Short Communication 
 Creative Commons, CC-BY
Creative Commons, CC-BY
LCPT in Tacrolimus-Fast Metabolizing Pancreas Recipients Compared to High Dose Prolonged Release Tacrolimus Versus Not-Fast Metabolizers: A Single Center Experience
*Corresponding author: Claudia Bösmüller, Department of Visceral, Transplant and Thoracic Surgery, Medical University of Innsbruck, Austria.
Received: February 12, 2020;Published: May 29, 2020
DOI: 10.34297/AJBSR.2020.09.001348
Abstract
We compared conversion to LCPT vs high dose prolonged release (PR)-Tacrolimus (Tac) in Tac fast-versus non-fast metabolizing patients. A total of 45 pancreatic graft recipients (43 combined kidney-pancreas, two pancreas transplant alone) were retrospectively analyzed. Immunosuppression consisted of a lymphocyte depleting agent, Tac+MMF+steroids. In response to subtherapeutic Tac-levels despite incremental increase of PR-Tacdosage, 15 patients were converted to LCPT (median day 10.9; range 5-21), after being identified as Tac-fast metabolizers (group 1) by the ratio of concentration/dosage (c/d)<1.05 (median: 0.69; range 0.3 0.9). Another 15 patients with a median c/d ratio 0.22 (range 0.4–0.9) were given high dosed IR-/PR-Tac (group 2). Group 3 consisted of 15 non-fast metabolizing patients (median c/d ratio 1.75; range 1.2–3.8). At 17.2 months, all study patients are alive. In group 1, no pancreatic and renal graft was lost. In group 2, two pancreata were lost to thrombosis, in group 3 one pancreas was lost for necrotizing pancreatitis and one for thrombosis, one kidney for primary non-function. All patients with a pancreas graft in situ are insulinfree. Complications were comparable between all groups. LCPT is a promising alternative for Tac fast metabolizers in pancreatic transplantation.
Keywords: Pancreas Transplantation; LCPT; Tacrolimus; Fast metabolizer
Abbreviations: CD: Concentration Dosage; CMV: Cytomegalovirus; CT: Computer Tomography; CYP: Cytochrome P; DSA: Donor Specific Antibodies; HLA: Human Leucocyte Antigen; IR: Immediate Release; MMF: Mycophenolatemofetil; PR: Prolonged Release; PRA: Panel Reactive Antibodies; PTA: Pancreas Transplantation Alone; SPK: Simultaneous Pancreas Kidney Transplantation; Tac: Tacrolimus
Introduction
Pancreatic transplantation is an effective therapy for type 1 diabetes and kidney failure. A stable therapeutic trough level of Tacrolimus (Tac) is a key factor for graft survival in these patients. Previous studies identified a subgroup of patients in whom a therapeutic range of Tac-level cannot be achieved despite a dose increase of Tac immediate release (IR) or prolonged release (PR) formulations. These Tac “fast metabolizers’’ are characterized by a Tac concentration/dose (c/d) ratio<1.05, basing on a genetic variation of Cytochrome P (CYP) 450 3A5 [1-5]. The CYP 3A5*1allele is associated with rapid Tac metabolism whereby the inferior susceptibility of LPCT to the CYP 3A5 genotype probably results from the LCPT absorption in the more distal gastrointestinal tract where CYP 3A5 activity is decreased [4,5]. A randomized multicentre study and several published clinical trials suggest that LCPT as a feasible immunosuppressant in kidney, liver and pancreas transplantation since it features a 30% increased bioavailability [1- 11].
In contrast to published trials assessing LCPT in larger cohorts of kidney and liver transplantation, only small populations in pancreatic transplantation have been previously reported [2-4,6-10]. Promising results after have been achieved after conversion from PR-Tac to LCPT for tremor or lesser bioavailability. Kerstenetzky et al. postulated the need of further studies on LCPT in pancreatic recipients [10]. In our retrospective center analysis, we investigated pancreas graft recipients after conversion from PR-Tac to LCPT for subtherapeutic Tac levels despite a dose increase of oral PR Tac. Patient, pancreas and renal graft survival and postoperative complications were assessed. This cohort was compared to Tac fast metabolizers who were given high dose IR or PR-Tac and a control group of normal metabolizers.
