Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Comparative Efficacy of Aerosolized Particle Filtration by Non-Invasive Ventilation Modalities: Simulation of SARS-CoV-19 Transmission
*Corresponding author: Steven Harry Cataldo, Physician/Anesthesiologist, CareMount Medical, P.C. Yorktown Center for Specialty Surgery, New York, USA.
Received: August 01, 2020; Published: August 17, 2020
DOI: 10.34297/AJBSR.2020.09.001460
Abstract
Introduction: Patients with COVID-19 with severe respiratory disease may require non-invasive ventilation (NIV) devices, and selection should consider the greatest ability to reduce coronavirus-sized particles aerosolization. The objective of this study was to characterize the aerosolization of coronavirus-sized particles using different oxygen delivery systems.
Methods: To simulate real-life clinical scenarios, a surrogate head with adult morphology within an airtight containment box reproduced Nasal-only (N) and Nasal/Oral (N/O) Normal Breathing as well as Coughing for six respiratory support devices: SuperNO2VA with and without HEPA filter; High-Flow Nasal Cannula (HFNC); Non-Rebreather; NV-NIV Mask, both single-and dual-limb; and no device.
Results: Fit Factor between devices significantly differed (P<0.0001) but not between Normal Breathing and Coughing (P=0.15). SuperNO2VA with HEPA (N) and NV-NIV Mask with single-limb circuit each had significantly larger FF compared to all other devices (P<0.005). SuperNO2VA without HEPA (N), Non-Rebreather, and NV-NIV Mask with dual-limb circuit had no significant differences between FFs (P=1.0). SuperNO2VA with HEPA (N/O), SuperNO2VA without HEPA (N/O), and HFNC had significantly lower FF than other devices (P<0.05).
Conclusion: This model demonstrated in NIV settings the most important factor for minimizing particle spread is a strong mask seal. The SuperNO2VA device offered the strongest mask seal among those tested but, remains ineffective at trapping particles during oral breathing or coughing. Use of SuperNO2VA with a surgical mask can be safely used on patients with COVID-19 with no further risk of transmission.
Introduction
Coronavirus disease 2019 (COVID-19) is a viral respiratory tract infection caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The treatment and management of patients with COVID-19 is not well understood and is continuously evolving [1,2]. While most symptomatic cases are mild and self-limited, upwards of 14% of patients experience severe disease requiring hospitalization and 5% require critical care admission [3]. Of the critically ill, 71-79% require mechanical ventilation and mortality is between 50-61.5% [4-6]. For mechanically ventilated patients, mortality increases to 81% [4].
The pathophysiology of severe respiratory disease in COVID-19 has been likened to a form of Acute Respiratory Distress Syndrome (ARDS) [1]. The mainstay treatment of ARDS revolves around increased inspired oxygen concentration (FiO2), high positive end-expiratory pressure (PEEP), and low-tidal volume ventilation [7]. With the high mortality rates among ventilated patients, it is important to look to non-invasive respiratory support devices capable of improving FiO2 and PEEP, particularly in patients with mild-to-moderate disease. Three non-invasive ventilation (NIV) techniques may be useful for COVID-19: high-flow nasal oxygenation (HFNO); NIV via continuous positive airway pressure (CPAP) or bi-level positive airway pressure (BiPAP); and high FiO2/PEEP nasal positive airway pressure (nPAP) via the SuperNO2VA™ device (Vyaire Medical, Mettawa IL).
HFNO and NIV/CPAP have been used for many years to treat patients with advanced lung disease and hypoxemic respiratory failure. They have demonstrated efficacy in pulmonary edema, acute exacerbations of Chronic Obstructive Pulmonary Disease, and ARDS, as well as decreased rates of extubating failure in patients weaning from mechanical ventilation [8-11]. Increasing FiO2 and PEEP in patients with COVID-19 may slow disease progression and may prevent intubation and mechanical ventilation. The novel SuperNO2VA™ device provides high FiO2 and PEEP using wall oxygen through a non-vented, sealed nasal mask [12]. This device demonstrated efficacy in providing positive pressure and decreasing hypoxemia in high-risk patients, making its use a potential first step prior to mechanical ventilation in patients with COVID-19 [13-15].
