Case report 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Safety of Sofosbuvir and Daclatasvir in Patients having Ischemic Heart Disease for Treatment of HCV Compensated Cirrhosis
*Corresponding author: Ahmed Rezq, Lecturer of cardiology, Ain Shams University, Cairo, Egypt.
Received: May 30, 2020; Published: July 15, 2020
DOI: 10.34297/AJBSR.2020.09.001420
Condensed Abstract
Various biomarkers were used to evaluate the progression and prognosis of atherosclerosis. Safety of new therapeutic modalities of HCV needs more systematic studies. 80 patients were enrolled in our study to demonstrate the safety margin of the the new treatment of HCV in the form of Sofosbuvir and Daclatasvir in patients with coronary artery disease. CIMT, as well as different biomarkers that predict progression of atherosclerosis were analyzed at the beginning of therapy and after receiving the final dose.
Introduction
Background: Sofosbuvir is indicated for the treatment of chronic hepatitis C infection as a component of a combination antiviral treatment regimen. However, its safety in ischemic patients still needs more studies focusing on the inflammatory status that might worsen atherosclerosis. Objectives: to demonstrate the safety of HCV therapy in ischemic patients.
Methods: 80 patients with history of ISHD received HCV therapeutic regimen, with assessment of cardiac biomarkers, ejection fraction, as well as carotid intima media thickness before and after the therapeutic protocol. Other laboratory test including CRP, RDW, serum creatinine, liver function test and lipid profile were done as well.
Results: No significant differences between cardiac biomarkers or the ejection fraction before and after the treatment course. Slight nonsignificant elevation of C-reactive protein (p=0.1) was noticed. Right carotid IMT before and after the treatment was 0.78±0.14 mm and 0.8±0.13 mm respectively (p=0.25). Left carotid IMT was 0.76±0.12 mm and 0.81±0.12 mm before starting and after finishing the therapy (p=0.2). Significant reduction of the liver enzymes after the treatment (p=0.000). LDL and cholesterol levels were significantly increased at the end of the therapeutic course (p=0.017 and 0.03 respectively).
Conclusions: HCV therapy seems to be safe in ischemic patients with no increase of the inflammatory parameters or the CIMT that might be a predictor of atherosclerosis progression.
Keywords: HCV ischemic patients; Sofosbuvir in ischemia; CIMT
Abbrevations: ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase; CIMT: Carotid Intima-Media Thickness; CK: Creatine Kinase; CK-MB: Creatine Kinase Myocardial band, CRP: C-reactive Protein; CVD: Cardiovascular Disease; DAA: Direct Antiviral Agent; DM: Diabetes Mellitus; EDHS: Egypt Demographic and Health Survey; GIT: Gastrointestinal Tract; HCV: Hepatitis C Virus; HDL-C: High Density Lipoprotein Cholesterol; IFN: Interferon; ISHD: Ischemic Heart Disease; LDL-C: Low Density Lipoprotein Cholesterol; MI: Myocardial Infarction; RDW: Red Cell Distribution Width; ULN: Upper Limit of Normal
Introduction and background
The Egyptian Demographic Health Survey (EDHS), a cross sectional survey including hepatitis C virus (HCV) biomarkers, was conducted in 2008 on a large nationally representative sample and estimated HCV prevalence among the 15–59 years age group to be 14.7%. Accordingly, Egypt has the highest HCV prevalence in the world [1].
It has been postulated that the epidemic has been caused by widespread iatrogenic transmission during the era of parenteralantischistosomal- therapy (PAT) mass-treatment campaigns. Today, HCV infection and its complications are among the principal public health challenges in Egypt [1].
Hepatitis C virus (HCV) modulates intra hepatic cholesterol biosynthetic pathways to encourage viral replication. Chronic HCV infection is associated with distorted metabolism, including dyslipidemia and insulin resistance, which contributes to disease progression and influences response to therapy [2].
