Case Report 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Nivolumab Induced Autoimmune Like Gastritis: A case report
*Corresponding author: Mohamad Mubder, University of Nevada Las Vegas, Department of internal medicine, University medical center, USA.
Received: May 29, 2020; Published: June 04, 2020
DOI: 10.34297/AJBSR.2020.09.001358
Abstract
Immune checkpoint inhibitors (ICPI) such as ipilimumab (anti-cytotoxic T-lymphocyte antigen-4 (CTLA-4) antibody) and nivolumab (antiprogrammed cell death protein-1 (PD-1) antibodies) are novel chemotherapeutic agents used in multiple advanced metastatic malignancies. They are well known for their immune-related adverse events [1]. Although diarrhea and enterocolitis have been documented as gastrointestinal side effects of ICPI, gastritis has been rarely reported as a side effect of ICPI. Here, we present a case of a 65-years old female with advanced hepatocellular carcinoma who developed autoimmune like gastritis after treatment with Nivolumab [2].
Keywords: Immune check point inhibitors, anti-programmed cell death protein-1 antibodies, Nivolumab, Autoimmune like gastritis.
Introduction
Immune-checkpoint inhibitors are a novel class of chemotherapy currently approved for treatment of multiple metastatic cancers. This class of medications includes anti programmed cell death protein-1 (PD-1) antibody, anti-cytotoxic T-lymphocyte antigen-4 (CTLA-4) antibody, and anti PD-L1 which have shown to improve survival rate of cancer patients in clinical trials [3]. As a result, their clinical use has increased
Significantly. Nivolumab is a fully human IgG4 programmed death 1 (PD-1) immune-checkpoint inhibitor antibody that works by disrupting PD-1 mediated signaling and may restore antitumor immunity [6]. Blockade of immunity checkpoints is associated with inflammatory side effects, known as immune-related adverse events (irAEs) [4,5,6]. These events can affect any organ system and typically target the gastrointestinal, hepatic, skin, and endocrine systems [7,8].With respect to the gastrointestinal tract, the colon is the most commonly affected. To our knowledge few case reports have shown gastric involvement. Here, we present a rare case of Nivolumab induced autoimmune like gastritis.
Case Report
A 65-year-old female with recurrent hepatocellular carcinoma (HCC) on Nivolumab presented with intractable nausea, vomiting, and abdominal pain. Her medical history was also notable for a remote history of ulcerative colitis in deep remission for which she was not on treatment and hepatitis C [9]. She initially presented in 2013 with pT1Nx cholangiocarcinoma s/p resection. In 2016 she was found to have new liver lesions consistent with HCC on biopsy for which she underwent ablation [10,11]. In January 2018 she endorsed recurrent right upper quadrant pain. An MRI of the abdomen and pelvis without MRCP sequence was done during this presentation and she was diagnosed with advanced Stage C HCC [12]. She was started on Sorafenib in March 2018, however, follow up MRI in August showed disease progression [13]. In October 2018, her oncologist initiated Nivolumab as a second line treatment option for metastatic HCC [12,13].The patient had received a total of 6 doses of Nivolumab when she began to have intractable nausea, vomiting and abdominal pain resulting in anorexia in February 2019.She was directly admitted from a follow up appointment due to the severity of her symptoms [10].