High-flow and positive pressure devices have thus far been underutilized in the clinical fight against COVID-19 due to fears of aerosolizing the otherwise droplet-contained SARS-CoV-2, thus increasing the risk of transmission [16,17]. These actions are based primarily on previous data analyzing the exhaled air dispersion distances of simulated and non-simulated clinical scenarios using different types of oxygenation devices [18-21]. These studies did not use a size-matched particle generator to mimic coronavirus aerosolization and did not include a surgical mask placed over the “patient’s” mouth and nose, as is becoming common procedure during oxygen therapies in SARS-CoV-2-positive patients [22].
This study is the first to characterize the aerosolization of coronavirus-sized particles using different types of oxygen delivery systems with and without surgical masks, using a simulation of real-life COVID-19 clinical scenarios.
Materials/Methods
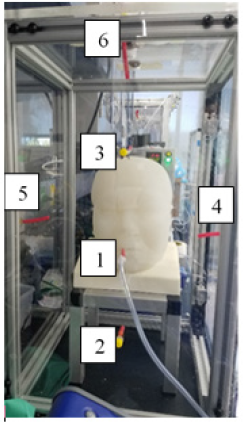
A custom-built surrogate adult head, with human face morphology and realistic nasal and oral cavities, was placed inside an approximately 1’ x 1’ x 2’ airtight containment box for the aerosolization tests.
Flow valves at the rear of the head simulated Nasal/Oral (N/O) and Nasal-only (N) breathing and served to attach an ASL 5000 Lung Simulator (IngMar Medical, Pittsburgh, PA) to simulate a breathing patient. A particle generator (Particle Generator 8026, TSI Incorporated, Shoreview, MN) was connected via a luer connection near the rear input to the surrogate head. A respiratory fit tester (PortaCount Pro+8038, TSI Incorporated) was used to quantify the concentration and spread of particles that escaped through the surrogate head’s mouth and nose.
The number of particles was sampled at six different locations within the test box (CSample, Figure 1) and compared to the number of particles sampled inside the surrogate head (CHead). A mean Fit Factor (FF), calculated as the ratio of CHead to CSample, estimated how well the oxygen delivery device setups contained particles from escaping into the environment. A larger FF is desirable and indicates release of fewer particles.
Five difference device setups were tested in defined conditions and breathing scenarios (Table 1). The SuperNO2VA was tested both with and without a HEPA filter (AirLifeTM 303HEPA) and at oxygen “Low” and “High” flow rates of 8 and 15 liters per minute (L/min). A digital manometer (Series 490, Dwyer, Michigan City, IN) connected to the port on the SuperNO2VA measured the pressure inside the mask. A High-Flow nasal cannula (HFNC, AcuCare 23004) was tested with rates of 30 and 60L/min and a Non-Rebreather (AirLifeTM Adult Oxygen Mask) was tested at rates of 8 and 15L/min. Finally, a Non-Vented NIV (NV-NIV) Mask (AirLifeTM NIV Non-Vented Full-Face Mask without Anti-Asphyxiation Valve) was used with both a single-limb and dual-limb circuit configuration. For SuperNO2VA and HFNC tests, a surgical mask was hooked over the surrogate head’s ears and over the device.
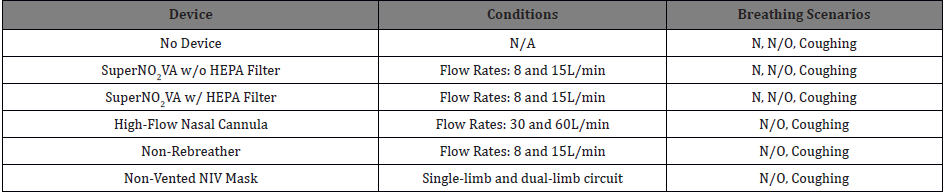
Table 1: Tested device with corresponding conditions and breathing scenarios.
