Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
PCSK9 Deficiency Modulates Glucose Homeostasis and Insulin Secretion Via Pancreatic Ldl Receptors And Cholesterol Accumulation
*Corresponding author: Jianbo Wu, Department of Medical Pharmacology and Physiology, University of Missouri, USA
Received: July 11, 2020; Published: July 15, 2020
DOI: 10.34297/AJBSR.2020.09.001421
Abstract
Intracellular lipid accumulation contributes to β-cell dysfunction in type 2 diabetes. How PCSK9 interacts with lipid metabolism to alter insulin availability/resistance is not well studied. Genetic deficiency of PCSK9 mice was fed a high fat (HFD; 45% Kcal from fat) for 14 weeks, and the metabolic phenotype including glucose, insulin, GTT, ITT, and cholesterol was examined. In addition, the expression of PCSK9 and LDLR was were examined by RT-qPCR, and immunohistochemistry. We provide data supporting a role for PCSK9 in pancreatic islets. In HFD mice, PCSK9 deletion led to impaired glucose tolerance and decreased insulin secretion. Pancreatic islets of HFD PCSK9-/ mice showed impaired insulin production and increased LDLR mRNA. Cholesterol was higher in islets of both HFD-fed WT and PCSK9 KO mice compared to low-fat diet controls. Glucose intolerance in PCSK9 deficiency was reversed when the LDLR was also lacking. Further, islets isolated from PCSK-/-LDR-/- mice showed improved insulin secretion. Our findings support a tissue-specific role for PCSK9 in pancreatic cholesterol homeostasis such that PCSK deficiency may accentuate LDLR-dependent β-cell dysfunction.
Keywords: PCSK9; Pancreatic Islet Lipid Handling; Cholesterol Accumulation; Insulin Secretion; High Fat Feeding
Abbrevations: PCSK9: Proprotein Convertase Subtilisin Kexin Type 9; LDLR: Low Density Lipoprotein Receptor; HFD: High-Fat Diet; GTT: Glucose Tolerance Tests; ITT: Insulin Tolerance Tests; IP: Intraperitoneal; DKO: Double Knockout
Introduction
Proprotein Convertase Subtilisin Kexin Type 9 (PCSK9) acts as an inhibitor of the low density lipoprotein receptor (LDLR) [1,2]. This action occurs due to PCSK9 being involved in the translocation of proteins to cellular endosomes for degradation [3]. In the case of the LDLR, PCSK9-mediated degradation can lead to markedly elevated cholesterol levels, as evidenced in a subset of human subjects with genetic mutations that manifest as Familial Hypercholesterolemia [4,5]. As a result of this, considerable effort has been directed towards developing clinically effective PCSK9 inhibitors as cholesterol-lowering agents [6-8]. The major site of synthesis for PCSK9 is the liver, however it is now appreciated that it is produced in other tissues/cell types, including pancreatic islets, intestine, kidneys, and blood vessels [9-11]. These observations have raised questions regarding circulating (predominantly liverderived) possible roles and locally-produced pools of this enzyme [12]. Further, as cholesterol accumulation in pancreatic islets has been associated with impaired insulin release interest has developed in the local role of PCSK9 within pancreatic b cells and effects that may be mediated through its inhibition [13-15]. Thus, global inhibition of PCSK9 may lead to cholesterol accumulation within the pancreatic islets impairing insulin release and glucose metabolism [16,17].
In this regard, studies have suggested that PCSK9 inhibition could increase the risk for diabetes although doubt has been cast on this suggestion by the conclusions of a recent meta-analysis of 35 randomized controlled trials [18,19]. Pre-clinical studies in rodents on the role of PCSK9 have produced conflicting results with knockout models showing both unchanged and impaired glucose tolerance [20,21]. Further, while pancreatic islet morphological differences have been reported, most PCSK9 deficiency situations are associated with normal insulin sensitivity. In the current study, using genetically modified mice, we aimed to examine relationships between PCSK9 and glucose/insulin homeostasis in the setting of a high fat diet. This translationally relevant diet was used to simulate the feeding environment often associated with the development of abnormal metabolic states as in obesity, hyperinsulinemia/ insulin resistance, and ultimately overt type 2 diabetes. The studies utilized PCSK9-/- mice cross-bred onto an LDLR deficient background to create a double knockout as recently also used by others [22]. Collectively our data suggest that in the presence of a high fat diet, an increased interaction between PCSK9 and the LDLR in pancreatic islet β cells leads to cholesterol accumulation, impaired glucose tolerance, and pancreatic islet morphological abnormalities. Interestingly similar changes were not observed in PCSK-/- mice fed a normal chow diet.
