Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Fact-finding Survey of GS1 Data Bar Labeling of Ethical Drugs in Hospital Pharmacy Departments
*Corresponding author: Mikio Murata, Department of Clinical Pharmacy, Yokohama University of Pharmacy, 601, Matano-Cho, Totsuka-Ku, Yokohama, Kanagawa, Japan.
Received: June 10, 2020; Published: June 30, 2020
DOI: 10.34297/AJBSR.2020.09.001389
Abstract
In Japan, JAN codes were used for the packaging of ethical drugs, but in September 2006, the MHLW issued “Guidelines for implementation of bar-code labeling of ethical drugs” for mechanical checking of drugs using GS1 Data Bar to prevent mix-ups and to ensure the traceability of ethical drugs.
In Japan, tablets and capsules of ethical drugs are mostly delivered to patients, particularly outpatients, in PTP or SP. In the present survey, the printing conditions of GS1 DataBar on PTP or SP of oral tablets and capsules, and on the containers and labels of external preparations were investigated.
The height and width of GS1 DataBars were most frequently 3 mm and 19 mm, respectively, regardless of the size of the PTP sheet itself. The color of GS1 DataBars was most frequently black, and the background was most frequently white.
For drugs for internal use, improvements in printing technology have led to the increased diversity of the design of labeling with GS1 DataBars and preservation of the original design image. For drugs for external use, however, how to overcome the “smallness of the printing area” and “unique shape of the dosage form” is an important challenge for the future.
It is important to evaluate the state of use of GS1 DataBars and package designs of drugs by reflecting on the viewpoints of both pharmacists and patients.
Keywords: GS1 DataBar, Bar Code, PTP, SP, Press Through Package, Strip Package, Package Designs, Ethical Drugs
Abbreviations: JAN code: Japanese Article Number code; MHLW: Ministry of Health, Labour and Welfare, Japan; PTP: Press Through Package; SP: Strip Package
Introduction
Securing the safety of medical services by preventing health damage and serious accidents due to mix-ups of ethical drugs is one of the important health care policies of our country. Causes of mix-ups of ethical drugs include similarities of the names, appearances of drugs and shapes, and printing colors of their packages. Notifications calling for attention to these causes have been issued by the MHLW, and measures to prevent medical accidents related to similarities of names and appearances have been taken [1-4].
In addition, to prevent medical accidents due to mix-ups of ethical drugs, a new measure to identify ethical drugs mechanically and automatically without dependence on human judgment was suggested to be necessary, and thorough implementation of safety management in handling drugs through drug management using 2-dimensional codes and IC tags, establishment of databases for the evaluation of similarities of drug names and appearances, and clarification of conditions of prescription of drugs that need particular caution in handling, such as anticancer drugs, was demanded [5]. Numerous measures and education of medical workers for the prevention of mix-ups of ethical drugs have also been attempted overseas, but the patients’ safety has not been fully protected [6-9], and the use of a medication management system using bar codes is recommended in countries such as the United States [6, 9-11].
In Japan, JAN codes were used for the packaging of ethical drugs, but in September 2006, the MHLW issued “Guidelines for implementation of bar-code labeling of ethical drugs” for mechanical checking of drugs using GS1 DataBar to prevent mix-ups and to ensure the traceability of ethical drugs [12]. This notification urged holders of marketing authorization to label all packaging units, including the prescription packaging units (minimum packaging units for manufacturing and sales: ampoules, PTP), of specified biological products, biological products, and injection drugs among the ethical drugs and sales packaging units (sales units: individually packaged boxes) of drugs for internal and external use with GS1 DataBar, and “GS1 DataBar” labeling in compliance with this notification was implemented for the products shipped in and after September 2008.
As the technical development of labeling for each packaging form of drugs for internal and external use was not completed at this point, their labeling with the new GS1 DataBar was not included in the notification.
As it became technically possible to label these packaging forms with GS1 DataBar thereafter, the guidelines were revised in 2012 based on the circumstances, and labeling of drugs for internal and external use by the preparation packaging unit was incorporated [13].
In Japan, tablets and capsules of ethical drugs are mostly delivered to patients, particularly outpatients, in PTP or SP. Currently, the size of PTP or SP is not standardized, and there are no detailed regulations about printing on aluminum foil. In the present survey, the printing conditions of GS1 DataBar on PTP or SP of oral tablets and capsules, and on the containers and labels of external preparations were investigated.