Abbreviations: N: Nasal; N/O: Nasal/Oral; HEPA: High-Efficiency Particulate Air Filter; NIV: Non-Invasive Ventilation; L/min: Liters per minute
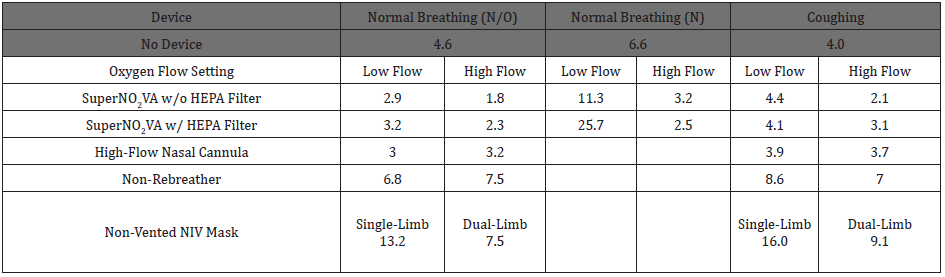
Table 2: Mean fit factor across tested devices, corresponding conditions and breathing scenarios.
Abbreviations: N: Nasal; N/O: Nasal/Oral; HEPA: High-Efficiency Particulate Air Filter; NIV: Non-Invasive Ventilation Fit Factor, CHead/CSample, is an estimate of how well the device setup contains particles from escaping into the environment, and larger estimates mean less particles are released into the environment.
A specialized mechanical ventilator (bellavistaTM 1000, Vyaire Medical) delivered gas for both the HFNC as well as NIV to minimize differences in experimental circuit set up. For NIV, the ventilator was set to Pressure Assist Control mode with a PEEP of 5 millibar (mbar), rate of 12 breaths per minute (BPM) and an inspiratory pressure of 9 mbar (single-limb) or 6 mbar (double-limb).
Both Normal Breathing and simulated coughing tests (Coughing) were tested for each condition of each device. For the Normal Breathing tests, the ASL 5000 simulated normal adult spontaneous breathing with a rate of 12BPM and tidal volume of 500 milliliter (ml). Simulated N/O breathing was conducted for all devices. For the SuperNO2VA tests, Normal Breathing (N) also was conducted because the patient’s mouth is usually closed during clinical use involving the SuperNO2VA mask. During Coughing, the ASL 5000 was programed to deliver a repeating pattern of four breaths followed by two coughs. As a control, the N, N/O, and Coughing breathing tests also were conducted with no device attached to the surrogate head.
Data and Statistical Analysis
The mean FF, calculated across the six sampling locations for each test, permitted comparison of the degree of aerosolization across the six device configurations and both Normal Breathing and Coughing breathing patterns. A two-way ANOVA of FFs used fixed effects of “Device Configuration” and “Breathing Type.”
Tukey’s post-hoc test was performed for pairwise comparisons between devices. Paired t-tests compared FFs between single-limb and dual-limb patient circuits during both breathing protocols using the NV-NIV Mask. Finally, a three-way ANOVA compared the FFs when using the SuperNO2VA mask under different conditions with fixed effects of HEPA filter presence, Breathing Type (i.e., N and N/O), and Flow Rate (i.e., 8 and 15L/min).
Because the production released devices in this simulation have 510(k) approval and CE marks, a single sample of each device was used, with each device sampled at six locations with different flow and breathing patterns. The device configurations were: No Device, SuperNO2VA without HEPA filter (N/O), SuperNO2VA without HEPA filter (N), SuperNO2VA with HEPA filter (N/O), SuperNO2VA with HEPA filter (N), HFNC, Non-Rebreather, NV-NIV Mask with single-limb circuit, and NV-NIV Mask with dual-limb circuit.
Results
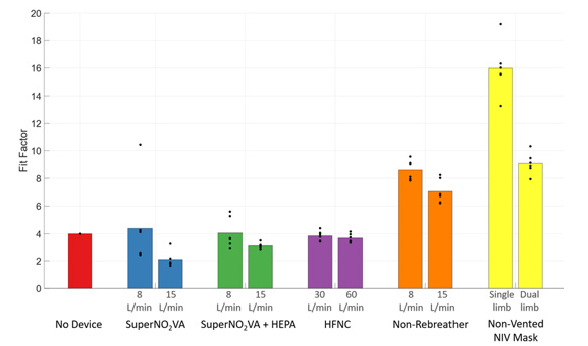
Mean FF, representing the estimated efficacy of each setup to contain particles from escaping into the environment are reported for all devices across breathing scenarios and flow settings in Table 2. When no device was attached to the surrogate head, the Coughing test had the lowest FF (4.0), followed by N/O (4.6) and N (6.6) breathing, indicating the poorest control of particle aerosolization. The NV-NIV Mask had the highest FF rates for Normal Breathing N/O (13.2 single-limb and 7.5 dual-limb) and Coughing (16.0 single-limb and 9.1 dual-limb), the best particle aerosolization control.
