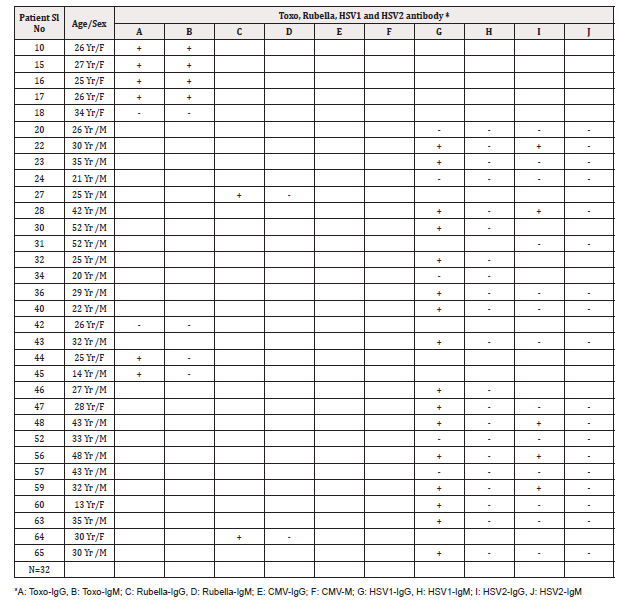
Table 3: Laboratory investigations for components of TORCH i.e. Toxo, Rubella, HSV1 and HSV2 infections singly or together/.
As per request from the ward or OPD, 32 specimens were analysed for one or more components of TORCH infections separately rather than the complete profile Table 3. Of the 8 specimens analysed for Toxoplasma infection only, 04 of them (i.e. No. 10, 15, 16, 17) were positive for both IgG and IgM classes of antibodies indicating acute/ recent infection,02 specimens were positive for IgG class antibody suggestive of past infection and 02 specimens were negative for both classes (IgG and IgM) of antibodies indicating no infection with Toxoplasma. Only 2 specimens (i.e. specimen no. 27, 64) were analysed for Rubella antibodies alone which were positive for IgG class antibody only suggesting past infection or vaccination with rubella virus and no specimen for only CMV antibodies. Out of the remaining 22 specimens, 17 were analysed for both HSV1 and HSV2, 4 were analysed for HSV1 alone and 1 was analysed for HSV2 alone as per request. Of the 17 specimens, 5 were positive for IgG class and negative for IgM class antibodies indicating past/ old infections for both HSV1 and HSV2, 8 were positive for past infections with HSV1 but negative for HSV2 and 4 specimens were negative for both HSV1 and HSV2 infections. Out of the 3 specimens analysed for HSV1 only, 2 were indicative of past infection and 1 was negative for HSV1 infection. The 01 specimen analysed for HSV2 alone was found negative for both classes of antibodies indicating no exposure to HSV2.
Discussion
Since diagnostic immunology laboratory was reorganized and some more versatile tests were introduced at MCW&H in August 2002 with competitive prices, a large number of requests were received for various biochemical and immunodiagnostic tests. The highest number of specimens received were for HBsAg followed by TFTs and other tests as stated in Table 1.
Regarding the autoantibody profile, 3/29(10.3%) and 1/7 (14.3%) were positive for ANA and anti-dsDNA respectively. The anti-dsDNA antibody test is highly specific for differential diagnosis of systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). Although ANA test is nonspecific, it is very useful and diagnostic of many rheumatoid and inflammatory autoimmune disorders. Among the complements, C3 was low in 16/20 (80.0%) specimens. Low C3 level is considered as an useful indicator for diagnosis of a wide range of diseases such as SLE, RA with vasculitis, post-streptococcal glomerulonephritis (PGN), membranoproliferative glomerulonephritis (MPGN), acute and chronic infections, inflammatory disorders, complement deficiencies and pregnancy [1-3]. Serum total IgE level was positive (>100 iu / ml) in 22/33 (66.1%) specimens indicating probable allergic and parasitic infections Table 1 [7-11].
Among the hepatitis profile, HBsAg, HBc-IgM ab and HBeAg were positive in 43/828 (5.2%), 3/14 (21.5%) and 3/19 (15.5%) respectively indicating the importance of these parameters in the diagnosis and follow-up of patients with HBV infection. HBs ab was positive in 1/10 (10%) specimens indicating probable natural recovery of these patients from HBV infection eventually. HCV-ab and HAV-IgM ab and HEV-IgM ab were positive in 5/47 (12.1%), 4/50 (8.0%) and 44/74 (59.4%) respectively suggesting high rate of exposure to other hepatitis viruses particularly HEV. HEV-IgM ab was positive in 59.4% of our specimens and this high incidence was probably due to the fact that HEV is an enterically transmitted virus Table 1 [3,4,20-23].