Hereby, we aim to study whether the treatment regimen in the form of oral Daclatasvir (60 mg) plus oral Sofosbuvir (400 mg) given for 12 weeks could have a deleterious effect on vasculature and possible development or progression of atherosclerosis in patients with coronary artery disease by evaluating carotid intima media thickness and other inflammatory markers before and after receiving the treatment.
Study design and methodology
Study design
This is a two center single cohort study conducted on 80 consecutive patients with history of ischemic heart disease presented to the gastroenterology outpatient clinic of Galaa Military Medical Complex and Ain Shams University hospitals and found eligible for the new regimen including Sofosbuvir as well as daclatasvir.
Study populations
Inclusion criteria: Compensated Cirrhotic patients with INF ineligibility will be treated according to the following criteria
a. Child Score up to 6, which is used to assess the prognosis of chronic liver disease, mainly cirrhosis. Although it was originally used to predict mortality during surgery, it is now used to determine the prognosis, as well as the required strength of treatment and the necessity of liver transplantation [3].
b. Total bilirubin ≤ 2
c. Albumin ≥ 3
d. Platelet count ≥ 100000
e. Prothrombin concentration ≥ 80%
f. Hemoglobin concentration ≥ 10 mg
Patients with history of coronary artery disease. (Previous percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG))
Patients having ischemic cardiomyopathy controlled on medical treatment.
Exclusion criteria
a. Child C patients with scores ≥ 6.
b. Presence of ascites (except after control), this is the buildup of fluid in the space between the lining of the abdomen and abdominal organs, ascites results from portal hypertension and hypoalbuminemia. Diseases that can cause severe liver damage can lead to ascites including include long-term hepatitis C [4].
c. Patients with Hepato-cellular carcinoma.
d. Presence of large risky oesophageal varices (Except after prophylactic treatment).
e. Age limits for treatment legibility will be above 18 years and below 70 years.
f. BMI will be accepted up to 35.
g. Patients having acute coronary syndrome or presented with acute heart failure.
Methodology
a. All patients provided written informed consent before inclusion in the study after approval of the scientific and ethical committees in the cardiology as well as gastroenterology department and university hospital.
b. Detailed medical history regarding other risk factors like hypertension and diabetes mellitus was obtained.
c. Carotid intima – media thickness (IMT) by carotid Duplex (before and after the therapeutic regimen).
The IMT is calculated as the mean distance between the leading edges of the inner and outer echoes of the double-line pattern of the far artery wall along a 1-cm portion of the vessel [5,6].
d. ECG, echocardiography as well blood tests including cardiac biomarkers, lipid profile, liver and kidney function test, ESR, CRP and complete blood picture including red cell distribution width (RDW), which quantifies the variability in size of circulating red blood cells (i.e. ,anisocytosis), defined as the standard deviation of erythrocyte size divided by the mean corpuscular volume(MCV). A close relationship between high RDW and intima media thickness, and the inci¬dence of carotid plaque was identified [7,8]. All these tests were done before starting the treatment and after the final therapeutic session.
The Therapeutic regimens: HCV direct-acting antiviral (DAA) agent in the form of daily oral Daclatasvir (60 mg) plus oral Sofosbuvir (400 mg) will be given to all patients for 12 weeks
Regular visits to the outpatient clinic at the first, second and fourth week during the first month, then monthly visits for the remaining 2 months.
Statistical analysis: Categorical variables are expressed as number and percentage of patients. Continuous data are reported as means and standard deviation as well as 95% confidence intervals when appropriate. Statistical analyses were performed with SPSS 18.0 (SPSS Inc., Chicago, Illinois).