Materials and Methods
Animals
C57BL/6J mice were obtained from Chongqing Tengxin Bioscience Inc. (Chongqing, China). PCSK9-/- mice (strain: B6.129S6-PCSK9tm1jdh/J; Stock number: 005993) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). LDLR-/- mice (strain: B6.129S7-Ldlrtm1Her/JNju; Stock number: J002207) were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China). PCSK9-/- mice and ldlr-/- mice were mated to yield double-heterozygotes. PCSK9+/+, LDLR+/+ (i.e. wild-type, WT) mice and PCSK9-/-, LDLR-/- (DKO) mice were created by cross-breeding of the respective heterozygotes. Mice were maintained on a 12:12-h light-dark cycle. All protocols for animal use were approved by the Animal Care Committee of Southwest Medical University in accordance with institutional guidelines.
High Fat Fed Mouse Model
Four- to five-week-old male WT (C57B6/6J) mice and PCSK-/- and LDR-/- models were fed a commercially available high-fat diet (HFD; 45% fat by kcal) (D12451; Research Diet, New Brunswick, NJ) for 14 weeks. Age-matched male mice fed normal chow served as controls. Blood glucose levels were measured in tail vein blood samples using a glucometer (Accu-Check; Roche Diagnostics, Mannheim, Germany). Plasma levels of total cholesterol (C), LDL-C, HDL-C, and triglycerides were measured using an AU680 analyzer (Beckman Coulter, Indianapolis, IN).
Quantitative PCR
Following surgical removal of the pancreas islets were isolated by collagenase digestion as described previous [23]. Islets were cultured for 4 hours in 10 mM glucose RPMI media (containing 100 IU/ml penicillin, 100μg/ml streptomycin, and 10% FCS; pH was adjusted to 7.4 with NaOH), and total RNA was extracted from isolated islets using TRIzol reagent (Invitrogen). RNA samples were pre-treated with deoxyribonuclease I (Invitrogen Life Technologies) and cDNA synthesized using a Super Script kit (Invitrogen Life Technologies). qRT-PCR was performed using mi Script SYBR Green PCR Kits (TaKaRa, Dalian, China) and an ABI PRISM 7700 cycler (Applied Biosystems, Foster City, CA). Amplification reactions were performed at 950C for 10sec and followed by 40 cycles of denaturing (950C for 5sec), Dissociation curve analysis for the PCR products was determined in the final cycle of 55-950C. Each sample was analyzed in duplicate with ribosomal 18S RNA serving as an internal control. Fold changes in gene expression were determined using the 2−ΔΔCT method [24]. Sequences of all primers are shown in Supplementary (Table 1).
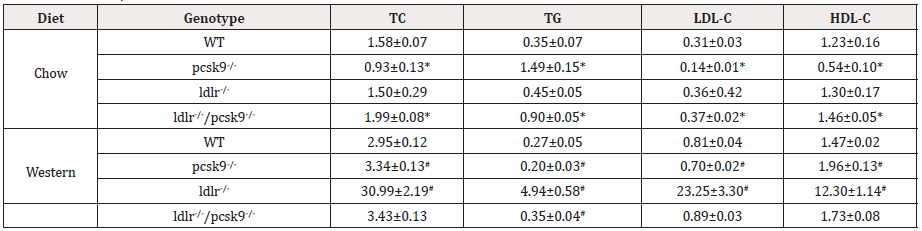
Table 1: Plasma Lipid Profiles.
