Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Sepiolite Enhances Biomineralization Activity of The Cementoblasts
*Corresponding author: Sema S Hakki, Faculty of Dentistry, Selcuk University, Konya, 42079, Turkey.
Received: July 01, 2020; Published: August 06, 2020
DOI: 10.34297/AJBSR.2020.09.001447
Abstract
Aim: The inductive potentials of graft materials are important for regenerative therapies. Thus, this study was conducted using cementoblasts (OCCM-30) tooth root lining cells to determine whether sepiolite affected proliferation, mRNA expressions of genes associated with cementum/bone and biomineralization.
Materials and Methods: The media containing released components of sepiolite (100 mg/ml ratio; waited 72 hrs in 5%FBS containing media) were used for cementoblast’s treatments. Proliferation of the cells was evaluated using a real-time cell analyzer (RTCA) for 170 hrs. After total RNA isolation on days 3 and 6, cDNA synthesis was performed. Bone sialoprotein (BSP), osteocalcin (OCN), collagen type I (COL-I), runt-related transcription factor 2 (Runx2), and alkaline phosphatase (ALP) transcripts were examined employing quantitative RT-PCR. Biomineralization of the cementoblasts was evaluated on day 8 via von Kossa staining.
Results: Sepiolite decreased proliferation of the cementoblasts when compared to untreated control group. While there was no change for BSP mRNA expression for both time points, sepiolite up-regulated OCN, Runx2, COL-I and ALP mRNA expressions (p <0.01) on days 3 and 6 when compared to control. Sepiolite stimulated mineralized nodule formation of the cementoblasts when compared to mineralization media group (positive control).
Conclusion: The findings of this study demonstrated that sepiolite enhances the functions of the cementoblasts involving new cementum formation which is critical for periodontal regeneration. Results suggested that sepiolite has potential as a graft material in dentistry and medicine.
Keywords: Sepiolite, Cementoblasts, Mineralized Tissue, Periodontal Regeneration, Cementum
Introduction
Huge numbers of biomaterials with/without growth and differentiation factors have been investigated in dentistry and orthopedics aiming regenerative procedures. Platelet derived growth factor (PDFG), basic fibroblast growth factor (bFGF) and/or bone morphogenetic proteins (BMPs), enamel matrix proteins containing graft materials have been widely assessed [1-8]. However, recombinant technologies and production cost of these osteoinductive materials in general are expensive. Researchers focus to find safe, cheap, easily accessible, and bioactive materials to replace with others in regenerative medicine [5-8].
To date several alternative biografts for regenerative purpose have been synthesized. Trace elements like; boron, selenium etc. were incorporated to the materials to induce mineralized tissue formation [9-11]. Gelatin, cuttlefish backbone, meerschaum sepiolite, hydroxyapatite and chitosan based scaffolds as graft substitutes were provided acceptable mechanical structure and cell viability rates [12,13]. Sepiolite known as meerschaum is nanoclay which has complex magnesium silicate. Sepiolite might have potentials for regenerative therapies due to extremely active surface area (~300 m2/gr) [14,15], safety and easily accessibility in nature.
The aim of regenerative therapies is to improve the migration, proliferation, and differentiation progenitor and/or stem cells to the differentiated cells synthesizing extracellular matrix components appropriate for targeted tissue/organ. Cementum is specialized calcified substance covering the root of a tooth and the part of the periodontium that attaches the teeth to the alveolar bone by anchoring the periodontal ligament [16]. The regeneration of periodontium requires key components including cells, signals, scaffolds, and an appropriate niche providing wound healing without scar formation. Osteoblasts, cementoblasts, progenitor cells/mesenchymal stem cells for new alveolar bone, cementum and periodontal ligament tissue formation respectively are the criteria of the periodontal regeneration [17]. Due to limited regenerative capacity and avascular structure of cementum; the induction of tooth root lining cells is very critical [16,17]. The aim of this study is to assess the effects of sepiolite on the proliferation, mRNA expressions of the mineralized tissue associated genes; including Bone sialoprotein (BSP), osteocalcin (OCN), collagen type I (COL-I), runt-related transcription factor 2 (Runx2), and alkaline phosphatase (ALP) and biomineralization of the cementoblasts.