Results
Baseline characteristics
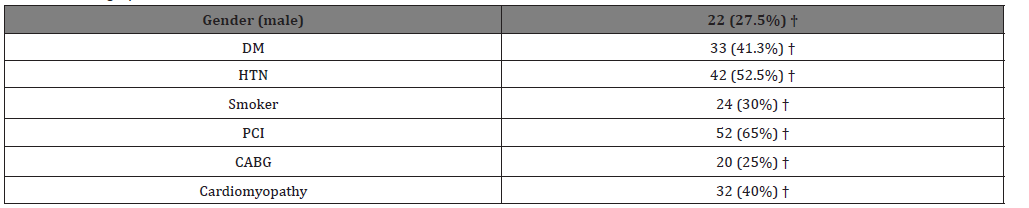
22 males (27.5%) were included, 33 (41.3%) diabetics, 42(52.5%) hypertensive, 24 (30%) smokers. Patients having coronary artery disease with history of previous PCI were 52 patients (65%) and those who had bypass grafting previously were 20(25%), the rest were on medical treatment (Table 1).
Blood tests results
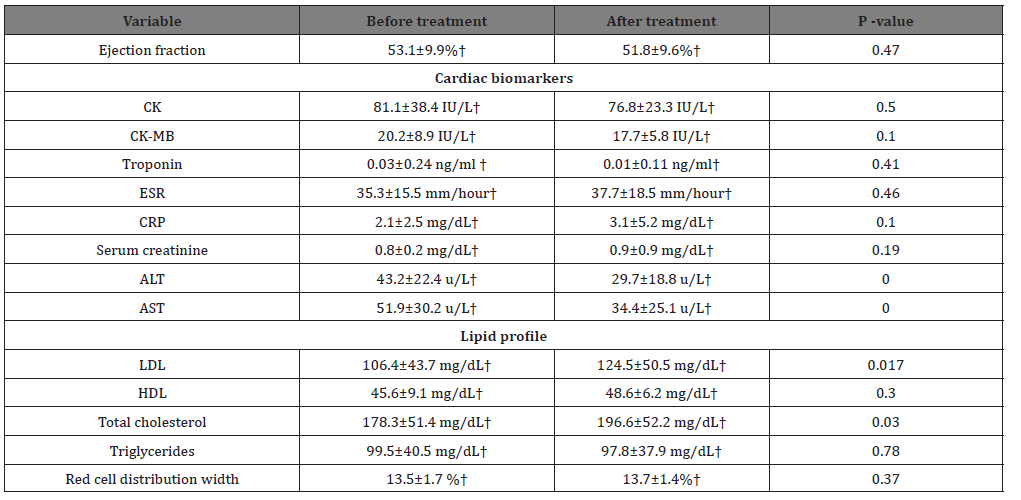
There were no significant differences between cardiac biomarkers before and after the treatment course. The treatment slightly elevated the C-reactive protein however non significantly (p=0.1).
Significant reduction of the liver enzymes with the values before starting the treatment 43.2±22.4 u/L and 51.9±30.2 u/L and after therapy 29.7±18.8 u/L and 34.4±25.1 u/L for ALT and AST respectively (p=0.000 for both). LDL and cholesterol levels were significantly increased at the end of the therapeutic course (p=0.017 and 0.03 respectively). The rest of the laboratory tests results are shown in table 2. (Table 2)
Ejection fraction
The treatment didn’t affect the systolic functions of the myocardium with a mean ejection fraction before and after the treatment of 53.1±9.9% and 51.8±9.6% respectively (p=0.47).
Carotid intima media thickness
Right carotid IMT before and after the treatment course was 0.78±0.14 mm and 0.8±0.13 mm respectively (p=0.25). Left carotid IMT was 0.76±0.12 mm and 0.81±0.12 mm before and after finishing the therapy (p=0.2). (Table 3).
Discussion
Our study was a prospective, two centers, single cohort study conducted on patients with chronic HCV infection presenting to the GIT outpatient clinic at the Galaa Military Medical Complex and Ain Shams University hospital for receiving the new direct antiviral agents regimen in the form of sofosbuvir and daclatsavir combination.