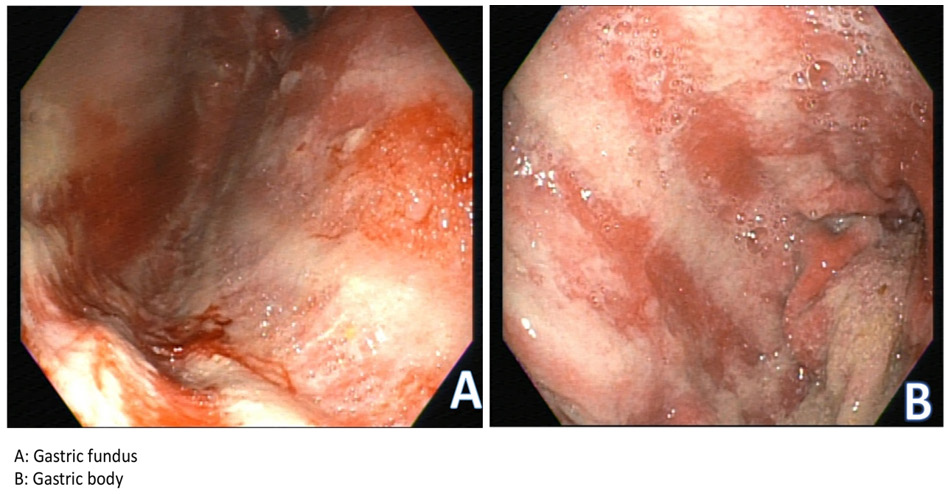
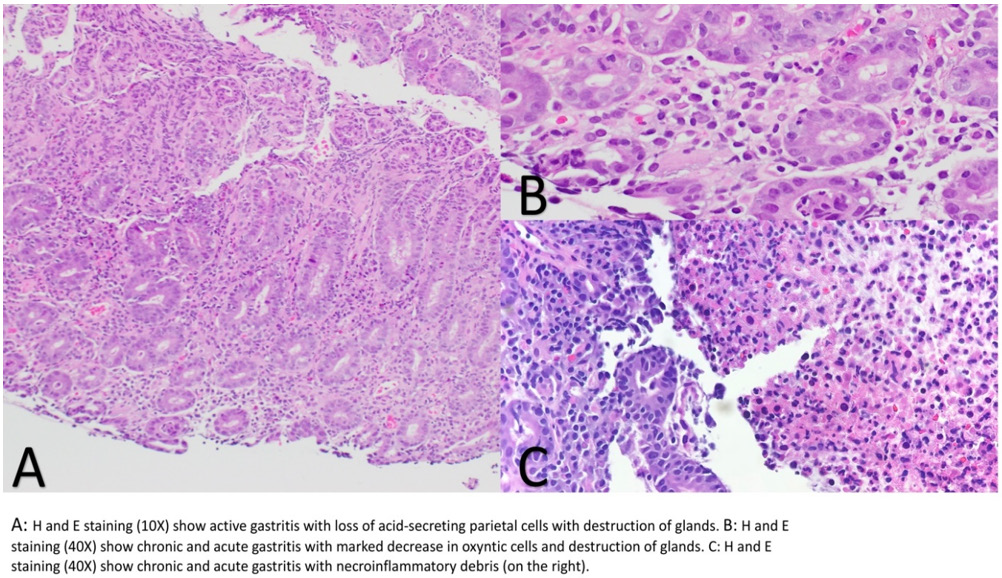
Upon initial inpatient evaluation, she was afebrile with normal vital signs. Labs were notable for WBC 4.7, Hgb 10.4 (patient’s baseline), BUN 8, Cr 0.7, K 3.6, LFTs and transaminases were within normal limits. Abdominal x-ray was negative for obstruction. CT of the abdomen and pelvis with contrast was notable for known metastatic HCC, with some evidence of improvement compared to prior imaging in January 2019. Subsequently, EGD was performed with multiple biopsies taken from gastric antrum, body and duodenum (Figure 1). Hematoxylin an eosin staining showed severe acute gastritis with necro-inflammatory debris diffusely involving the entire stomach and first part of the duodenum. Biopsies from the gastric body showed marked reduction of acid secreting cells with destruction of the glands in the background of diffuse lymphoplasmacytic infiltration (Figure 2). Biopsies were negative for H. Pylori infection. Overall, these findings were consistent with an autoimmune gastritis like picture. To rule our other causes of autoimmune gastritis, anti-parietal cell antibodies, anti-intrinsic factor antibodies, and anti-histone antibodies were drawn and found to be negative. On combining the histopathological, serological and clinical findings, we favor this to be Nivolumab related autoimmune like gastritis.