Materials and Methods
Culture of Cementoblasts
An immortalized mouse cementoblast cell line OCCM-30 (kindly provided by Dr. Martha J. Somerman) was cultured in Dulbecco’s modified Eagle’s medium (DMEM; GIBCO; Grand Island, NY, USA), 10% fetal bovine serum (FBS; GIBCO), L glutamine (600 mg/ml; GIBCO;), penicillin (100 U/ml; GIBCO), and streptomycin (125 mg/ ml; GIBCO) in a humidified atmosphere of 5% CO2.
Preparation of Graft Material’s Solutions
Sepiolite at 100 mg/mL concentrations were incubated in 5% DMEM medium for 72 hours at 37oC according to ISO 10993- 12:2007 and 7405:2008. After incubation period, media obtained containing bioactive components released from graft materials and this extract medium was filtered using 0.22 μm pore size filter for cell culture experiments.
Cell Proliferation Assay
The effect of sepiolite on the cementoblast’s proliferation, real time cell analyzer (RTCA-SP; xCELLigence) was used. 200 μL cell suspensions, (5 x103 cell/well) was seeded to the wells of a 96-well view E-plate for 24 hours. The following day, 200 μL DMEM containing released components of sepiolite was applied to each well and the proliferative curve of the cementoblasts was determined every 15 minutes for 170 hours.
RNA Isolation and Real-Time Polymerase Chain Reaction
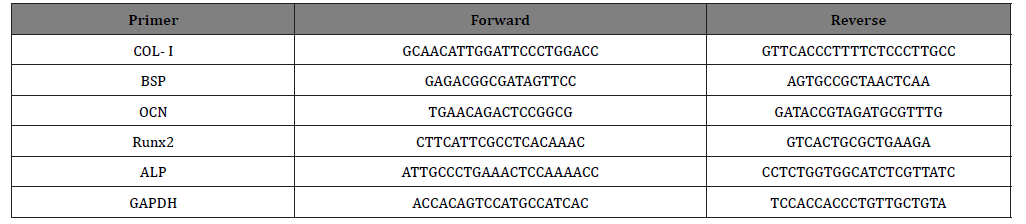
Cells were added in 60 mm cell culture dishes 25×103 cells/ cm2 for 24h. Then, OCCM-30 cells were treated with the DMEM with sepiolite and total RNA was isolated using a monophasic solution of phenol and guanidine isothiocyanate on day 3 and day 6. The RNA concentrations were measured at nanodrop and stored at -80oC until complementary DNA (cDNA) synthesis. First-strand cDNA was transcribed from 1μg of RNA by cDNA synthesis kit in accordance with the manufacturer’s protocol. Quantitative real-time polymerase chain reaction was also performed with the Strategene MX3000P with SYBR Green QPCR Master Mix in triplicate. The amplification profile included 95/600; 95/15; 60/60; 72/30 (temperature in °C/time in seconds), and 35 to 40 cycles. The primers for mouse BSP, OCN, COL-I, RunX2, ALP and GAPDH were used in PCR reactions (Table 1).
Mineralization Experiment
Von Kossa staining was performed to observe mineralized nodules. Cementoblasts was added 5x 104 cells/cm2 24-well plates in DMEM containing 10% FBS and was incubated at 37oC 5% CO2 for 24 hours. Following this incubation, cells were treated with 5% FBS DMEM+ 50 μg/ml ascorbic acid and 10 mM β-glycerophosphate Von Kossa staining was performed to observe mineralized nodules. Cementoblasts was added 5x 104 cells/cm2 24-well plates in DMEM containing 10% FBS and was incubated at 37oC 5% CO2 for 24 hours. Following this incubation, cells were treated with 5% FBS DMEM+ 50 μg/ml ascorbic acid and 10 mM β-glycerophosphate
Statistical analysis: The values for cell index and mRNA expressions were analyzed by ANOVA using Graph PadPrism software. The results were presented as means ±SEM. P values ≤0.05 were considered as statistically significant.