Note: All units are mM. Values are mean ±SEM. *p<0.01 vs. WT mice fed normal diet; #p<0.05 vs. WT fed western diet; n=6 mice per group. TC, total cholesterol; TG, triglycerides; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; WT, wild-type.
Glucose and Insulin Tolerance Testing
After an overnight fast, glucose tolerance tests (GTT) were performed in response to an intraperitoneal (IP) injection of D-glucose (Roth, Karlsruhe, Germany) (2g of glucose/kg body weight). Following a 4-h fast, insulin tolerance tests (ITT) were performed in response to an IP injection of insulin (0.75 U insulin/ kg body weight). Blood samples were obtained from the tail, and blood glucose levels measured at 0, 30, 60, and 120min using an Accu-Check glucometer (Roche Diagnostics).
Plasma Insulin Measurement
To determine in vivo insulin secretion, after a 4h fast mice were injected (IP) with glucose (3mg/kg) and plasma insulin measured by ELISA (Crystal Chem) as previously described [23].
Insulin secretion from Isolated Pancreatic Islets
To assess capacity for insulin secretion pancreatic islets were isolated by collagenase digestion four size-matched islets were transferred into a 24-well plate and incubated in Krebs-bicarbonate buffer with 2.8 mmol/L glucose and 0.2% BSA to equilibrate for 1h. The preincubation medium was then replaced with fresh buffer containing either 5 or 20mmol/L glucose. After incubation for 1h at 37°C, the supernatant was removed and insulin concentrations were determined by ELISA (Crystal Chem Inc. Downers Grove, IL, USA) as previously described [25]. The experiment was repeated four times.
Histology
Pancreatic tissue was embedded in OCT compound, frozen, serially sectioned (6μm) and cross-sections prepared for immunofluorescence analysis. As an index of cholesterol accumulation, filipin staining (Sigma-Aldrich Chemical Company) was performed as previously described [13] and counterstained with propidium iodide (Sigma-Aldrich). Insulin and LDLR were determined by immunostaining using anti-insulin and LDLR (1:250, Abcam, Cambridge, UK) antibodies, respectively. Goat antirabbit IgG Alexa Fluor 568-conjugated antibody (1:500, Molecular Probes, Invitrogen) was used as the secondary antibody. Images were captured using a fluorescence microscope (Leica, Germany). Levels of fluorescence were quantified in 5 microscopic fields in each of 3 cross-sections of each tissue using ImagePro Plus software (Microsoft Media Cybern8etics, Bethesda, MD).
Statistical Analyses
Data are presented as the mean ± SEM. Differences between groups were analyzed by Student’s t test (comparisons of two groups) or analysis of variance (ANOVA; multiple comparisons) using GraphPad Prism (La Jolla, CA, USA). P < 0.05 was considered to represent statistical significance.
Results
qHigh-Fat Feeding and Deficiency of PCSK9 Lead to Disturbed Glucose Homeostasis
Under control diet conditions (i.e. low fat intake) PCSK9- /- and WT mice exhibited similar levels of glucose and insulin tolerance suggesting that PCSK9 does not differentially regulate whole-body glucose homeostasis. (Figures 1A & 1B) show blood glucose responses following I.P. administration of either glucose (A) or insulin (B), respectively. After 14 weeks HFD feeding, both WT and PCSK9-/- mice showed a significant increase in fasting plasma glucose levels compared to their respective control groups (compare time = 0) (Figure 1A &1C). Fasting glucose levels were, however, not significantly different between WT and PCSK9-/- mice (8.4± 0.4 Vs. 7.4 ± 0.5 mM, p=0.15). Intraperitoneal glucose tolerance tests were similarly performed to further characterize the metabolic state of these animal groups. PCSK9-/-- HFD mice showed a significant impairment in glucose tolerance compared to WT- HFD mice (Figure 1C) while responsiveness to exogenous insulin was unchanged (Figure 1D). These observations were taken to suggest that in PCSK9 deficiency high fat feeding impaired glucose tolerance occurs as a result of a pancreatic deficiency rather than peripheral insulin resistance.