TORCH infections are some of the most common infections associated with congenital anomalies. Most of the TORCH infections cause mild maternal morbidity, but have serious foetal consequences. Treatment of maternal infection frequently has no impact on foetal outcome20,21. Maternal TORCH infections during pregnancy are a threat to pregnancy because they can be transmitted to the foetus while in the womb20,22. Also, congenital malformations caused by TORCH infections are important causes of morbidity, mortality and disability found in large percentage of neonates [20,23]. The consequence of these infections on foetus defend upon the type and virulence of the infecting agent and the stage of pregnancy. Therefore, recognition of maternal disease and then foetal monitoring are important for all relevant clinicians [23-25]. The prevalence of these infections varies from one geographical location to another [20,27,28]. Any patient infected with the TORCH agents, mainly two types of antibodies i.e. IgM and IgG classes are produced against the infective organisms. By measuring the antibody classes in mother’s blood, one can identify the type of infection [20,23]. When IgM class antibody is present with or without IgG class antibody, it invariably suggests acute/ recent infection and the presence of only IgG class antibody suggests mainly past or present active infection [20,29]. In the present study, an attempt was made to determine the prevalence of seropositivity in of TORCH in requested specimens sent to MRU Diagnostic Laboratory for investigations. However, laboratory evidence for TORCH infections, either multiple or single, was very low in the present study (Table 2, 3) contrary to some other reports from Bangladesh [20,22,25]. The probable reason may be that samples received at our MRU laboratory were from heterogeneous sources rather than concentrated from either Obstetrics and Gynecology or Neonatal units of hospitals and clinics.
American College of obstetrics and Gynecology (ACOG) currently recommended screening pregnant women for TORCH at the first prenatal visit. Clinicians must be well acquainted with the presentations of these infections, so that proper diagnosis can be made and treatment initiated, thus decreasing the likely wood of maternal and neonatal morbidity and mortality [20,28-30].
In conclusion, we were encouraged by the interest and confidence shown by the patients as well as the clinicians in the advanced tests introduced at MRU, MHWT, MCW&H Building, Uttara, Dhaka. The costs of these tests in our laboratory were one of the lowest and in the near future, we are inclined to introduce more immunodiagnostic tests with competitive prices. It is of paramount importance, however, to take clinical data into consideration whenever making interpretations of the laboratory (diagnostic) test results. Therefore, it would have been clinically and scientifically more meaningful if the specimens were sent to the laboratory with more clinical details as per as practicable, particularly the provisional diagnosis and other most relevant clinical features of the patients.
Acknowledgement
The authors would like to thank the authority of Medical Research Unit (MRU) and Board of Trustee, Medical & Health Welfare Trust (MHWT), Plot 4 Road 9 Sector 1, Uttara Model Town, Dhaka-1230, Bangladesh for the financial support. Also, the authors would like to thank Mr. Taposh Kumar Datta and Mr. Nawjes Ali, Medical Technologists, at MRU Laboratory for technical support.
References
- Chapel H, Haeney M, Misbah S, Snowden N (2006) Essentials of Clinical Immunology, Fifth Edition; Oxford: Blackwell Publishing Ltd.
- Roitt I (1997) Roitt's Essential Immunology, Ninth Edition; Oxford: Blackwell Science Ltd.
- Gooi HC, Chapel H (1990) Clinical Immunology: A Practical Approach; Oxford: IRL press (Oxford university press).
- Giasuddin ASM (2003) Monoclonal antibodies: The magic bullets. J Med Coll Women Hosp 1(1): 26-31.
- Kohler G, Milstein C (1975) Continuous cultures of fused cells secreting antibodies of predetermined specificity. Nature 256(5517): 495-497.
- Giasuddin ASM, Haq AMM, Azim FA, Khasru MA, Khan MH (2005) Diagnostic immunology profile at MCW&H, Uttara Model Town, Dhaka, Bangladesh. JMCWH 3(1): 36-40.