‖Life Sciences/GIBCO, Paisley, UK
‖‖ Sigma, St. Louis, MO, USA
▲BD Biosciences, Bedford, MA, USA
ΣxCELLigence, ACEA Biosciences, Inc, CA, USA
§§§Stratagene, La Jolla, CA, USA
ΨApplied Biosystems, Carlsbad, CA, USA
ΦNanoDrop, Wilmington, DE, USA,
†InVitrogen, Camarillo, CA, USA
‡ Roche Diagnostics GmbH, Germany
*Insert, Millicell, Millipore, Billerica, MA, USA
††Zeiss EVO ® LS 10, Brock & Michelsen, Denmark
†††Thermo Fisher Scientific, RevertAid First Strand cDNA Synthesis
Kit Waltham, MA USA
‡‡‡Thermo Fisher Scientific, Fermentas Maxima SYBR Green
qPCR Master Mix (2X), Waltham, MA USA
Results
Proliferation
Until 60 hrs, the proliferation trend was similar. According to the statistical analysis, from 70 hrs to the end of the proliferation experiment, reduced cell proliferation was observed in sepiolite group (Figure 1).
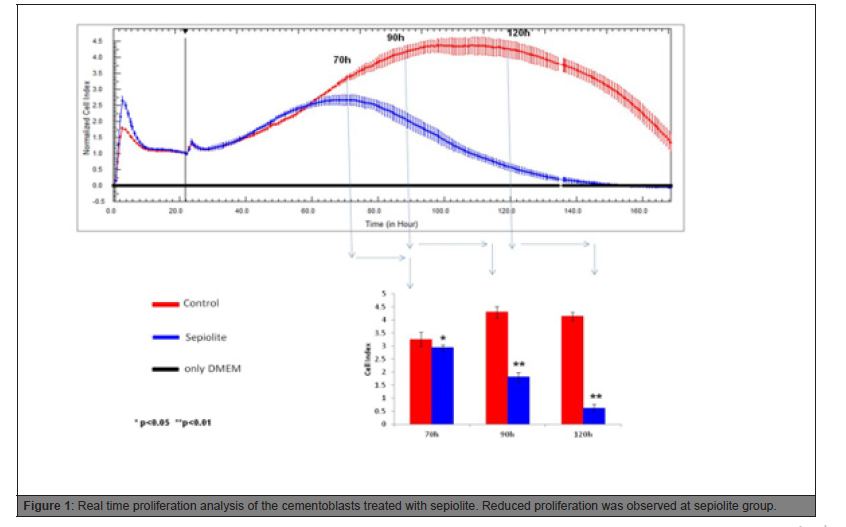
Figure 1: Real time proliferation analysis of the cementoblasts treated with sepiolite. Reduced proliferation was observed at sepiolite group.
mRNA expression
Sepiolite induced OCN, COL-I, osteoblastic transcription factor Runx2, and ALP mRNA expression on day 3 and 6 of the cementoblasts. BSP mRNA expression was not affected when the cells exposed to DMEM containing sepiolite released components (Figure 2).