To determine if the LDLR, a downstream target of PCSK9, contributes to impaired glucose tolerance in the presence of PCSK9 deficiency and HFD, LDLR-/- mice were crossed to PCSK9-/- mice to produce a double knockout (DKO) model. WT, PCSK9-/-, LDLR- /-, and LDLR-/-/PCSK9-/- mice were fed the HFD for 14 weeks, after which levels of glucose tolerance were assessed. Interestingly, DKO (i.e. LDLR-/-, PCSK9-/-) mice fed the high fat diet exhibited significantly decreased glucose levels compared to PCSK9-/- mice receiving the same diet (Figure 1E). As expected, plasma total cholesterol levels were decreased in PCSK9-/- mice compared with WT with most of the cholesterol appearing to be transported by HDL. Plasma lipid profiles of each experimental group are shown in Table 1. These results raised the question as to the importance of the LDLR in regulating glucose hemostasis in PCSK9 deficient mice, specifically in a situation of high fat feeding.
Impaired Pancreatic Islet Function in PCSK9 Deficiency During High Fat Feeding
Accumulation of cholesterol in pancreatic β-cells has been shown to reduce insulin secretion and to subsequently cause abnormal glucose metabolism [13,14]. To investigate the role of PCSK9 in β-cell function, we assessed cellular cholesterol levels in histologic sections of pancreas from PCSK9-/- mice. Using the cholesterol probe filipin, pancreatic sections showed a similar low intensity of staining for PCSK9-/- and WT mice fed the normal chow/low fat diet (Figure 2A). In contrast, after 14 weeks of high fat feeding, both WT and PCSK9-/- mice showed a marked increase in the intensity of filipin staining (Figure 2b). Following this, we next examined insulin content in sections of pancreas, Intensity of insulin staining was increased in sections from control diet fed WT mice compared to WT mice fed the high fat diet (Figure 2C).
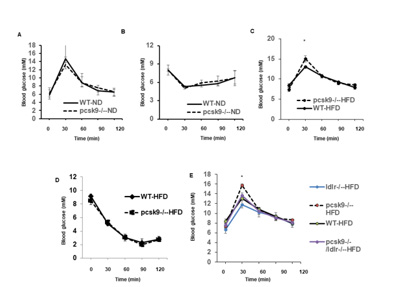
Figure 1: High-fat feeding and PCSK9 deficiency results in impaired glucose homeostasis.
a) Glucose and insulin
b) Tolerance tests (GTT and ITT, respectively) for experimental groups receiving the control diet (n=5 per group).
c) GTT for experimental groups receiving the high fat diet (n=5 per group). *P<0.05 compared to WT-HFD mice.
d) Responsiveness to exogenous insulin (administered I.P), as reflected by changes in blood glucose levels, in WT and PCSK9-/- fed HFD (n=6
per group).
e) Effect of LDR deletion on GTT in experimental groups fed the HFD( n=6 per group). *P<0.05 vs. WT-HFD, ldlr-/-/pcsk9-/--HFD, and ldlr-/--HFD
mice. Data in all panels are shown as mean ± SEM.
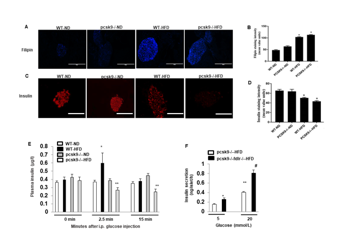
Figure 2: Impaired pancreatic islet function in PCSK9 deficiency during high fat feeding.
a) Representative images showing immunofluorescent staining of filipin (blue) as a marker of cholesterol accumulation in pancreatic islets
pancreas in histological sections from WT-ND, pcsk9-/--ND, WT-HFD, and pcsk9-/--HFD.
b) Group data quantifying fluorescence intensities for cholesterol. 13-49 islets, from n=3 animals, for each condition were quantified and
averaged.
c) Representative images showing immunofluorescent staining for insulin (red) content of pancreatic islets in WT-ND, WT-HFD, pcsk9-/--HFD,
and ldlr-/-/pcsk9-/--HFD mice.
d) Group data quantifying fluorescence intensities for insulin. 5-31 islets, from n=3 animals, for each condition were quantified and averaged.
e) Plasma insulin secretion following an i.p. injection of glucose (3 mg/kg) at indicated time points (n=3 per group). Data are shown as mean ±
SEM. *P<0.05 vs. pcsk9-/--ND.
f) Glucose-induced insulin secretion from islets isolated from HFD-fed PCSK9−/− mice and PCSK9-/-Ldlr−/− mice with glucose stimulation.