Discussion
Immune checkpoint inhibitors (ICPIs) have greatly contributed to the treatment of metastatic cancers. The physiological role of immune checkpoints, such as cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death protein-1 (PD-1), which are expressed primarily on T cells, is to regulate the degree of T cell activation to prevent exaggerated immune responses or autoimmunity[14,15] , but may also result in an acquired tolerance to certain pathogens. In particular some tumors demonstrate high levels of the ligand PD-L1, blunting the immune response against them. This interaction can be circumvented through the use of PD-1 or CTLA-4 inhibitors, facilitating cytotoxic T cell activity against such tumors [16,17].Since inhibition of PD-1 or CTLA-4 leads to nonselective activation of the immune system, immune-related adverse events (irAEs) are frequent and commonly include enterocolitis, thyroiditis, pneumonitis, hypophysitis, and pancreatitis [7,11]. Most irAEs are manageable, but they can occasionally be very severe and even fatal as in cases with enterocolitis that leads to intestinal perforation [14,18,19].
Severe or life-threatening irAEs are more common with CTLA- 4 blockers (reported rates of 20–27%) than with PD-1 blockers (13–16%), whereas with combination treatment the rate rises to around 50% [14,20,21].Gastrointestinal AEs are the most common irAEs associated with immune checkpoint inhibitor treatment, often resulting in diarrhea and colitis [14,22,23]. Clinically, PD-1 inhibitors have been shown to cause less severe side effects that are somewhat distinct from those of ipilimumab and idelalisib. In clinical trials, the reported incidence of diarrhea with CTLA-4 inhibitors was 23-33% (severe diarrhea 6-9%) while with PD-1 inhibitor it was 11-19% (severe diarrhea 1-3%). The incidence of colitis with CTLA-4 inhibitor was 8-12% (severe 7-9%) and with PD-1 inhibitor it was 1-4% (severe colitis <1-3%) (2, 26-28). In general, severe complications are less frequent in patients taking PD-1 inhibitors than in those taking CTLA-4 inhibitors [16, 24,25]. The treatment of irAEs is based on the severity of the symptoms. Mild diarrhea/enterocolitis can be managed with symptomatic therapy, but in moderate and severe cases, intravenous corticosteroids are used [14, 22, 23].
If corticosteroids are not sufficient, more potent immunosuppressive agents such as infliximab, mycophenolate mofetil, or calcineurin inhibitors are recommended [26,27]. Patients with pre-existing autoimmune disease (AID) who are being treated with PD-1 inhibitor are at higher risk of irAEs and can occur earlier than those without AID [28-30]. Recent study has shown 23% of patients with AID experienced a flare of their disease while being treated with PD-1 inhibitor [31].Autoimmune gastritis secondary to Nivolumab has rarely been reported. To our knowledge, few cases of autoimmune gastritis in patients on Nivolumab immunotherapy have been reported thus far [4, 7]. Previous reports have shown lymphocytic infiltration in the lamina propria and epithelium of the involved mucosa [4,7]. However, previous cases did not perform serological studies to rule out other causes of autoimmune gastritis as we did in our patient. Immunomodulation is becoming an increasingly common therapeutic approach in patients with advanced malignancy, and therefore its side effects are also becoming more prevalent [16]. Physicians need to be vigilant with regards to these complications in patients being treated with the new class of check points inhibitors who present with gastrointestinal symptoms, perhaps more so in those with pre-existing autoimmune disease [27,28,32].
Authors contributions
Mubder M., provided medical care for the patient and participated in writing the manuscript, Bach L., provided medical care for the patient and participated in writing the manuscript. Varshney N., reviewing pathology slides, participating in writing the manuscript. Banerjee B., Supervising the medical care for the patient and reviewing the manuscript
References
- Yamauchi (2018) the characteristics of nivolumab-induced colitis: an evaluation of three cases and a literature review. BMC Gastroenterology 18(1): 135.
- Hodi FS, O Day SJ, McDermott DF, Weber RW (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363(8): 711-723.
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366(26): 2455-2465.
- Justin Bioko, Todd Dejulio (2017) Severe Esophagitis and Gastritis from Nivolumab Therapy. ACG Case Rep J 12: 4:e57.
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26: 677-704.
- Borghaei H, Paz Ales L, Horn L (2015) Nivolumab versus Docetaxel in Advanced Non squamous Non–Small-Cell Lung Cancer. N Engl J Med 373: 1627-1639.