Materials and Methods
Between February 2016 and March 2019, 45 type 1 diabetic patients (43 simultaneous pancreas kidney transplantation (SPK), two pancreas transplantations alone (PTA)) underwent pancreatic transplantation at our center. Details on transplantation and demographic data are depicted in table 1. The surgical procedures were performed according to standard techniques (renal anastomosis: left iliacal vessels; pancreatic anastomosis: right A. iliaca com./V. cava inferior; exocrine drainage: pancreatoduodenojejunostomy). For pancreas retransplantation, the resection of previous grafts were performed for technical reasons (Table 1).
Immunosuppression consisted of a lymphocyte depleting agent, PR-Tac (in 15/14/14 patients in group 1/2/3, respectively) or immediate release (IR)-Tac (1 patient each in group 2 and 3 for logistic reasons), MMF, steroids (Table 1). The targeted Tac-level (ng/mL) was 12-14 during the first month and 4-6 after 1 year. The initial dose of oral PR-Tac was 0.07 mg/kg and gradually increased in order to achieve the targeted Tac trough level. In response to subtherapeutic Tac-levels despite incremental increase of PR-Tacdosage, 15 patients were converted to LCPT (median day 10.9; range 5-21). They were identified as Tac-fast metabolizers (group 1) by ac/d ratio<1.05 (median: 0.69; range 0.3–0.9). Another 15 fast metabolizers (median c/d ratio 0.22; range 0.4–0.9) received high dose of PR-Tac (group 2). Group 3 consisted of 15 Tac-non-fast metabolizing patients (median c/d ratio 1.75; range 1.2-3.8). Details are depicted in table 2. CYP 3A5 genotyping was not performed for logistic reasons.
The standard antimicrobial prophylaxis consisted of piperacillin-tazobactam or ciprofloxacin, anidulafungin, CMVprophylaxis of gancyclovir/valgancyclovir.
All groups were comparable regarding the postoperative regulation of digestion. All patients were heparinized and later converted to oral anticoagulation depending on the individual risk of bleeding, coronary heart disease and peripheral vasculopathy. Postoperative graft monitoring included assessment of serum creatinine, urea, blood glucose, HbA1c, C-peptide, amylase, lipase, ultrasound and CT imaging in case of inconclusive ultrasound findings regarding pancreatic perfusion. Pancreatic delayed graft function was defined by the need of exogenous insulin in blood glucose>180 mg/dL until day 14 and >250 mg/dL thereafter. Renal delayed graft function was defined by the requirement of ≥2 dialysis post-transplant. All renal rejections were diagnosed clinically (apart from one biopsy proven case) as defined by decrease of urine output/increase of serum creatinine/deteriorated sonographic graft perfusion. All pancreatic rejections were diagnosed clinically as defined by an increase of serum amylase, lipase/hyperglycaemia. Subtherapeutic Tac levels mostly preceded rejection. No biopsies in pancreatic grafts were performed since the risk of bleeding and pancreatic fistula was considered significant.
The long-term pancreas function was calculated according to the Igls score [12]. Anti HLA DSA testing (ELISA or Luminex) was performed according to the availability of the test in the center performing the follow up visits. Steroid withdrawal was performed individually depending on side effects, stability of Tac level, incidence of infections and rejections.
Statistical methods
A descriptive analysis was applied for data assessment since the assessment was done in a relatively small study population.