Insulin concentrations in the medium were measured by ELISA (n = 4/group). *, **P < 0.05 Vs. PCSK9−/− mice; #P < 0.05 Vs. PCSK9−/− and
PCSK9-/-Ldlr−/− mice. Scale bar, 200m for all images.
Similarly, insulin staining was increased in sections from control diet fed PCSK9-/- mice compared to PCSK9-/- mice fed the high fat diet (Figure 2C). Moreover, there appeared to be a much lower staining in PCSK9-/--HFD mice compared with WT-HFD mice (Figure 2C). Furthermore, PCSK9-/-/LDLR-/- DKO mice exhibited a similar intensity in insulin staining compared with WT-HFD mice (Figure 2C). Group data quantifying fluorescence intensities for cholesterol and insulin staining are shown in Figures 2B & 2D, respectively. In vivo insulin secretion was further assessed by measurement of plasma insulin concentrations following a glucose (I.P.) challenge. No significant differences in plasma insulin were apparent at the early time-point (2.5mins) following glucose injection between WT and PCSK9-/--mice under either low or high fat feeding. In contrast, insulin levels at 15 mins (p<0.05) after glucose injection were significantly lower in the PCSK9-/--HFD mice compared to PCSK9-/--ND, WT-ND and PCSK9-/-- HFD mice, respectively (Figure 2E). Moreover, glucose-stimulated insulin release by isolated islets was significantly increased in PCSK9-/-/ ldlr-/- mice compared with PCSK9-/- mice (Figure 2F). Collectively these data are consistent with a scenario whereby PCSK9 deficiency leads to cholesterol accumulation in β-cells, which is associated with impaired insulin secretion and implicate involvement of the pancreatic LDLR in this process.
PCSK9 Regulates LDL Receptor Expression in HFD-Mice
Compared with mice fed the normal chow diet, the HFD WT mice group demonstrated increased liver PCSK9 mRNA expression (1.9-fold of ND control, p<0.05; (Figure 3A). Further, while the pancreas exhibited a much lower relative PCSK9 mRNA expression in both control and HFD WT mice, expression was significantly increased by high fat feeding. We further investigated the effect of PCSK9 deficiency on LDLR mRNA expression due to its role in the transport of LDL-C. As shown in Figure 3B, the expression of liver LDLR mRNA was notably decreased in HFD-mice compared to mice fed the normal chow diet from both WT and PCSK9-/-mice. On the other hand, there was no significant difference in pancreas LDLR mRNA from both WT and pcsk9-/-- mice fed normal chow. In contrast, the PCSK9-/---HFD mice demonstrated a significant increase in LDLR mRNA levels compared with WT-HFD (2-fold of WT-HFD, p<0.05) (Figure 3B). Furthermore, in pcsk9-/--HFD mice LDLR staining in pancreatic sections were significantly increased compared with that of WT-HFD mice (Figure 3C& 3D).
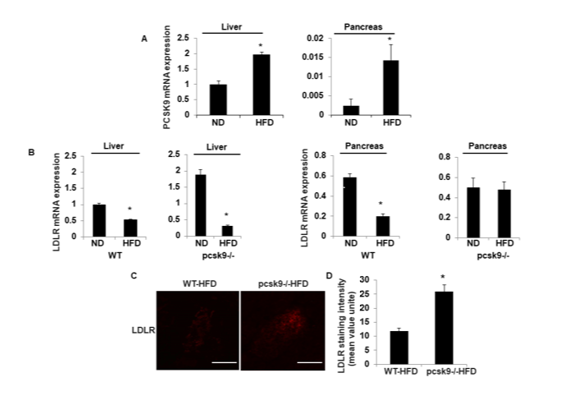
Figure 3: PCSK9 regulates LDL receptor expression in HFD-mice.
