Short Communication 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Deciphering Interactive Associations of Antiviral and Electron-Shuttling Characteristics of Flavonoid Compounds for Antiviral Drug Development
*Corresponding author: Bor-Yann Chen, Department of Chemical and Materials Engineering, National I-Lan University, I-Lan 26047, Taiwan.
Received: July 13, 2020; Published: August 14, 2020
DOI: 10.34297/AJBSR.2020.09.001455
Abstract
In face of the 2020 coronavirus COVID-19 pandemic, exploration of feasible medicines and effective medication is of course a top-priority issue to save lives worldwide. This study focused on antiviral properties of flavonoids to decipher synergistic interactions with electron-shuttling characteristics for possible herbal medication in clinical treatment. In fact, the numbers and relative positions of hydroxyl groups all strongly influenced antioxidant activities of flavonoid compounds. In particular, ortho-polyhydroxy benzene-bearing aromatics seemed to trigger antiviral characteristics to be expressed. Prior studies mentioned that ortho and para-polyhydroxy benzene-bearing aromatic structure could exhibit reversible and stable electron-shuttling characteristics. This comparative study suggested the rationale of initiatives for seeking possible methods and strategy of natural medicines with antiviral properties.
Keywords: COVID-19; Flavonoid; Antivirus; Electron shuttle
Abbreviations: ADV: Adenovirus; CHIKV: Chikungunya virus; CoV: Coronavirus; DENV: Dengue virus; EV: Enterovirus; ES: Electron shuttle; EC: Epicatechin; EGC: Epigallocatechin; ECG: Epicatechin gallate; EGCG: Epigallocatechin gallate; EBV: Epstein-barr virus; HHV: Human herpesvirus; HIV: Human immunodeficiency virus; IV: Influenza virus; JEV: Japanese Encephalitis virus; MFCs: Microbial fuel cells; NDV: Newcastle Disease Virus; QPD: Qingfei paidu decoction; RSV: Respiratory syncytial virus; VSV: Vesicular stomatitis virus
Introduction
Since the end of 2019, the new coronavirus COVID-19 pandemic took more than half million lives among 12+ million confirmed cases worldwide. As clinical trials recently indicated, remdesivir seemed to be an encouraging coronavirus drug. However, there were still currently not much progress on drug medicines that could effectively inhibit COVID-19. To develop effective medication in such a short period of time to save millions of lives, natural herbs (e.g., Chinese herbal medicine) should first be considered for aspect of not only anti-COVID-19 infection, nut also primary health care. As mentioned in literature on natural herbs, flavonoids in polyphenolic compounds (e.g., flavonols-quercetin[1-6], myricetin [2,5,7] fisetin [3,5] and flavones-Baicalein [5], Luteolin [2,5,6] and Flavanols-catechin [2,4,5,8], epicatechin (EC)[2], epigallocatechin (EGC) [4,6,8], epicatechin gallate Epicatechin gallate (ECG) [4,8], epigallocatechin gallate (EGCG) [4-6,8] would exhibit significant antiviral activities (Table 1). For instance, the flavonoids could effectively inhibit viral protein synthesis and enhance the immune system of host.