Materials and Methods
The study protocol was reviewed and approved by the institutional review boards of the participating institutions.
Surveyed Drugs
The state of GS1 DataBar labeling of drugs for internal and external use dispensed at Saiseikai Yokohamashi Tobu Hospital was surveyed during the period of June 26-August 6, 2017. Saiseikai Yokohamashi Tobu Hospital is a core hospital providing emergency medical service in the northern area of Yokohama City (population in the area: 1,570,303 persons [14]). The items to be surveyed were determined using the report by Sato et al. [15] as a reference.
Drugs for internal use: The survey was made concerning 451 tablets/capsules in PTP. As GS1
DataBars with different sizes were printed on one PTP sheet in 14 drugs, a total of 465 DataBars were collected.
Drugs for external use: The survey was made concerning 144 preparations and 144 GS1
Databars were collected. The preparations consisted of 52 tubed ointments, 18 ophthalmic solutions, 15 liquid preparations, 14 transdermal preparations, 13 bottled ointments, 8 inhaled drugs, 8 lotions, and others (suppositories, vaginal suppositories, ear solutions, nasal solutions, lozenges, and powders).
Surveyed items concerning GS1 DataBars
Surveyed items concerning GS1 DataBars
Length and width of PTP sheets: As the length and width of GS1 Databar may be affected by
the size of the PTP sheet itself, the length and width of each PTP sheet, defined as the dimensions parallel and perpendicular, respectively, to the drug name printed on the PTP sheet, were measured in mm according to the method by Sato et al. [15]
Height and width of GS1 DataBars: The height and width of GS1 DataBars were measured in
The dimension parallel to the numerical string of the GS1 application identifier (from the bar on the extreme left to the bar on the extreme right) and the dimension perpendicular to it were defined as the width and height, respectively.
Number of GS1 DataBars: The number of GS1 DataBars was counted if they were printed by
the pitch method and expressed as “multiple” if they were repeatedly printed by the endless method.
Package form
Position, pattern, and substrate of printing of GS1 DataBars: For drugs for internal use,
data of the position of printing of GS1 DataBars were collected by classifying it as “upper middle”, “lower middle”, or “upper left”. Concerning GS1 DataBars printed perpendicularly to the drug name and those expressed as “multiple” on counting, details were described in the space for the printing pattern. The substrate of printing of GS1 DataBars was classified as “aluminum”, “plastic”, or “paper”, and it was classified as “paper” when a paper sticker on which a GS1 DataBar was printed was attached to an ointment tube, etc.
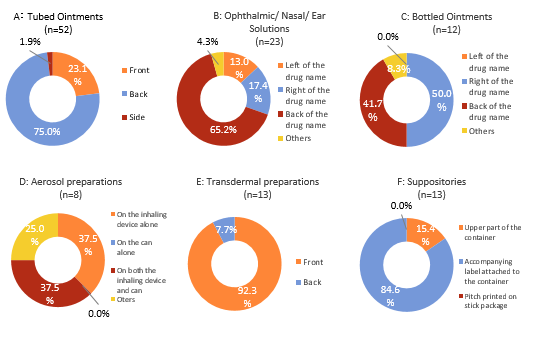
Concerning drugs for external use, the position of printing of GS1 DataBars was classified as follows by the dosage form: In tubed ointments, the position was classified into front, back, and side by defining the surface on which the drug name was indicated as front. For ophthalmic/nasal/ear solutions and bottled ointments, as the labels on which GS1 DataBars were printed were attached to the containers, the position was classified as left of the drug name, right of the drug name, or back of the drug name with the position of the drug name as the reference point. If the front of the container was unable to be determined for reasons, such as too large or too small labeling of the drug name, the front and back of the container were judged by defining the gap between labels or the window for checking the amount of the remaining drug as the side. For aerosol preparations, the position of GS1 DataBars was classified as “on the inhaling device alone”, “on the can alone”, and “on both the inhaling device and can”. For transdermal preparations, the surface on which the drug name was indicated was defined as the front, and the position was expressed as front or back. For suppositories, the position of GS1 DataBars was classified as the upper part of the container, accompanying label attached to the container, and pitch printed on stick package.