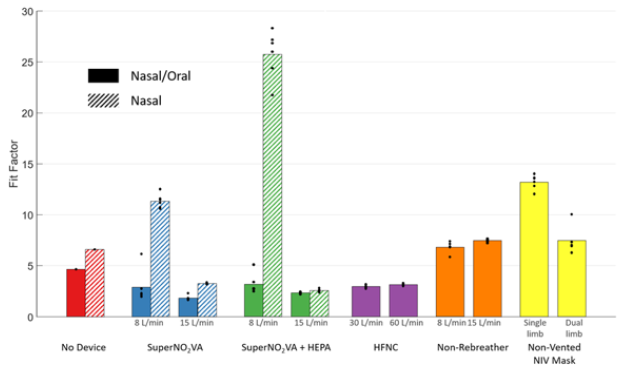
Figure 2 visualizes the FF during Normal Breathing and Figure 3, for the simulated Coughing tests. Both figures plot each of the five devices tested in addition to the No Device control, with separated Low-Flow and High-Flow rate tests, as are the single-limb and dual-limb circuits for the NV-NIV Mask.
The two-way ANOVA showed a significant difference in FF between devices (P<0.0001) but no significant difference between Normal Breathing and Coughing (P=0.15). Tukey’s post-hoc test showed the SuperNO2VA with HEPA filter (N) and NV-NIV Mask with single-limb circuit each had FFs indicating significantly reduced particle aerosolization compared to all other devices (P<0.005). No significant FF differences occurred with the SuperNO2VA without HEPA filter (N), Non-Rebreather, and NV-NIV Mask with dual-limb circuit (P=1.0). FFs for tests with SuperNO2VA without HEPA filter (N/O), SuperNO2VA with HEPA filter (N/O), and HFNC were significantly lower than other devices (P < 0.05).
For both N/O and N breathing tests with the SuperNO2VA, the use of a HEPA filter yielded significantly higher FF than without (P<0.0001). Furthermore, significantly higher FF occurred for N breathing tests compared to N/O tests (P<0.0001), and Low-Flow rates compared to High-Flow rates tests (P<0.0001). During N breathing, the pressure within the SuperNO2VA was 9.7±0.3cmH2O (mean±SD) at 8L/min and 19.2±0.6cmH2O at 15L/min. For the NV-NIV Mask, use with a single-limb patient circuit had significantly higher FFs for both Normal Breathing (P=0.0004) and Coughing (P= 0.0004, paired t-tests).
Discussion
Understanding particle spread with the use of different respiratory support devices is critical to managing viral transmission during the COVID-19 pandemic. The World Health Organization recommends NIV for the treatment of patients with COVID-19 disease in certain clinical scenarios but with cautious use secondary to the risk of aerosolization and transmission. If NIV use increases to curtail mechanical ventilation of patients, healthcare providers must be armed with all available knowledge to use these therapies safely, including the risk for viral particle spread.
In this experimental model, compared to no device at all, a standard non-rebreather mask seems to offer some protection from particle spread. This finding is not surprising as a non-rebreather mask forms a barrier seal on the patient’s face and likely limits the spread of respiratory droplets, thereby reducing aerosolization. However, a non-rebreather mask when treating patients with COVID-19 often is insufficient to meet the patient’s oxygen requirements, even in mild-to-moderate disease states [4].