Results and Discussion
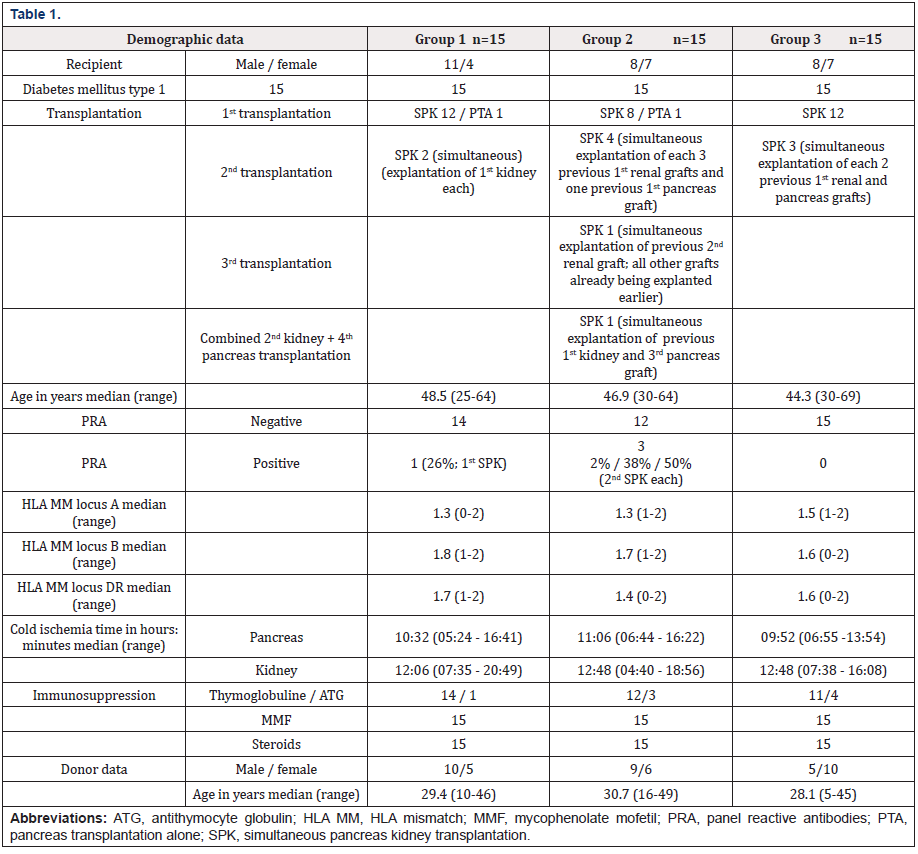
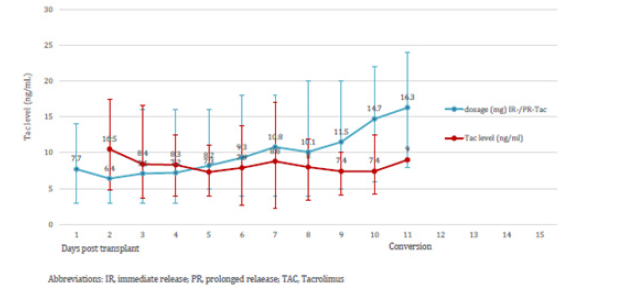
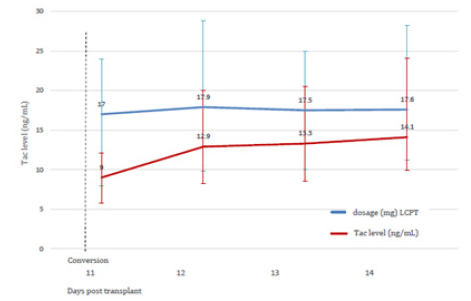
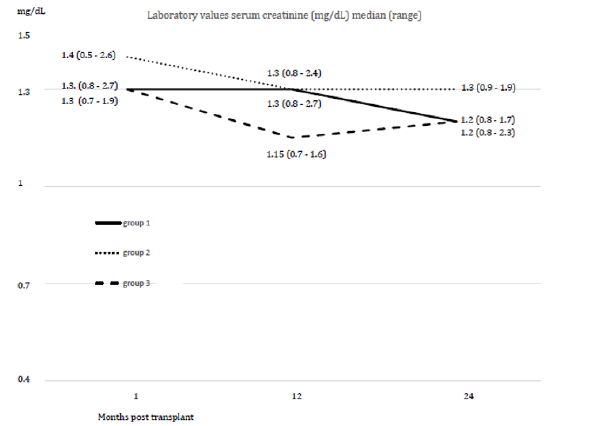
Within an observation time of median 17.6 months (range 6-24) all study patients are alive. In group 1 no pancreatic and renal graft was lost. Patients with subtherapeutic Tac-levels despite of a gradual increase of PR-dosage were switched to LCPT at day 10.9 (median). This resulted in Tac-level reaching the target at day 2.3 (median) after switch (Figures 1a & 1b, Table 2). The dosage of PRTacat conversion was 0.22 mg/kg (median) and converted to LCPT at a starting dosage of 0.23 mg/kg (median). Totally, 4 pancreatic and 1 clinically suspected renal rejection were reversed by steroids and increase of Tac-level. In three cases, pancreatic rejection occurred as a result of a low Tac-level median 8.7 ng/mL) and prior to conversion to LCPT. The median creatinine between month 1-24 was normal, all patients were insulin-free and classified as “optimal” function according to the Igls score (Figures 2a & 2b). The analysis of DSA against HLA class I (ELISA) was negative in 11 patients and not performed in four for logistic reasons. One re- SPK patient reconverted herself from LCPT to IR-Tac at month 2 for recurring diarrhea. Reconversion did not result in an improvement and eventually, a psychosomatic disorder was diagnosed and considered to contribute to the somatic disturbances. Concerning concomitant immunosuppression at month 12, 12 patients were on MMF, three were converted to azathioprine for diarrhea and 1 patient was steroid-free.