Repetition, boxing, and white outline: Multiple GS1 DataBars printed on the scanning line
of the bar-code scanner were regarded as “repeated” and multiple GS1 DataBars printed on a sheet by the endless method were regarded as “not repeated” if multiple GS1 DataBars were not printed on one scanning line. Whether the area in which a GS1 DataBar was printed was boxed or whitened to highlight the DataBar was also investigated.
Printing and background colors of GS1 DataBars
Holder of marketing authorization
Printing method: The printing method on the PTP sheet was classified as “endless” or “pitch”.
Results and Discussion
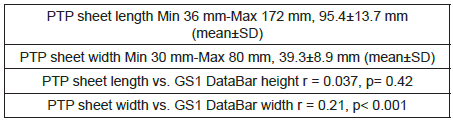
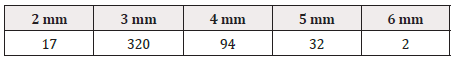
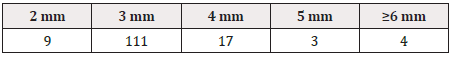
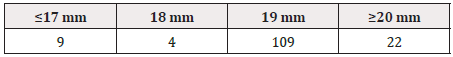
The height and width of GS1 DataBars were most frequently 3 mm and 19 mm, respectively, regardless of the size of the PTP sheet itself (Tables 1-3), (Figure 1).
Of the drugs with GS1 Data Bars printed, one GS1 DataBar was printed on each PTP sheet in 54.8% (247/451), 2 were printed in 5.3% (24/451), and 3 or more were printed in 39.9% (180/451). GS1 Data Bars were printed on the aluminum foil of the PTP sheet by the endless method in 33.9% (153/451) and by the pitch method in 66.1% (298/451). Printing was by the pitch method on all PTP sheets on which only 1 GS1 Data Bar was indicated. On these PTP sheets, the position of printing was the lower middle in 55.9% (138/247), being the most frequent (Figure 1) (Table 4). Of the sheets on which multiple GS1 Data Bars were printed, GS1 Data Bars were printed by the pitch method for each tablet in some and for every 2 tablets in others (Figure 2) (Table 5).
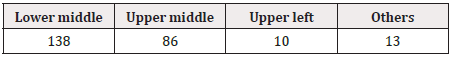
Table 4: Position of printing on PTP sheets on which 1 GS1 DataBar was printed (all by the pitch method) (n=247).

Figure 2: Examples of printing of GS1 DataBars: for each tablet (Amlodipine OD tab.2.5 mg EMEC), for every 2 tablets (Enalapril M tab.2.5 EMEC)
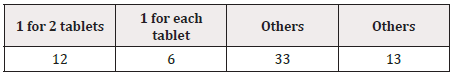
Table 5: Position of printing on PTP sheets on which multiple GS1 DataBars were printed (all by the pitch method) (n=51).
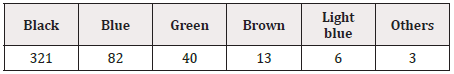
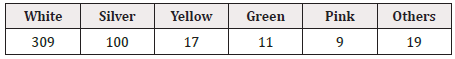
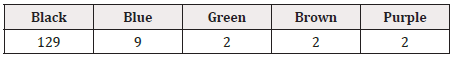
The substrate on which GS1 DataBars were printed was aluminum for all 450 drugs except 1 (plastic). GS1 DataBars were printed by the endless method on PTP sheets for all products in which they were printed repeatedly. Of the drugs for which GS1 DataBars were printed by the endless method, they were printed repeatedly in 68.6% (105/153). GS1 DataBars were boxed in 37.4% (174/465) and were highlighted with a white background in 34.0% (158/465) of all products. The color of GS1 DataBars was most frequently black, and the background was most frequently white (Table 6, 7).
Drugs for external use
A total of 144 DataBars of 144 drugs for external use were surveyed. The height of GS1 DataBars was most frequently 3mm and the width was most frequently 19mm (Table 8, 9).
The substrate of printing was plastic in 37.5% (54/144) of all products, followed by aluminum in 35.4% (51/144), and the difference was small. The substrate was “paper” in 27.1% (39/144) of the drugs for external use, although rarely for drugs for internal use because paper labels were attached to bottle containers in many drugs for external use. Little difference was observed in the printing substrate among drugs for external use. However, in consideration of the dosage form, 33.3% (18/54) of the 54 drugs for which GS1 DataBars were printed on plastic were “ophthalmic solutions”. In addition, of the 51 drugs for which the printing substrate was aluminum, 78.4% (40/51) were “tubed ointments”.