- Feltkamp TE (1996) Antinuclear antibody determination in a routine laboratory. Ann Rheum Dis 55(10): 723 -727.
- Hietarinta M, Lassila O (1996) Clinical significance of antinuclear antibodies in systemic rheumatic disease. Ann Med 28(4): 283-291.
- Nakamura RM, Tan EM (1992) Update on autoantibodies to intracellular antigens in systemic rheumatic diseases. Clin Lab Med 12(1): 1-23.
- Hoffman DR (1973) Estimation of serum IgE by an Enzyme linked Immunosorbent Assay (ELISA). J Allergy Clin Immunol 51(5): 303-307.
- Campbell ML (1992) More cancer found with sensitive PSA assay. Urol Times 20: 10-14.
- Schwartz MK (1987) Tumour markers in diagnosis and screening. In : Ting SW, Chen JS, Schwartz MK (Editors). Human Tumour Markers; Amsterdam: Elsevier science: 3-16.
- Sell S (1990) Cancer markers of the 1990s Comparison of the New Generation of Markers Defined by Monoclonal Antibodies and Oncogene Probes to Prototypic Markers. Clin Lab Med 10(1): 1-37.
- Schuurs AH, Van Weeman BK (1977) Review: Enzyme-immunoassay. Clin Chem Acta 81(1): 1-40.
- Giasuddin AS, El Sherif AI, EI Ojali SI (1998) Prolectin: Does it have a role in the pathogenesis of psoriasis? Dermatology 197(2): 119-122.
- Fody EP, Johnson DF (1987) The serologic diagnosis of viral hepatitis. J Med Technol 4: 54-59.
- Dienstag JL, Isselbacher KJ (1998) Acute viral hepatitis. In: Fauci AS, Braunwald E, Isselbacher KJ, et al (Editors). Harrison's Principles of Internal Medicine, Vol 2, 14th Edition; New York: McGraw-Hill 1677-1692.
- Fry L (1988) Psoriasis. Br J Dermatol 119(4): 445-461.
- Baker BS, Fry L (1992) The immunology of Psoriasis. Br J Dermatol 126(1): 1-9.
- Nabi SN, Wasey AFSA, Haider KMTS, Khan AA, Hoque MM (2012) Seroprevalence of TORCH antibody in pregnant women. Journal of Armed Forces Medical College Bangladesh 8(1): 35-39.
- Stegmann BJ, Carey JC (2002) TORCH infections. Toxoplasmosis, Other (Syphilis, Varicella-Zoster, Parvovirus B19), Rubella, Cytomegalovirus (CMV), and Herpes Infections. Curr Womens Health Rep 2(4): 253-258.
- Rawshan A, Mahmuda K (2001) TORCH infection: A real threat to pregnancy. Journal of Bangladesh College of Physicians and Surgeons (JBCPS) 19: 101-105.
- Turbadkar D, Mathur M, Rele M (2003) Seroprevalence of TORCH infection in bad obstetric history. Indian J Med Microbiol 21(2): 108-110.
- Rajendra B, Surpam (2006) Seroprevalence of TORCH infection in bad obstetric history. J Obs & Gynae 56: 41-43.
- Arif A, Motiur R, Nizamuddin A (2003) Seroprevalence of Toxoplasma gondii amongst pregnant women in Bangladesh. Bangladesh Armed Forces Medical Journal 31: 75-79.
- Burtis CA, Bruns DE (2015) Tietz Fundamentals of Clinical Chemistry and Molecular Diagnostics, Seventh Edition; Elsevier Saunders: 1-1075.
- Ko RC, Wong FW, Todd D, Lam KC (1980) Prevalence Toxoplasma gondii antibodies in the ethnical population of Hong Kong. Trans R Soc Trop Med & Hyg 74(3): 351-354.
- Biswas SK, Badruddoza M, Sultana N (2018) Seroprevalence of TORCH infections in children. Chattogram Maa-O-Shishu Hospital Medical College Journal 17(1): 25-29.
- Padmavathy M, Gowen M, Malini J, Umapathy BL, Navaneeth BV, et al. (2013) Seroprevalence of TORCH infections and adverse reproductive outcome in current pregnancy with bad obstetric history. J Clin Biomed Sci 3(2): 62-71.
- De Jong Ep, Vossen ACTM, Walther FJ, Lopriore E (2013) How to use neonatal TORCH testing. Arch Dis Child Educ Prct Ed 98(3): 93-98.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.