Mini Review 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Influence of Organismal Aging in Mesenchymal Stem Cell Therapy
*Corresponding author: Techung Lee, Department of Biochemistry, University at Buffalo, NY 14203, USA.
Received: June 04, 2020; Published: June 10, 2020
DOI: 10.34297/AJBSR.2020.09.001363
Introduction
Mesenchymal stem cells (MSCs) from several tissue sources have been investigated in clinical trials for multiple disorders, and mixed results from these trials have been documented [1], indicating that critical variables that can affect the therapeutic outcome remain to be defined. Organismal aging represents a potential impediment to stem cell therapy. Aged tissue often exhibits telomere shortening, increased Wnt signaling, and fibrosis [2,3], and may thus be more refractory to stem cell therapy. Accumulation of extracellular matrix (ECM) components, which invariably causes thickened lamina in aged tissue, can potentially impede the actions of the many growth/trophic factors secreted by the transplanted MSCs. Indeed, the aged heart often exhibits significant functional deteriorations contributed in part by cardiac stem cell senescence and lower capacity for angiogenesis [4]. Impaired HGF/c-Met and Delta/Notch signaling is also prominent in aged tissue [5].
This host tissue deficit remains a major challenge in regenerative medicine because the aging population usually require the therapy. We previously used a hamster (TO2 strain) heart failure model to study cardiac repair mechanisms mediated by MSCs [6]. However, these therapeutic studies were conducted using young animals (~4 months). Since the TO2 hamster heart is known to exhibit an early aging phenotype due to progressive loss of cardiomyocytes and functional decline, it is important to determine whether the aging heart of older TO2 hamster may be able to achieve functional improvement in response to MSC therapy. Echocardiography performed 1 month after MSC injection shows that both the saline (HBSS) - and MSC-treated old TO2 hamsters exhibited a similar decline in function as indicated by indistinguishable fractional shortening (FS) and left ventricular end-diastolic dimension (LVDd) between the two groups (Figure 1).
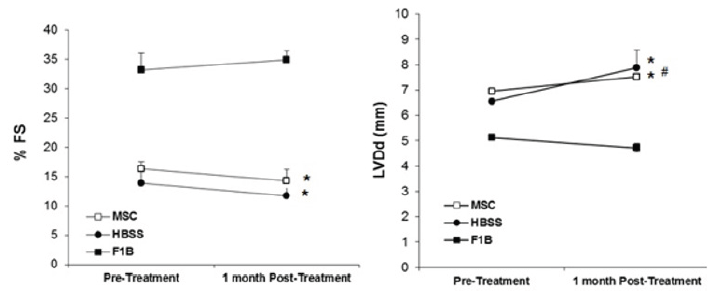
Figure 1: MSCs failed to improve cardiac function in old cardiomyopathic hamster. Ten-month-old F1B (normal) and TO2 hamsters were used for the study. TO2 hamsters received injections of Hanks Balanced Salt Solution (HBSS) or MSCs (n=5 per group). Echo measurements of %FS and LVDd were performed before injection and 1 month after injection. *p<0.05 vs. F1B; #p<0.05 vs. pre-injection.
Thus, although MSC therapy is effective in treating younger TO2 hamsters, it is ineffective in treating the older cardiomyopathic hamsters. Advanced age typically exhibits more prevalent adverse events in humans. Although the human heart has been known to harbor a significant number of resident stem cells possessing limited regeneration capacity, age-related ECM remodeling and stem cell senescence can lead to declining cardiomyocyte populations and myocardial dysfunction [7, 8]. Similar to our finding here, the cardiovascular beneficial effects of G-CSF and ischemic preconditioning were found to be impaired by aging [9,10]. Since the failing hamster heart exhibits abnormally active Wnt signalingmediated fibrosis, excessive fibrosis in the 10-month TO2 heart may profoundly interfere with the growth factor signaling cascade mediated by the administered MSCs. The finding highlights the progressive nature of the fibrogenic process in the cardiomyopathic heart, which can interfere with the regenerative therapy. Thus, host tissue aging/fibrosis represents a major consideration in the design of MSC therapy.
References
- Allison M (2009) Genzyme backs Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, et al. (2007) Augmented Wnt signaling in a mammalian model of accelerated aging. Science 317: 803-806.
- Osiris, despite Prochymal flop. Nat Biotechnol. 27: 966-977.
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, et al. (2007) Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317: 807-810.
- Loubani M, Ghosh S, Galinanes M (2003) The aging human myocardium: tolerance to ischemia and responsiveness to ischemic preconditioning. J Thorac Cardiovasc Surg 126(1): 143-147.
- Conboy IM, Conboy MJ, Smythe GM, Rando TA (2003) Notch-mediated restoration of regenerative potential to aged muscle. Science 302(5650): 1575-1577.
- Mastri M, Lin H, Lee T (2014) Enhancing the efficacy of mesenchymal stem cell therapy. World journal of stem cells 6(2): 82-93.
- Torella D, Rota M, Nurzynska D, Musso E, Monsen A, et al. (2004) Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res 94(4): 514-524.
- Biernacka A, Frangogiannis NG (2011) Aging and Cardiac Fibrosis. Aging Dis 2(2): 158-173.
- Lehrke S, Mazhari R, Durand DJ, Zheng M, Bedja D, et al. (2006) Aging impairs the beneficial effect of granulocyte colony-stimulating factor and stem cell factor on post-myocardial infarction remodeling. Circ Res 99(5): 553-560.
- Boengler K, Buechert A, Heinen Y, Roeskes C, Hilfiker Kleiner D, et al. (2008) Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Circ Res 102(1): 131-135.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.