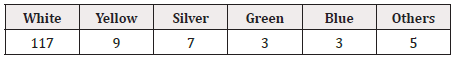
Concerning repeated printing, boxing, and whitening of the background of GS1 DataBars, the pitch method was used for all drugs for external use, and repeated printing was not observed (0/144). GS1 DataBars were boxed in 6.9% (10/144) and were highlighted with a white background in 16.7% (24/144). The color of GS1 DataBars was most often black and the background was most often white (Table 10, 11). The position of GS1 DataBars on drugs for external use is shown in Figure 3.
In the report by Sato et al. [15], the “height of GS1 DataBars” was most frequently 2 mm, but it was most frequently 3 mm in our present survey. Other differences from their report [15] were that the background color of GS1 DataBars was most frequently white, and that there were wider variations in the printing color of GS1 DataBars and background color.
As the height of GS1 DataBars was most frequently 3 mm, we examined the length of the PTP sheet. The length was 175 mm at the maximum and 36 mm at the minimum, with a difference of 172 mm. This suggests that the size of GS1 DataBars is not affected by the size of the PTP sheet itself.
The background color of the aluminum foil of the PTP sheet was often silver, but the areas on which DataBars were printed or the entire background were colored white to reduce reflection in most drugs. For both internal and external drugs, the GS1 DataBars were more often “not highlighted with a white background” because the entire background was white in many products. For the products with a non-white background, DataBars were “highlighted with a white background” in 87.5% (133/152) of drugs for internal use and 81.5% (22/27) of drugs for external use.
The background color of GS1 DataBar most often being silver or white was considered to be due to the widespread adoption of white coloring of the PTP sheet, which was developed to avoid difficulty in reading GS1 DataBars printed directly on the surface of aluminum foil due to specular reflection. Furthermore, the increased variation of the printing color and background color of GS1 DataBars is largely due to technical innovation, e.g., the marketing of products such as READ-MAX® (Toyo Aluminium K.K.). READ-MAX® is PTP aluminum foil that has made reading of GS1 DataBars possible even without a white colored layer by special direct OP coating on the aluminum foil rather than a white colored surface due to diffused reflex [16]. This technology has made a wide variety of PTP available, which was reflected in this survey. The four changes revealed in this survey are all considered to have been brought about by improvements in the printing technology observed during the last few years. In addition, these improvements in printing technology have made printing in smaller areas and on surfaces with unique shapes specific to drugs possible. As it has become possible to print GS1 DataBars anywhere, the diversity of the position of their printing has increased to cater to the needs of holders of marketing authorization and drug types.
For tubed ointments and transdermal preparations, the position of GS1 DataBars was classified as “front” or “back”. The position of DataBars was most frequently “back” in tubed ointments and “front” in transdermal preparations, probably because of the smallness of the printing area on tubed ointments. On tubed ointments, the drug name, specifications, ingredients, holder of marketing authorization, etc., are printed in addition to the GS1 DataBar, and printing all of them on the front surface complicates the labeling design. For this reason, to improve the readability, DataBars are considered to be printed on the front side of transdermal preparations, which have a wide printing area, and on the back side of tubed ointments, which have a small printing area.
For ophthalmic/nasal/ear solutions and bottled ointments, the position of GS1 DataBar was classified as “left of the drug name”, “right of the drug name”, or “back of the drug name”. It was most often “back of the drug name” for ophthalmic/nasal/ear solutions but “right of the drug name” for bottled ointments. These two dosage forms differed markedly in “size of printing area”.
For aerosol preparations, the position of GS1 DataBars was most frequently “on the inhalation device alone” and “on both the inhalation device and can” but was “on the can alone” in none of the preparations examined. For suppositories, GS1 DataBars were mostly prepared separately as a sticker inside or outside the individual package to be attached at the time of dispensation. These two dosage forms had a “unique shape specific to the dosage form” as a common characteristic. In particular, for suppositories, if the GS1 DataBar is printed on each preparation, it is curved along its shape. As a curved GS1 DataBar leads to reading errors, they are considered to be attached according to the amount used. However, this labeling method is not considered optimal because it increases the complexity of the dispensing procedure.