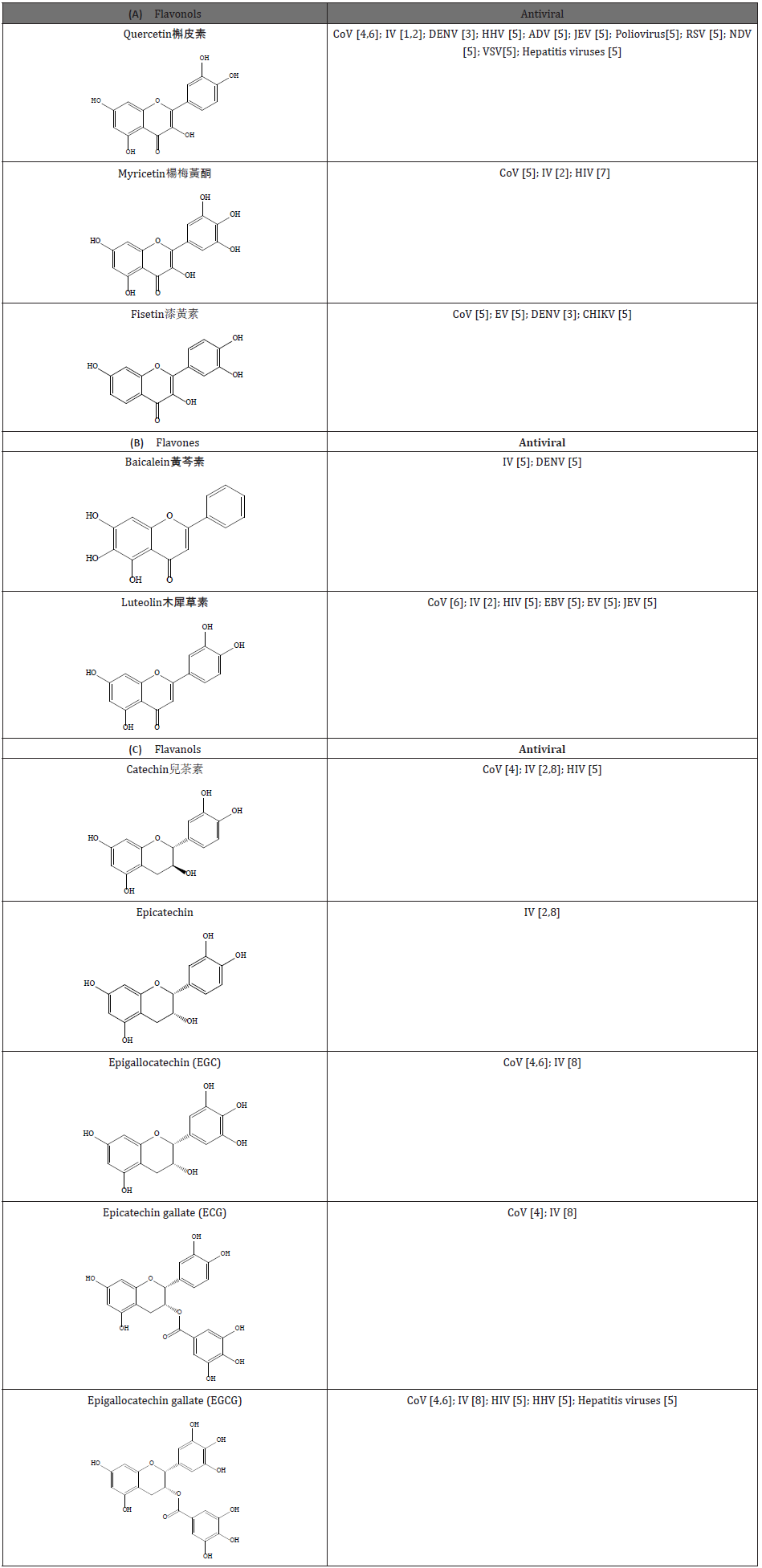
Table 1: Comparative list for antiviral characteristics of ortho di-hydroxyl group-bearing flavonoid compounds. (CoV(Coronavirus); IV(Influenza virus); DENV(dengue virus); HHV(Human Herpesvirus); ADV(Adenovirus); JEV(Japanese encephalitis virus); RSV(Respiratory syncytial virus); NDV( Newcastle Disease Virus); VSV(Vesicular stomatitis virus); HIV(Human immunodeficiency virus); CHIKV(Chikungunya virus); EBV(Epstein-Barr virus); EV(Enterovirus).
In addition, kaempferol and luteolin could effectively inhibit 3a protein and S2 protein of coronavirus and hemagglutinins of influenza virus [9]. Among flavonoid compounds, quercetin seemed to be more favorable to inhibit wide-range viruses (e.g., Adenovirus, Japanese encephalitis virus, Respiratory syncytial virus, Newcastle Disease Virus, Vesicular stomatitis virus, dengue virus, Coronavirus, Influenza virus). As [5] pointed out, quercetin could block the fusion and penetration between the viruses and host cells, and quercetin could exhibit anti-human herpes virus properties. As quercetin could interact with influenza virus hemagglutinin protein, thereby quercetin inhibits fusion of influenza virus and host cells [5]. Furthermore [6] revealed that the flavonoids could inhibit SARS-CoV 3CL protease [10] mentioned that the Chinese herbal medicine “Qingfei Paidu Decoction” (QPD) could be used to regulate immune system to reduce lung damage. According to composition analysis, the main components were flavonoids (e.g., quercetin, luteolin, kaempferol) [10].