Carotid IMT
In their study in the early 90’s, J.Persson et al [5] stated that a thicker intima media complexes in the common carotid artery in patients with plaques in the bifurcation is a sign of early generalized atherosclerosis. And they concluded that measurement of CIMT with ultrasound will reflect early atherosclerotic lesions in a valid way and can give valuable information on the progression and regression of such disease. In our study, CIMT showed no significant increase demonstrating safety in ischemic patients with atherosclerotic coronary artery disease.
Ejection fraction
The treatment seems to be out of harm’s way on the cardiac ejection fraction. This is of particular importance in patients with ischemic cardiomyopathy. Many hepatologists were apprehensive to start the treatment in this category of patients (with impaired LV systolic functions) because they were worried about the possible side effects on the myocardium. Nevertheless, the drug interaction with beta blockers, which is a fundamental part of ischemic heart diseases or cardiomyopathy therapy was always questioned. In spite of the fact that our study has a small cohort number, but we could still state that, it seems safe and sound not to defer patients with ischemic cardiomyopathy or those taking beta blockers from receiving this HCV therapeutic regimen.
Cardiac biomarkers
Many studies demonstrated that even mild troponin-I elevations, including levels below the 99th percentile of a reference population, may predict the presence of angiographic CAD and future cardiovascular events [9,10]. The new antiviral regimen didn’t even affect the myocardial cells, and proved safety, demonstrated by stable Troponin-I levels before and after the treatment.
CK-MB, a subdivision of CK enzymes, is an enzyme found mainly in the heart muscle, but also found in tongue, diaphragm, uterus, prostate and skeletal muscle, although in low amounts of only 1% to 3% [11].
Creatine kinase was assessed in the FISSION and NEUTRINO trials. Isolated, asymptomatic creatine kinase elevation of greater than or equal to 10xULN was observed in less than 1% and 2% of subjects in the peginterferon alfa sofosobuvir + peginterferon alfa + ribavirin 12 weeks and sofosobuvir + ribavirin 12 weeks groups, respectively.
Although CK and CK-MB levels can be elevated in myocardial damage, it may also be released as a result of damage to non-cardiac muscle which decreases its specificity [12].
Neither CK or CK-MB levels showed significant changes in our study. This might express more safety margin for the new regimen compared to the previous ones. However, it might be attributed to the small cohort number of our study.
CRP
CRP is not only an excellent biomarker of inflammation, but it is also a direct participant in atherogenesis. Many studies have demonstrated that increased CRP concentrations are associated with an increased risk of MI, stroke, peripheral arterial disease, and sudden cardiac death. Unlike other markers of inflammation, CRP levels are stable over long periods, have no diurnal variation, can be measured inexpensively and have shown specificity in terms of predicting the risk of cardiovascular disease. When combined with lipid screening, CRP improves global risk prediction in patients who would otherwise not be identified for primary prevention by lipid assessment alone [13].
Marcin Skowroński et al demonstrated that diabetic patients with HCV infection seem to have decreased inflammatory markers compared with diabetic subjects without the infection. Hypothetically, this finding could be attributed to various HCV immuno inhibitory effects, for example via induction of interleukin-10, one of the cytokines associated with Th2 response favoring chronic infection [14].
The therapy didn’t affect the C-reactive protein levels, which supports the idea that the treatment doesn’t initiate any inflammatory response that might affect the progression of atherosclerosis in ischemic patients.
Lipid profile
HCV infection, in particular with genotype-3, is associated with liver steatosis and hypocholesterolaemia. In addition, HCV replication affects insulin sensitivity and may result in a higher incidence of cardiovascular events [15,16].
In their recent study, as well as ours, Stefan Mauss et al, demonstrated a rapid increase of total and LDL-cholesterol in a larger number of patients treated with a variety of DAA combinations, these changes of total cholesterol were driven by changes in LDL-cholesterol, whereas HDL-cholesterol remained essentially unchanged. Triglycerides as well remained unchanged during and after interferon-free regimen. In their study, there was no association between the effect on cholesterol levels and a specific DAA regimen. A possible interpretation of the increase in LDL- cholesterol after eradication of HCV may be a return to ‘normal’ pre-infection lipid patterns after elimination of the effect of HCV on lipid levels [16].