In group 2, no renal but two pancreatic graft were lost at month 1 and 2, respectively. Both organs were lost as a result of venous thrombosis. One PTA had DSAs against HLA class I+II in the Luminex assessment. In the other case, DSA were not assessed. Both grafts were eventually removed. Two pancreatic rejections and one case each of biopsy proven (Banff IIa) renal rejection were reversed by steroids (plus IR-/PR-Tac adaption to a therapeutic level), and an additional series of 10 immunaphereses in the latter patient. The median Tac-level at time of rejection was 10.2 ng/ mL. Three out of 4 patients with positive DSAs against HLA class I+II (Luminex; 3 patients preoperatively sensitized with PRA 2%/38%/50% PRA, respectively; all 2nd SPK; one renal acute rejection Banff IIa) had a stable graft function while one patient lost his graft due to thrombosis (PTA; preoperatively PRA negative). The median creatinine between month 1-24 was normal, the Igls score of the remaining pancreatic grafts was “optimal“ (figures 2a, 2b). At month 12, three patients were converted from MMF to azathioprine for diarrhea, five were steroid-free.
In group 3, one renal graft was lost for primary non function (while pancreas function was good), one pancreas was lost as a result of necrotizing pancreatitis (month 2; positive HLA class I+II antibodies in Luminex) and one after thrombosis (month 12; negative HLA class I antibodies in ELISA). The latter 2 were re- SPKs, both were removed. The remaining pancreas grafts display “optimal” function according to the Igls score (figure 2a). The median serum-creatinine between month 1-24 was normal (figure 2b). Five pancreatic rejections were reversed by pulsed steroids and PR-Tac dosage adaptation to the therapeutic range. The median Tac level at the time of rejection was 10.0 ng/mL. Amongst two patients with positive DSA against HLA-class I+II (Luminex), one patient lost the pancreatic graft as a result of thrombosis at month 2 (2nd SPK; stable kidney graft; preoperatively PRA negative). The function of the remaining pancreatic grafts is “optimal“ according to the Igls score (figure 2a). Nine patients had no DSAs against HLA class I (ELISA) one patient had DSAs against class I+II (Luminex). One patient was converted from IR-Tac to CyA for suspected drug fever. As this assumption could not be proven he was reconverted to IR-Tac for hirsutism as a probable CyA side effect, and Tac was tolerated well. At month 12 one patient was switched from MMF to azathioprine for diarrhea, one patient steroid-free. In all three groups the infectious and other complications were controllable (Table 3).
The Following Aspects are Noteworthy for Discussion
Tac fast metobolism is a pharmacokinetic phenomenon resulting from a genetic variation in the CYP 3A5 system, whereby patients porting the CYP 3A5*1 allel are considered to meet the Tac trough levels with LCPT since it is resorbed in the distal gastrointestinal tract where less CYP 3A5*1 is expressed (1-5). CYP 3A5 genotyping might be considered at the time of patient registration on the waiting list. In our study population, a genotyping was not done and we defined Tac-fast metabolizers through a c/d ratio <1.05 (1- 5). The c/d ratio seems to be a feasible denominator at about day 5 when the post operativebol function has recovered. Promising results were published in kidney and liver transplant patients where Tac fast metabolizing recipients were effectively treated with LCPT, but only relatively small groups of Tac fast metabolizing pancreatic graft recipients are reported so far [2-4,6-11]. We therefore retrospectively analyzed our center cohort by comparing 15 Tac fast metabolizing pancreatic graft recipients converted to LCPT with patients treated with high dose IR-/PR-Tac and regular Tac metabolizers.