a) PCSK9 mRNA was assessed by quantitative RT-PCR in liver and pancreas tissue from WT-ND and WT-HFD mice. Data (mean ± SEM) are for
3 experiments and are expressed as fold-control. *P<0.05 vs. WT-ND.
b) LDLR mRNA was assessed by qPCR in liver and pancreas tissues from WT-ND, WT-HFD, pcsk9-/--ND, and pcsk9-/--HFD mice. Data (mean ±
SEM) are for 3 experiments and are expressed as fold-control. *P<0.05 vs. WT-ND; #P<0.05 vs. WT-HFD.
c) Representative images showing immunofluorescent staining of LDLR (red) in pancreatic islets pancreas in histological sections from WT-HFD,
and pcsk9-/--HFD.
d) Quantification of the immunostaining intensity of islets in each group. *P<0.05 vs. WT-HFD. Scale bar, 200m.
Discussion
PCSK9 acts as an inhibitor of the LDLR by promoting the translocation of proteins to cellular endosomes for degradation. As a result, PCSK9 inhibition is actively being studied to enhance LDLR levels and reduce circulating LDL-cholesterol levels in hypercholesterolemic subjects. While PCSK9 is predominantly formed in the liver, its production in other cell types raises the possibility that its inhibition may more broadly affect cellular function. Of specific relevance, cholesterol accumulation within pancreatic islets has been implicated in negatively impacting insulin secretion, thereby leading to impaired glucose handling [13]. In the current study we examined the effects of PCSK9, and the LDLR in mice fed a HFD for 14 weeks.
This approach was taken as this model is widely used to mimic Western/industrialized diets and predisposes weight gain and insulin resistance [26]. Importantly, and consistent with a recent study [22], our data suggest that in the presence of a high fat diet, an increased interaction between PCSK9 and the LDLR in pancreatic islet β cells leads to cholesterol accumulation and impaired glucose tolerance. Interestingly in the current studies, similar changes were not observed in PCSK-/- mice fed a normal chow diet although HFD fed WT mice showed islet cell cholesterol accumulation but did not exhibit an apparent increase in pancreatic LDLR expression. As shown in Figure 1, when fed a normal chow/low fat diet, globally PCSK9 deficient mice showed similar levels of glucose tolerance and peripheral insulin responsiveness. On feeding the high fat diet (14 weeks) PCSK9-/- mice showed impaired glucose tolerance relative to WT mice fed the same diet while also showing decreased glucose-induced plasma insulin levels consistent with a decrease in pancreatic secretion. Interestingly the high fat diet-induced impairment of glucose tolerance was ameliorated in PCSK9-/- mice also deficient in the LDLR (Figure 1E). Relevant to this, deletion of the LDLR in PCSK9-/- mice also improved in vitro glucosestimulated insulin secretion from isolated pancreatic islets (Figure 2F). PCSK9-/- mice, regardless of the diet fed, showed normal responsiveness to exogenous insulin as indicated by the decline in blood glucose levels. High fat feeding reduced pancreatic islet immunostaining for insulin in both WT and PCSK9 deficiency with the effect being more marked in the KO mice. Collectively these observations suggest that under conditions of PCSK9 deficiency high fat feeding results in impaired availability of pancreatic insulin through decreased synthesis and/or release.
While this appears to relate, at least in part, to increased pancreatic LDLR and cholesterol accumulation, PCSK9 deficiency in a setting of high fat feeding may exert additional effects on insulin production. Consistent with this, of the experimental groups studied, insulin staining was lowest in islets of PCSK9-/- mice fed the high fat diet. Consistent with published studies, PCSK9 mRNA expression was markedly higher in the liver than in the pancreas (less than 1% of relative values) [21,26]. While Langhi et al. [20] previously reported pancreatic levels to be 30% of that for liver, this difference may relate their studies being performed on isolated islets while in the present studies measurements were performed on homogenates of the whole pancreas. PCSK9 mRNA expression levels increased in both liver and pancreas when mice were fed the HFD. LDLR mRNA expression in the liver decreased in response to the HFD in both WT and PCSK9-/- mice. In contrast, high fat feeding decreased pancreatic LDLR mRNA expression in the WT animals while being unchanged in PCSK9 deficient mice.