RDW
It is worthy to mention that increased RDW has been associated with an increased risk of a wide spectrum of cardiovascular diseases in a large number of studies [17]. It was clearly demonstrated in our study, that RDW showed no alteration, which adds to the safety profile of this treatment in patients having coronary artery disease.
Kidney function tests
Since sofosbuvir is the only DAA with renal elimination, concerns for potential nephrotoxicity have been raised mainly for this agent. There have been reports suggesting that sofosbuvir might have a negative impact on renal function in patients at high renal risk (e.g., decompensated cirrhosis, liver transplant, proteinuria). However, renal function decline in such high renal risk patients does not necessarily reflect drug related toxicity. In addition, improvement in renal function after treatment has also been reported in patients who achieved sustained viral response despite the scarcity of long follow-up data after the end of therapy. Only nephrotoxicity related to sofosbuvir has been observed but only in patients with GFR < 30 ml/min and seems to be minimal given the short duration of therapy. Therefore, no definite conclusion can be drawn, while it seems reasonable to apply nephroprotective measures and careful renal monitoring during treatment with sofosbuvir-based regimens in patients at high renal risk [18]. Hereby, serum creatinine was not influenced by the treatment and all patients demonstrated normal serum creatinine levels.
Finally, there was a significant reduction in the serum levels of both ALT and AST across the study groups, indicating normalization of the aminotransferases after 12 weeks of the therapeutic regimen, that reflects improvement of the liver performance and efficacy of this regimen.
Study limitations
Small cohort number as well as the absence of long term follow up data is the main weaknesses of our study.
Conclusion
HCV direct-acting antiviral (DAA) agent in the form of daily oral Daclatasvir (60 mg) plus oral Sofosbuvir (400 mg) seems to be safe in ischemic patients with history of coronary artery disease. It doesn’t affect any of the cardiac biomarkers, or the inflammatory markers that might predict worsening/progression of coronary atherosclerosis. This safety has been demonstrated in high risk patients, including patients with ischemic cardiomyopathy and previous CABG.
References
- Domenico Cucinotta, Maurizio Vanelli (2020) WHO Declares COVID-19 a Pandemic. Acta bio-medica 91(1): 157.
- Ahmed H, Allaf M, Elghazaly H (2020) COVID-19 and medical education. Lancet Infect Dis 20(7): 777-778.
- Augestad KM, Lindsetmo RO (2009) Overcoming distance: Videoconferencing as a clinical and educational tool among surgeons. World J Surg 33(7): 1356-1365.
- Rose S (2020) Medical Student Education in the Time of COVID-19. JAMA 323(21): 2131-2132.
- Kaup S, Jain R, Shivalli S, Pandey S, Kaup S (2020) Sustaining academics during COVID-19 pandemic: The role of online teaching-learning. Indian J Ophthalmol 68(6): 1220-1221.
- Kay D, Pasarica M (2019) Using technology to increase student (and faculty satisfaction with) engagement in medical education. Adv Physiol Educ 43(3): 408-413.
- Abrahamson SD, Canzian S, Brunet F (2006) Using simulation for training and to change protocol during the outbreak of severe acute respiratory syndrome. Crit Care 10(1).
- Thangasamy IA, Loeb S, Sathianathen NJ, Leveridge M, Stork B, et al. (2019) Evaluating the effectiveness of an online journal club: Experience from the International Urology Journal Club. Eur Urol Focus.
- Theoret C, Ming X (2020) Our education, our concerns: The impact on medical student education of COVID-19. Med Educ 54(7): 591-592.
- Broyles IL, Cyr PR, Korsen N (2005) Open book tests: Assessment of academic learning in clerkships. Med Teach 27(5): 456-462.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.