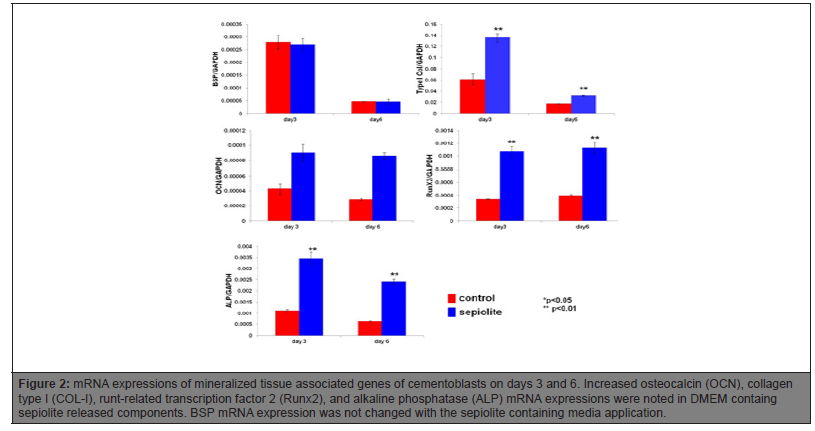
Figure 2: mRNA expressions of mineralized tissue associated genes of cementoblasts on days 3 and 6. Increased osteocalcin (OCN), collagen type I (COL-I), runt-related transcription factor 2 (Runx2), and alkaline phosphatase (ALP) mRNA expressions were noted in DMEM containg sepiolite released components. BSP mRNA expression was not changed with the sepiolite containing media application.
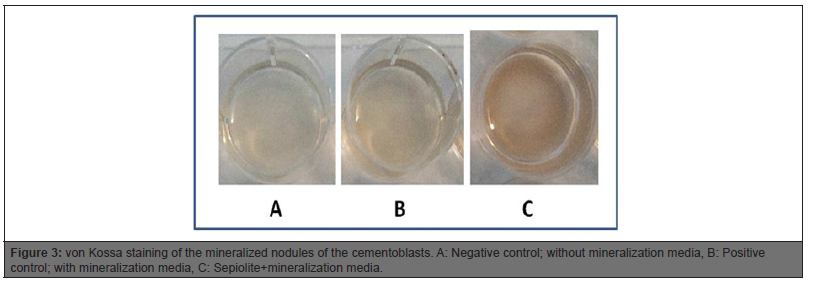
Mineralization
Mineralization media treated positive control group had apparent mineral-like nodules when compared to negative control. DMEM containing sepiolite material induced mineral-like nodules when compared to positive control group (Figure 3).
Discussion
Sepiolite up-regulated significantly important mineralized tissue associated gene’s mRNA expressions and biomineralization of the cementoblasts whist it reduced cell proliferation. While the differentiation of the cells was stimulated, reduced proliferation was expected. Cells stop the proliferation, while they synthesized extracellular matrix which was required for differentiation [18].We may conclude that Sepiolite is a promising graft material when the inductive properties taken into account in biomineralization process of the cementoblasts. In our previous study [19], we evaluated different regenerative graft materials (Nanobone®, BioOss®, Emdogain ®, Endobon® ) on the cementoblasts and we found that Bio- Oss apparently regulated better gene expressions playing role in the formation mineralized tissues compared to other graft materials. Sepiolite and BioOss were comparable in both mRNA expression and biomineralization experiment. While BioOss bovine derived particulated bone graft, sepiolite is clay materials and absolutely the cost is low and easily accessible when compared to BioOss [19].
In our previous studies, we evaluated the effects of BMP-7, recombinant amelogenin, bFGF, dexamethasone on the cementoblasts [6-8]. According to our experiences on the cementoblasts, sepiolite might be a good candidate material for periodontal tissue engineering in dentistry. Further animal studies should be conducted to evaluate potential of sepiolite in the critical size defects for orthopedic usage. Other differentiation and/or growth factors containing graft materials are very expensive. If the targeted effects were possible with the sepiolite, and the response of the cells was comparable, we should prefer cost effective treatment alternatives considering the biological response of the cells to the materials. However, in this study we evaluated only one cell and the cells were mouse originated. And in vitro studies have some limitations to mimic clinical conditions in human. Further studies are required evaluating osteoblasts, periodontal ligament mesenchymal stem cells as well. Moreover, bioactive properties of sepiolite can be enhanced including some element to these materials like boron. In future boron or selenium containing sepiolite biomaterials can be investigated. Sequential elemental releasing can be also considered for future applications.
Acknowledgements
This work was performed at Selcuk University, Research Center of Dental Faculty, Konya, Turkey and presented European Biotechnology Congress, April 11-13, 2019 Barcelona, Spain. Authors declare no conflict of interest.