Moreover, the flavonoids could enhance antiviral properties due to interactive synergism. For example, synergies gained from compound prescription of kaempferol and luteolin, or quercetin and antiviral medicine-acyclovir could effectively enhance anti-human herpes virus properties [11]. Thus, this study tended to explore the chemical mechanism about how and why flavonoids could effectively augment antiviral consequence. Considering pathology, oxidative stress induced by viruses [12] could damage the redox balance in the host, and then host immune system would be weakened to favor viral infection to be taken place. Therefore, antioxidant-abundant natural substances may be one of top ranked candidates to be selected as appropriate medicines to against viruses (e.g., COVID-19). That is, antiviral properties of flavonoids may be strongly associated to antioxidant or redox-rebalancing properties [12].
Regarding redox-associated characteristics of flavonoids, chemical structure-activity relationships should be disclosed. The molecular structure of flavonoid is composed of two benzene rings (A ring, B ring) and one pyran heterocycle (C ring; A ring and C pyran heterocycle fused together). The higher numbers of hydroxyl groups would own more significant activity of antioxidant. Moreover, the relative positions of hydroxyl groups would significantly affect the antioxidant activity of flavonoids [2,5,13-16]. As literature indicated, the hydroxyl substituents on the B ring would be of great importance for flavonoids to considerably scavenge free radicals. Essentially, the hydroxyl groups on the B ring will transfer protons and electrons to hydroxyl radicals via resonance effect to form relatively stable flavonoid radical during redox reactions.
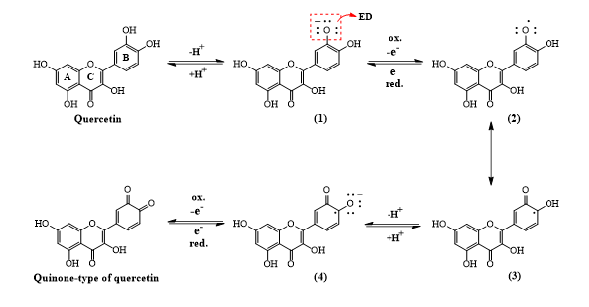
For the electrochemically steered reactions, ortho and para-polyhydroxy benzene-bearing aromatic compounds could stabilize the free radicals in the electron transfer via resonance effect, thereby promoting the redox reaction [17] (Figure 1). As electrochemical inspection (e.g., cyclic voltammetry assessment and power density analysis upon microbial fuel cells) [17-19] indicated, ortho and para-polyhydroxy benzene-bearing aromatic compounds were confirmed to have such redox-mediating (or electron-shuttling) properties, suggesting that ortho-dihydroxy benzene-bearing quercetin was a potential electron shuttle (ES). As proposed in Fig 1 for mechanisms of inter-conversion of quercetin, quercetin was first deprotonated (e.g., chemical (1) in Figure 1), then oxidized to form radical intermediates (e.g., chemical (2) in Figure 1). The following “free radical” electrons would be stably resonant to the carbon bound to be carbonyl group, and the radical electron was transferred to the adjacent carbon attached the strong electron-releasing hydroxyl group to be stabilized (e.g., chemical (3) in Figure 1). Next, deprotonation of this hydroxyl substituent was taken place (e.g., chemical (4) in Figure 1) and was followed to be oxidized for further formation of quinone-type of quercetin. Due to the formation of stable intermediates (Figure 1), the resonant chemical species could be stably and reversibly redox mediating to shuttle electrons between electron donor(s) and acceptor(s). Such mediating characteristics could be considered as redox catalysts, leading to significantly decreasing internal electron-transfer resistance in MFCs. Thus, the formation of intermediates was more electrochemically favorable to mediate electrons, stimulating the performance of power generation in MFCs (Figure 1).
Conclusion
The first-attempt study suggested the associations between antiviral features of flavonoids and electron shuttles (or redox mediators) from the perspective of electrochemistry. Considering chemical structure, flavonoid compounds (e.g., quercetin and catechin) with ortho-dihydroxyl groups on B ring would simultaneously own antioxidant and antiviral properties due to their electron-shuttling activities. As combined synergies gained from compound medicines with flavonoids could enhance physiological activity, this study proposed the flavonoid compounds would also function as electron shuttles (ESs) to catalyze the electron transferrelated reactions in cellular metabolism of humans. That is, followup studies should focus on detailed examinations of associations between drug medication and electron mediation.