It is important to note that in this study, we chose to use a relatively mild dietary intervention, although the HF-fed animals did exhibit impaired glucose tolerance and fasting hyperglycemia, as shown in Figure 1. Future studies could extend the findings to a more severe phenotype by extending the period of high fat feeding, adding a high level of refined sugars to the diet, or studying a mouse model of overt type 2 diabetes. Further, as the current studies were limited to male mice, studies of female mice should be conducted to examine any possible sexual dimorphism effects. In summary, our findings are consistent with a tissue-specific role for PCSK9 in pancreatic islet cholesterol homeostasis and insulin secretion. When PCSK9 is deficient, as may occur when PCSK9 inhibitors are used therapeutically, pancreatic LDLR levels increase in the presence of a HFD presumably altering pancreatic lipid handling leads to a reduction in insulin availability and impaired glucose tolerance. This defect in glucose handling occurs despite the maintenance of consistent peripheral responsiveness to insulin. Finally, the data support growing evidence that cholesterol accumulation in type 2 diabetes may accentuate b-cell dysfunction through the LDLR [21,27,28].
Funding
This work was supported by research grants from the National Natural Science Foundation of China (81172050).
Acknowledgements
JH and JW conception and design of research; JH, YL, KL, and RL performed experiments; JH, YL, LW, MAH, and JW analyzed data; JH, YL, and JW interpreted results of experiments; JH, YL, KL, RL, and JW prepared figures; YL, JW drafted manuscript; JH, MAH, JW edited and revised manuscript; JH, MAH, and JW approved final version of manuscript.
References
- Ramin-Mangata S, Blanchard V, Lambert G: Key aspects of PCSK9 inhibition beyond LDL lowering (2018) Curr Opin Lipidol 29(6): 453-458.
- Lagace TA (2014) PCSK9 and LDLR degradation: regulatory mechanisms in circulation and in cells: Curr Opin Lipidol 25(5): 387-39.
- Seidah NG, Awan Z Fau, Chrétien M, Mbikay M (2014) PCSK9: a key modulator of cardiovascular health. Circ Res 14(6): 1022-1036.
- Melendez QM, Krishnaji ST, Wooten CJ, Lopez D (2017) Hypercholesterolemia: The role of PCSK9. Arch Biochem Biophys 625-626:39-53.
- Di Taranto MD, Benito-Vicente A, Giacobbe C, Uribe KB, Rubba P, et al. (2017) Identification and in vitro characterization of two new PCSK9 Gain of Function variants found in patients with Familial Hypercholesterolemia. Sci Rep 7:15282.
- Tziomalos K (2017) The Role of Proprotein Convertase Subtilisin-Kexin Type 9 Inhibitors in the Management of Dyslipidemia. Curr Pharm Des 23(10):1495-1499.
- Pokrywka G (2018) PCSK9 inhibitors: a non-statin cholesterol-lowering treatment option. Postgrad Med 130(3): 287-298.
- Svatikova A (2017) Cholesterol Management in the Era of PCSK9 Inhibitors. Curr Cardiol Rep 19(9): 83.
- Norata GD, Tavori H, Pirillo A, Fazio S, Catapano AL (2016) Biology of proprotein convertase subtilisin kexin 9: beyond low-density lipoprotein cholesterol lowering. Cardiovasc Res 112(1): 429-442.
- Ding Z, Liu S, Wang X, Mathur P, Dai Y, et al. (2016) Cross-talk between PCSK9 and damaged mtDNA in vascular smooth muscle cells: Role in apoptosis. Antioxid Redox Signal 25(18): 997-1008.
- Mbikay M, Mayne J, Chrétien M (2013) Proprotein convertases subtilisin/kexin type 9, an enzyme turned escort protein: hepatic and extra hepatic functions. J Diabetes 5(4): 391-405.