For drugs for internal use, improvements in printing technology have led to the increased diversity of the design of labeling with GS1 DataBars and preservation of the original design image. For drugs for external use, however, how to overcome the “smallness of the printing area” and “unique shape of the dosage form” is an important challenge for the future. Under the present circumstances of labeling with GS1 DataBars, we will evaluate the viewpoint from which pharmacists, who are using GS1 DataBars in actual practice, rate the designs of drugs and how they perceive the present designs to make package designs including GS1 DataBars more convenient for use in medical practice.
Conclusion
The current state of labeling of PTP sheets with GS1 DataBars used in medical practice was surveyed. The use of GS1 DataBars in the field of traceability and medical safety is also expected to progress further in Japan. The end users of drugs are patients who consume drugs by an analogue action of “taking drugs”. It is important to evaluate the state of use of GS1 DataBars and package designs of drugs by reflecting on the viewpoints of both pharmacists and patients.
Conflict of Interest
There are no conflicts of interest influencing the work reported in this paper.
References
- Handling of Labels and Brand Names of Drugs for the Prevention of Medical Accident (2000) PMSB Notification No. 935 of Secretary-General of Pharmaceutical and Medical Safety Bureau (PMSB), Ministry of Health and Welfare (MHLW).
- Reinforcement of preventive measures against medical accidents in medical institution (2003) Joint HPB Notification No. 1127004 and PFSB Notification No. 1127001 of Secretary-General of the Health Policy Bureau (HPB), and of the Secretary-General of the Pharmaceutical and Food Safety Bureau (PFSB), Ministry of Health and Welfare (MHLW).
- (2003) Thoroughness of Measures for the Prevention of Medical Accident Due to the Similarity of Drug Brand Names and Appearances, PFSB Notification No. 1127003 of Secretary-General of Pharmaceutical and Food Safety Bureau (PFSB), Ministry of Health and Welfare (MHLW).
- (2008) Reinforcement and Thorough implementation of Measures for the Prevention of Medical Accident Due to the Similarity of Drug Brand Names (Request for alert), Joint HPB Notification No. 1204001 and PFSB Notification No. 1204001 of Secretary-General of the Health Policy Bureau (HPB), and of the Secretary-General of the Pharmaceutical and Food Safety Bureau (PFSB), Ministry of Health and Welfare (MHLW).
- (2002) Comprehensive Management for Medical Safety Promotion a report by the Medical Safety Management Council, Ministry of Health and Welfare (MHLW).
- Bates DW, Spell N, Cullen DJ, Burdick E, Laird N, et al. (1997) The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. JAMA 277(4): 307-311.
- Leape LL (1995) Systems analysis of adverse drug events. JAMA 274(1): 35.
- Baker GR (2004) The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ 170(11): 1678-1686.
- Shah K, Lo C, Babich M, Tsao NW, Bansback NJ (2016) Bar Code Medication Administration Technology: A Systematic Review of Impact on Patient Safety When Used with Computerized Prescriber Order Entry and Automated Dispensing Devices. Can J Hosp Pharm 69(5): 394-402.
- Bates DW (2000) Using information technology to reduce rates of medication errors in hospitals. BMJ 320(7237): 788-791.
- Bates DW, Gawande AA (2003) Improving safety with information technology. N Engl J Med 348(25): 2526-2534.
- (2006) Practice of Bar code Labeling on Prescription drugs, PFSB/SD Notification No. 0915001, by the Director of the Safety Division (SD), Pharmaceutical and Food Safety Bureau (PFSB), Ministry of Health and Welfare (MHLW).
- (2012) Partial Amendment of the “Guidance for Bar Code Labeling on Prescription Drugs, Joint HPB/EAD Notification No. 0629-1 and PFSB/SD Notification No. 0629-1, by the Director of the Economic Affairs Division (EAD), Health Policy Bureau (HPB) and by the Director of the Safety Division (SD), Pharmaceutical and Food Safety Bureau (PFSB), Ministry of Health and Welfare (MHLW).
- (2015) Population classification of medical services in the northern part of Yokohama City in Kanagawa Prefecture, Japan Medical Analysis Platform, Japan Medical Association, Japan.
- Sato H, Ueda Y, Kawaharabayashi J, Yamamoto S, Nakano F, et al. (2015) Verification of GS1 Data Bar Scanning on Dispensing Package Units of Oral Medicine. J Pharm Health Care Sci 41(12): 880-887.
- (2012) Technical Report, Toyo Aluminium KK.





 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.