Acknowledgements
The authors sincerely appreciate financial supports from Taiwan’s Ministry of Science and Technology (MOST 106-2221-E-197-020-MY3, MOST 106-2923-E-197-002-MY3, MOST 106-2621-M-197-001, MOST 107-2621-M-197-001, MOST 109-2221-E-197-016-MY3).
Conflict of Interest
The authors declare no potential competing financial interests.
References
- Savov VM, Galabov AS, Tantcheva LP, Mileva MM, Pavlova EL, et al. (2006) Effects of rutin and quercetin on monooxygenase activities in experimental influenza virus infection. Exp Toxicol Pathol 58(1): 59-64.
- Liu AL, Wang HD, Lee SMY, Wang YT, Du GH, et al. (2008) Structure–activity relationship of flavonoids as influenza virus neuraminidase inhibitors and their in vitro anti-viral activities. Bioorg Med Chem 16(15):7141-7147.
- Zandi K, Teoh B, Sam S, Wong PF, Mustafa MR, et al. (2011) Antiviral activity of four types of bioflavonoid against dengue virus type-2. Virol J 8: 560.
- Nguyen TTH, Woo HJ, Kang HK, Nguyen DV, Kim YM et al. (2012) Flavonoid-mediated inhibition of SARS coronavirus 3C-like protease expressed in Pichia pastoris. Biotechnol Lett 34(5): 831-838.
- Zakaryan H, Arabyan E, Oo A, Zandi K (2017) Flavonoids: promising natural compounds against viral infections. Arch Virol 162(9): 2539-2551.
- Jo S, Kim S, Shin DH, Kim MS (2020) Inhibition of SARS-CoV 3CL protease by flavonoids. J Enzyme Inhib Med Chem 35(1): 145-151.
- Pasetto S, Pardi V, Murata RM (2014) Anti-HIV-1 Activity of Flavonoid Myricetin on HIV-1 Infection in a Dual-Chamber In Vitro Model. PLoS One 9(12): e115323.
- Song JM, Lee KH, Seong BL (2005) Antiviral effect of catechins in green tea on influenza virus. Antiviral Res 68(2): 66-74.
- Lalani S, Poh CL (2020) Flavonoids as Antiviral Agents for Enterovirus A71 (EV-A71). Viruses 12(2): 184.
- Guo G, Ye L, Pan K, Chen Y, Xing D, et al. (2020) New Insights of Emerging SARS-CoV-2: Epidemiology, Etiology, Clinical Features, Clinical Treatment, and Prevention. Front Cell Dev Biol 8(410).
- Kumar S, Pandey AK (2013) Chemistry and Biological Activities of Flavonoids: An Overview. ScientificWorldJournal 2013:162750.
- Liu M, Chen F, Liu T, Chen F, Liu S, et al. (2017) The role of oxidative stress in influenza virus infection. Microbes Infect 19 (12): 580-586.
- Heim KE, Tagliaferro AR, Bobilya DJ (2002) Flavonoid Antioxidants: Chemistry, Metabolism and Structure-Activity Relationships. J Nutr Biochem 13(10): 572-584.
- Procházková D, Boušová I, Wilhelmová N (2011) Antioxidant and prooxidant properties of flavonoids. Fitoterapia 82(4): 513-523.
- Wang TY, Li Q, Bi KS (2018) Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian Journal of Pharmaceutical Sciences 13(1): 12-23.
- Nagula RL, Wairkar S (2019) Recent advances in topical delivery of flavonoids: A review. J Control Release 296: 190-201.
- Hsueh CC, Wu CC, Chen BY (2019) Polyphenolic compounds as electron shuttles for sustainable energy utilization. Biotechnol Biofuels 12.
- Chen BY, Ma CM, Liao JH, Hsu AW, Tsai PW, et al. (2017) Feasibility study on bio stimulation of electron transfer characteristics by edible herbs-extracts. Journal of the Taiwan Institute of Chemical Engineers 79: 125-133.
- Chen BY, Liao JH, Hsueh CC, Qu Z, Hsu AW, et al. (2018) Deciphering biostimulation strategy of using medicinal herbs and tea extracts for bioelectricity generation in microbial fuel cells. Energy 161: 1042-1054.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.