References
- Evans CH (2013) Advances in regenerative orthopedics. Mayo Clin Proc 88(11): 1323-1339.
- DiGiovanni CW, Petricek JM (2010) the evolution of rhPDGF-BB in musculoskeletal repair and its role in foot and ankle fusion surgery. Foot Ankle Clin 15(4): 621-640.
- Teng F, Yu D, Wei L, Su N, Liu Y, et al. (2019) Preclinical application of recombinant human bone morphogenetic protein 2 on bone substitutes for vertical bone augmentation: A systematic review and meta-analysis. J Prosthet Dent 122(4): 355-363.
- Ding T, Li J, Zhang X, Du L, Li Y, et al. (2020) Super-assembled core/shell fibrous frameworks with dual growth factors for in situ cementum-ligament-bone complex regeneration. Biomater Sci 8(9): 2459-2471.
- Hakki SS, Bozkurt B, Hakki EE, Kayis SA, Turac G, et al. (2014) Bone morphogenetic protein 2, -6, and -7 differently regulate osteogenic differentiation of human periodontal ligament stem cells. J Biomed Mater Res B Appl Biomater 102(1): 119-130.
- Hakki SS, Nohutcu RM, Hakki EE, Berry JE, Akkaya MS, et al. (2005) Dexamethasone and basic-fibroblast growth factor regulate markers of mineralization in cementoblasts in vitro. J Periodontol 76(9): 1550-1558.
- Hakki SS, Foster BL, Nagatomo KJ, Bozkurt SB, Hakki EE, et al. (2010) Bone morphogenetic protein-7 enhances cementoblast function in vitro. J Periodontol 81(11): 1663-1674.
- Hakki SS, Bozkurt SB, Türkay E, Dard M, Purali N, et al. (2018) Recombinant amelogenin regulates the bioactivity of mouse cementoblasts in vitro. Int J Oral Sci 10(2): 15.
- Hakki SS, Bozkurt BS, Hakki EE (2010) Boron regulates mineralized tissue-associated proteins in osteoblasts (MC3T3-E1). J Trace Elem Med Biol 24(4): 243-250.
- Gümüşderelioğlu M, Tunçay EÖ, Kaynak G, Demirtaş TT, Aydın ST, et al. (2015) Encapsulated boron as an osteoinductive agent for bone scaffolds. J Trace Elem Med Biol 31: 120-128.
- Karahaliloglu Z, Kilicay E (2020) In vitro evaluation of bone cements impregnated with selenium nanoparticles stabilized by phosphatidylcholine (PC) for application in bone. J Biomater Appl.
- Demirel M, Aksakal B (2018) Effect of porosity on the structure, mechanical properties and cell viability of new bioceramics as potential bone graft substitutes. Acta Bioeng Biomech 20(2): 11-22.
- Lauritano D, Limongelli L, Moreo G, Favia G, Carinci F, et al. (2020) Nanomaterials for Periodontal Tissue Engineering: Chitosan-Based Scaffolds. A Systematic Review. Nanomaterials (Basel) 10(4): 605.
- Handbook of Mineralogy
- https://www.ima-europe.eu/about-industrial-minerals/industrial-minerals-ima-europe/sepiolite
- Saygin NE, Giannobile WV, Somerman MJ (2000) Molecular and cell biology of cementum. Periodontol 24: 73-98.
- Foster BL, Popowics TE, Fong HK, Somerman MJ (2007) Advances in defining regulators of cementum development and periodontal regeneration. Curr Top Dev Biol 78: 47-126.
- Korkusuz P, Hakki SS, Purali N (2008) Interaction of MC3T3-E1 cells with titanium implants. Joint Dis Rel Surg 19(2): 84-90
- Mutafcilar E, Bozkurt SB, Götz W, Hakki SS. The effects of different regenerative materials on the tooth root lining cells; cementoblasts. Manuscript in preparation. Unpublished data.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.