Tac fast metabolizers were kept on IR-/PR-Tac, but a significant dose increase was applied. The therapeutic Tac level was reached on day 11.6 (median; table 3). In group 3 (regular Tac metabolizers), the therapeutic level was reached at day 5.7 (median; table 2). The importance of a stable therapeutic Tac-level was illustrated by the number of rejections in patients with subtherapeutic Taclevels, although reversed by pulsed steroids plus increase of Taclevel up to a therapeutic range. In the LCPT-group, no pancreatic or renal graft was lost in contrast to two pancreatic graft losses each in group 2 and 3. Due to the relatively small patient population a statistical significance was not calculated. All LCPT patients had a stable pancreas and renal function. Tremor as an established indication for conversion to LCPT was not relevant in this series [10]. Complications were comparable between all groups.
Conclusion
We can summarize from our study that a conversion from PRTac in fast-metabolizing pancreatic graft recipients is feasable and safe. Long-term results are warranted.of providing the needed solution through research and training.
Disclosure
The authors of this manuscript have no any financial or other conflicts of interest to disclose.
References
- Thölking G, Gerth H, Schuette-Nuetgen K, Reuter S (2017) Influence of tacrolimus metabolism rate on renal function after solid organ transplantation. World J Transplant 7(1): 26-33.
- Glander P, Waiser J, Kasbohm S, Friedersdorff F, Peters R, et al. (2018) Bioavailability and costs of once-daily and twice-daily tacrolimus formulations in de novo kidney transplantation. Clin Tranplant 32(8): e13311.
- Du Bay D, Teperman L, Ueda K, Silverman A, Chapman W, et al. (2019) Pharmacokinetics of once-daily extended-release tacrolimus tablets versus twice-daily capsules in de novo liver transplant. Clin Pharmacol Drug Dev 8(8): 995-1008.
- Kamar N, Cassuto E, Piotti G, Govoni M, Ciurlia G, et al. (2019) Pharmacokinetics of prolonged-release once-daily formulations of tacrolimus in de novo kidney transplant recipients: a randomized, parallel-group, open-label, multicenter study. Adv Ther 36(2): 462-477.
- Trofe-Clark J, Brennan DC, West-Thielke P, Milone MC, Lim MA, et al. (2018) Results of ASERTAA, a randomized prospective crossover pharmacogenetic study of immediate-release versus extended-release tacrolimus in African American kidney transplant recipients. Am J Kidney Dis 71(3): 315-326.
- Budde K, Bunnapradist S, Grinyo JM, Ciechanowski K, Denny JE, et al. (2014) Novel once-daily extended-release tacrolimus (LCPT) versus twice-daily tacrolimus in de novo kidney transplants: one-year results of Phase III, double-blind, randomized trial. Am J Transplant 14(12): 2796-2806.
- Rostaing L, Bunnapradist S, Grinyo JM, Ciechanowski K, Denny JE, et al. (2016) Novel once-daily extended-release tacrolimus versus twice-daily tacrolimus in de novo kidney transplant recipients: two-year results of Phase 3, double-blind, randomized trial. Am J Kidney Dis 67(4): 648-659.
- Altieri M, Delaval G, Kimmoun E, Allaire M, Salamé E, et al. (2018) Conversion from once-daily prolonged-release tacrolimus to once-daily extended-release tacrolimus in stable liver transplant recipients. Exp Clin Transplant 16(3): 321-325.
- Torabi J, Konicki A, Rocca JP, Ajaimy M, Campbell A, et al. (2020) The use of LCP-Tacrolimus (Envarsus XR) in simultaneous pancreas and kidney (SPK) transplant recipients. Am J Surg 219(4): 583-586.
- Kerstenetzky L, Descourouez JL, Jorgenson MR, Felix DC, Mandelbrot DA, et al. (2018) A single-center experience with Tacrolimus LCP (Envarsus XR) in pancreas transplant recipients. Ann Pharmacother 52(4): 392-396.
- Oberbauer R, Bestard O, Furian L, Maggiore U, Pascual J, et al. (2020) Optimization of tacrolimus in kidney transplantation: New pharmacokinetic perspectives. Transplant Rev (Orlando) 34(2): 100531.
- Rickels MR, Stock PG, de Koning EJP, Piemonti L, Pratschke J, et al. (2018) Defining outcomes for β-cell replacement therapy in the treatment of diabetes: a consensus report on the Igls criteria from the IPITA/EPITA opinion leaders workshop. Transplantation 102(9): 1479-1486.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.