- Luo Y, Warren L, Xia D, Jensen H, Sand T, et al. (2009) Function and distribution of circulating human PCSK9 expressed extrahepatically in transgenic mice. J Lipid Res 50(8): 1581-1588.
- Brunham LR, Kruit JK, Pape TD, Timmins JM, Reuwer AQ, et al. (2007) Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat Med 13(3) :340-347.
- Ishikawa M, Iwasaki Y, Yatoh S, Kato T, Kumadaki S, et al. (2008) Cholesterol accumulation and diabetes in pancreatic beta-cell-specific SREBP-2 transgenic mice: a new model for lipotoxicity. J Lipid Res 49(12): 2524-2534.
- Filippatos TD, Filippas-Ntekouan S, Pappa E, Panagiotopoulou T, Tsimihodimos V, et al. (2017) PCSK9 and carbohydrate metabolism: A double-edged sword. World J Diabetes 8(7): 311-316.
- Ibarretxe D, Girona J, Plana N, Cabré A, Ferré R, et al. (2016) Circulating PCSK9 in patients with type 2 diabetes and related metabolic disorders. Clin Investig Arterioscler 28(2): 71-78.
- Athyros VG,Tziomalos KJ, Doumas M, Sfikas G, Karagiannis A (2015) The Effect of Proprotein Convertase Subtilisin-Kexin Type 9 and its Inhibition on Glucose Metabolism and Cardiovascular Risk. We Should do Better the Second Time After Statins. Clin Sci (Lond) 128: 877-882.
- Momtazi AA, Banach M, Pirro M, Stein EA, Sahebkar A (2017) PCSK9 and diabetes: is there a link? Drug Discov Today 22(6): 883-895.
- Karatasakis A, Danek BA, Karacsonyi J, Rangan BV, Roesle MK, et al. (2017) Effect of PCSK9 Inhibitors on Clinical Outcomes in Patients With Hypercholesterolemia: A Meta-Analysis of 35 Randomized Controlled Trials. J Am Heart Assoc 6(12): 006910.
- Langhi C, Le May C, Gmyr V, Vandewalle B, Kerr-Conte J, et al. (2009) PCSK9 is expressed in pancreatic delta-cells and does not alter insulin secretion. Biochem Biophys Res Commun 390(4): 1288-1293.
- Mbikay M, Sirois F, Mayne J, Wang GS, Chen A, et al. (2010) PCSK9-deficient mice exhibit impaired glucose tolerance and pancreatic islet abnormalities. FEBS Lett 58(4): 701-706.
- Da Dalt L, Ruscica M, Bonacina F, Balzarotti G, Dhyani A, et al. (2019) PCSK9 deficiency reduces insulin secretion and promotes glucose intolerance: the role of the low-density lipoprotein receptor. Eur Heart J 40(4): 357-368.
- Johnson JD, Ahmed NT, Luciani DS, Han Z, Tran H, et al. (2003) Increased islet apoptosis in Pdx1+/- mice. J Clin Invest 111(8): 1147-1160.
- Livak KJ, Schmittgen T (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4): 402-440.
- Zhu M, Wei Y, Geißler C, Abschlag K, Corbalán Campos J, et al. (2017) Hyperlipidemia-induced microRNA-155-5p improves B-cell function by targeting Mafb. Diabetes 66(12): 3072-3084.
- Zaid A, Roubtsova A, Essalmani R, Marcinkiewicz J, Chamberland A, et al. (2008) Proprotein convertase subtilisin/kexin type 9 (PCSK9): hepatocyte-specific low-density lipoprotein receptor degradation and critical role in mouse liver regeneration. Hepatology 48(2): 646-654.
- Kruit JK, Kremer PH, Dai L, Tang R, Ruddle P, et al. (2010) Cholesterol efflux via ATP-binding cassette transporter A1 (ABCA1) and cholesterol uptake via the LDL receptor influences cholesterol-induced impairment of beta cell function in mice. Diabetologia 53(6): 1110-1119.
- Yu Q, Chen Y, Xu CB (2017) Statins and New Onset Diabetes Mellitus: LDL Receptor May Provide a Key Link. Front Pharmacol 8:372.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.