- Yoshito Nishimura, Miho Yasuda, Kazuki Ocho (2018) severe gastritis after administration of Nivolumab and Ipilimumab. Case Rep Oncol 11(2): 549-556.
- O Connor A, Marples M, Mulatero C, Hamlin J, Ford AC, et al. (2016) Ipilimumab-induced colitis: experience from a tertiary referral center. Therap Adv Gastroenterol 9(4): 457-462.
- Messmer M, Upreti S, Tarabishy Y, Mazumder N, Chowdhury R, et al. (2016) Ipilimumab-induced enteritis without colitis: a new challenge. Case Rep Oncol 9(3): 705-713.
- Brilli L, Danielli R, Ciuoli C (2017) Prevalence of hypophysitis in a cohort of patients with metastatic melanoma and prostate cancer treated with ipilimumab. Endocrine 58(3): 535-541.
- Friedman CF, Clark V, Raikhel AV (2016) Thinking critically about classifying adverse events: incidence of pancreatitis in patients treated with nivolumab + ipilimumab. J Natl Cancer Inst 109(4): djw260.
- Marrero JA, Kulik LM, Sirlin CB (2018) Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the americal association for the study of liver disease. Hepatology 68(2): 723-750.
- El khoueiry A, Sangro B, Yau T (2017) Nivolumab in patients with advanced hepatocellular carcinoma (checkmate040): an open-label, non-comparative, phase ½ dose escalation and expansion trial. Lancet 389(10088): 2492-2502.
- Bergqvist V, Hertervig E, Gedeon P (2017) Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol Immunother 66(5): 581-592.
- Buchbinder EI, Desai A (2016) CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol 39(1): 98-106.
- Gonzalez RS, Salaria SN, Bohannon CD, Huber AR, Feely MM & Shi C, et al. (2017) PD-1 inhibitor gastroentercolities: case series and appraisal of immunomodulatory gastroenterocolities. Histopathology 70(4): 558-567.
- McDermott DF, Atkins MB PD-1 as a potential target in cancer therapy. Cancer Med 2(5): 662-673.
- Kwon ED Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicenter, randomized, double-blind, phase 3 trial. Lancet Oncol 15(7): 700-712.
- Eggermont AM Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomized, double-blind, phase 3 trial. Lancet Oncol 16(5): 522-530.
- Larkin J, Chiarion Sileni V, Gonzalez R Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373(1): 23-34.
- Robert C, Schachter J, Long G V (2015) Pembrolizumab versus Ipilimumab in advanced melanoma. N Engl J Med 372(26): 2521-2532.
- Weber JS, Kahler KC, Hauschild A (2012) Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 30(21): 2691-2697.
- Spain L, Diem S, Larkin J (2016) Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev 44: 51-60.
- Dranoff G (2013) Immunotherapy at large: balancing tumor immunity and inflammatory pathology. Nat Med 19(9): 1100-1101.
- Mitchell KA, Kluger H, Sznol M, Hartman DJ (2013) Ipilimumab induced perforating colitis. JClin Gastroenterol 47: 781-785.
- Maughan BL, Bailey E, Gill (2017) DM Incidence of immune-related adverse events with program death receptor-1- and program death receptor-1 ligand-directed therapies in genitourinary cancers. Front Oncol 7: 56.
- Wolchok JD, Neyns B, Linette G, (2010) Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomized, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 11(2): 155-164
- Larkin J, Chiarion Sileni V, Gonzalez R, (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373: 23-34.
- Wei Wei, MDa Zhibin Luo, PhDb, Risk of gastrointestinal toxicities with PD-1 inhibitors in cancer patients: A meta-analysis of randomized clinical trials. Medicine Baltimore 96(48): e8931.
- Halis Kaan Akturk (2018) PD-1 Inhibitor Immune-Related Adverse Events in Patients with Preexisting Endocrine Autoimmunity. Clin Endocrinol Metab 103(10): 3589-3592.
- Danlos (2018) Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. European Journal of Cancer 91: 21e29.
- Giulia C, Leonardi (2018) Safety of Programmed Death-1 Pathway Inhibitors Among Patients with Non-Small-Cell Lung Cancer and Preexisting Autoimmune Disorders. J Clin Oncol 36(19): 1905-1912.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.