Use of HFNO and SuperNO2VA for N/O breathing resulted in the greatest concentration of viral-like particles detected, represented as the lowest FF, and was worse with increased oxygen flow rates. In fact, greater particle detection was seen even compared with no device at all. For HFNO, this is likely attributed to the high oxygen flows and the non-sealed characteristics of the nasal cannula interface, consistent with previously reported NIV aerosolization data [18-20]. In the SuperNO2VA, one explanation is that nasopharyngeal positive pressure transmitted to the patient’s oropharynx may cause pressurized release during exhalation, which may increase particle spread during use. However, patients during SuperNO2VA use typically do not breathe regularly from their mouths, as their oral breathing is an intermittent, short-lived occurrence. As such, the related risk calculated via this model may be overestimated.
Tighter mask seal qualities seem to offer protection from particle spread, as demonstrated by the higher FF of both SuperNO2VA during N breathing and the NV-NIV mask. The SuperNO2VA device during N breathing offered the greatest FF among all devices. This is likely explained by its strong mask seal around the nose, designed to mimic an anesthesia mask seal versus an NIV mask seal, which is softer and typically reserved for long-term use in patients, accommodating treatment for greater than 24 hours. The more robust the mask seal of a device used on an infected patient, the more likely the mask interface will keep aerosolized viral particles contained, thereby limiting particle travel and transmission to others.
Of note, the HEPA filter further increased the FF of the SuperNO2VA device, providing adequate evidence to consider its use when treating patients diagnosed with COVID-19. Considering the effectiveness and location of the filter on the breathing circuit, it demonstrates that without the filter, leakage of viral-like particles may occur along the entire length of the breathing circuit where exhaled air is released, not solely from the mask itself. HEPA filters, therefore, should be placed as close to the mask interface as possible, preferably before any exhalation or PEEP valve.
Previous investigations during the SARS outbreak of the early 2000s demonstrated that viral transmission when using NIV may not be significant. With proper use of PPE, Folwer [23] reported no significant association occurred between use of NIV/HFNO and increased transmission of the SARS virus to ICU healthcare workers. Cheung [24] saw similar results when studying NIV in SARS patients, reporting no new infections among their providers. This experimental model demonstrates that in the settings in which aerosolized viral spread is a concern and NIV is used, the most important factor for minimizing particle spread is a strong mask seal. The SuperNO2VA device offers the strongest mask seal among those respiratory support set ups tested here but remains ineffective at trapping particles during oral breathing or coughing. Interestingly, no statistical difference in FF occurred between normal breathing compared to SuperNO2VA when a surgical mask was used, suggesting it can be safely used on patients with COVID-19 with no further risk of transmission.
Conclusion
Data from this experimental model supports that NIV may not pose as high a risk for aerosolization of SARS-CoV-2 viral particles as previously thought. Although the use of NIV trended toward lower FFs compared to no device or a supplemental oxygen facemask, this difference did not meet statistical significance. In fact, FF were significantly higher with SuperNO2VA N breathing and CPAP via a NV-NIV mask. When using any form of NIV on patients with COVID-19, these findings also support recommendations use of the lowest possible fresh gas flow and the most robust mask seal.
Acknowledgment
- Author Contributions: Cataldo had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis
- Concept and design: Cataldo, Pedro, Harvey
- Acquisition, analysis, or interpretation of data: Cataldo, Pedro, Harvey
- Drafting of the manuscript: Cataldo and Pedro
- Critical revision of the manuscript for important intellectual content: Cataldo and Pedro
- Statistical analysis: Harvey
- Obtained funding: Pedro
- Administrative, technical, or material support: Pedro
- Supervision: Cataldo and Pedro
Conflict of Interest Disclosures
Dr. Cataldo serves on the medical advisory board of Vyaire Medical and was coinventor of the uperNO2VA. Dr. Pedro is an employee of Vyaire Medical and coinventor of SuperNO2VA. Brian Harvey is a paid consultant for Vyaire Medical. No other disclosures were reported.
Additional Contributions
The authors thank Marion E. Glick for editorial assistance in preparing the manuscript for which compensation was received.
References
- WHO (2020) Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: Interim Guidance v1.2, Geneva.
- Ferioli M, Cisternino C, Leo V, Pisani L, Palange P, et al. (2020) Protecting healthcare workers from SARS-CoV-2 infection: practical indications. Eur Respir Rev 29(155): 200068.
- Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention (2020) The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi 41(2): 145-151.
- Yang X, Yu Y, Xu J, Shu H, Xia J, et al. (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 8(5): 475-481.
- ICNARC (2020) ICNARC report on COVID-19.
- Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, et al. (2020) Covid-19 in critically Ill patients in the seattle region-case series. N Engl J Med 382(21): 2012-2022.
- Briel M, Meade M, Mercat A, Brower RG, Talmor D, et al. (2010) Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA 303(9): 865-873.
- Antonelli M, Conti G, Esquinas A, Montini L, Maurizio S, et al. (2007) A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med 35(1): 18-25.
- Vital F, Ladeira MT, Atallah AN (2019) Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database Syst Rev 5: CD005351.
- Frat JP, Thille AW, Mercat A, Girault C, Ragot S, et al. (2015) High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 372(23): 2185-2196.
- Thille AW, Muller G, Gacouin A, Coudroy R, Decavèle M, et al. (2019) Effect of postextubation high-flow nasal oxygen with noninvasive ventilation vs high-flow nasal oxygen alone on reintubation among patients at high risk of extubation failure: a randomized clinical trial. JAMA 322(15):1465-1475.
- Pedro MJ, Gidaro M, Cataldo SH (2017) Nasal variable positive airway pressure, a novel non-invasive airway technique to supplement deep sedation during endovascular therapy in a compromised pulmonary patient: A case report. J Anaesth Crit Care Case Reports 3(2): 34-38.
- Kozinn R, Foley L, Feinleib J (2018) SuperNOVA nasal mask ventilation maintains oxygenation during deep sedation in high-risk patients: a case series. Res Pract Anesthesiol Open J 3(1): 15-19.
- Dimou F, Huynh S, Dakin G, Pomp A, Turnbull Z, et al. (2019) Nasal positive pressure with the SuperNO2VA™ device decreases sedation-related hypoxemia during pre-bariatric surgery EGD. Surg Endosc 33(11): 3828-3832.
- Bai Y, Xu Z, Chandrashekar M, Jacques P, Liang Y, et al. (2019) Comparison of a simplified nasal continuous positive airways pressure device with nasal cannula in obese patients undergoing colonoscopy during deep sedation: A randomised clinical trial. Eur J Anaesthesiol 36(9): 633-640.
- WHO (2020) Rational use of personal protective equipment for coronavirus disease 2019 (COVID-19). Interim Guidance, Geneva.
- National Health Service (2020) Specialty guides for patient management during the coronavirus pandemic: Guidance for the role and use of non-invasive respiratory support in adult patients with coronavirus (confirmed or suspected), UK.
- Hui DS, Hall SD, Chan MT, Chow BK, Tsou JY, et al. (2006) Noninvasive positive-pressure ventilation: An experimental model to assess air and particle dispersion. Chest 130(3): 730-740.
- Hui DS, Chow BK, Ng SS, Chu LCY, Hall SD, et al. (2009) Exhaled air dispersion distances during noninvasive ventilation via different Respironics face masks. Chest 136(4): 998-1005.
- Hui DS, Chow BK, Lo T, Tsang OT, Fanny WK, et al. (2019) Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J 53(4): 1802339.
- Loh NW, Tan Y, Taculod J, Gorospe B, Teope AS, et al. (2020) The impact of high-flow nasal cannula (HFNC) on coughing distance: implications on its use during the novel coronavirus disease outbreak. Can J Anaesth 67(7): 893-894.
- Jing G, Li J (2020) Expert consensus on preventing nosocomial transmission during respiratory care for critically ill patients infected by 2019 novel coronavirus pneumonia. Chinese J Tuberculosis Respir Dis 17(0): E020.
- Fowler RA, Guest CB, Lapinsky SE, Sibbald WJ, Louie M, et al. (2004) Transmission of severe acute respiratory syndrome during intubation and mechanical ventilation. Am J Respir Crit Care Med 169(11): 1198-1202.
- Cheung TM, Yam LY, So LK, Lau AC, Poon E, et al. (2004) Effectiveness of noninvasive positive pressure ventilation in the treatment of acute respiratory failure in severe acute respiratory syndrome. Chest 126(3): 845-850.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.