Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
The Need of Early Detection of Positive COVID-19 Patients in the Community by Viral Tests (e.g. RT-PCR Tests) and Antibody Tests (Serological Tests) to Stop the Spread
*Corresponding author: Ahsan Ali Siddiqui, Consultant Preventive Medicine and Epidemiologist, Quality Management and Patient Safety Department, Ministry of Health, Riyadh, Saudi Arabia
Received: May 27, 2020; Published: June 03, 2020
DOI: 10.34297/AJBSR.2020.09.001357
Abstract
Background and Objective: The Aim of this article is to tell the Importance of early testing for
COVID-19 novel corona virus of the suspected communities. The earlier to find out the Positive results of the COVID-19 novel corona virus more robust preventions against COVID-19 could be implemented.
Methods: The Author of this article has randomly selected 15 Articles from PUBMED Central and others sources with search word COVID-19 Test Kits. After reviewing the research articles and looking at their results it clearly shows the Importance of use of COVID-19 Test Kits. The earliest the use of COVID-19 Test Kits it prevents the whole community from getting infection from infected people. The Positive people with COVID-19 Test Kits would be Quarantined and treated in the health facilities or at home depending upon the patient condition. And then the work of Epidemiologists and other HCW started to find the Contacts and trace them to mass test in the community which comes in contact with Positive people with COVID-19 novel corona virus.(Wikipedia Article, 2020)A literature review or narrative review is a type of review article. A literature review is a scholarly paper that presents the current knowledge including substantive findings as well as theoretical and methodological contributions to a particular topic. The author of the article has chosen integrative literature review as a methodology for this research article (Wikipedia Article, 2020).
Results: Table 1 shows the review of 15 distinguished articles resulting all of the articles agrees 100 percent for the Preventive importance of COVID-19 Test Kits. Table 2 shows the results of the 5 Articles about the number of people tested COVID-19 positive after using COVID-19 testing kits.Author of this article has used SPSS 19 software diagrammatic presentation of the data and results in this article.
Conclusion: As we all know that current Pandemic of COVID-19 novel corona virus is deadly and without vaccine and proven Treatment. More than quarter million people recently has died and more than four million people suffering from COVID-19 all over the world till May 2020. World health organization has issued the warning in December 2019 for the Novel corona virus in Wuhan China. Governments of the countries including most developed countries such as the USA, the UK and EU Countries act slowly with ignoring the facts about COVID-19 severity. The lesson learned from the Pandemic COVID-19 is that our health systems and health agencies do not have abilities to save their citizens and they have to work hard to improve their abilities to save their citizens.
Keywords: Health care workers (HCW), Reverse Transcription-Polymerase Chain Reaction (RT-PCR), COVID-19 novel corona virus, Detection, RT-LAMP, SARS-CoV-2,ELISA, Immunoassays, Lateral flow assays, SARS-CoV-2.
Introduction
COVID-19 testing(Wikipedia Article, 2020)can identify the SARS-CoV-2 virus and includes methods that detect the presence of virus itself (RT-PCR, isothermal nucleic acid amplification, antigen) and those that detect antibodies produced in response to infection. Detection of antibodies (serological tests) can be used both for diagnosis and population surveillance. Antibody tests show how many people have had the disease including those whose symptoms were minor or who were asymptomatic. An accurate mortality rate of the disease and the level of herd immunity in the population can be determined from the results of this test. However, the duration and effectiveness of this immune response are still unclear and the rates of false positives and false negatives must be duly factored into the interpretation. There are two broad categories of test. Some look for the presence of the virus, e.g. the RT-PCR test. Others look for the antibodies which arise when a body is attacked by the virus. The purposes for which the test results are useful are different for the two kinds of test [1]. Another option is to look for lung damage via either CT scan or low oxygen take up since testing via RT-PCR requires the virus to be established and there are a number of false negatives especially in the initial stages (Figure 1).
Two kinds of tests are available for COVID-19 viral tests and antibody tests (CDC Centre of Disease control and prevention, 2020) [2]. A viral test tells you if you have a current infection and an antibody test tells you if you had a previous infection. An antibody test may not be able to show if you have a current infection because it can take 1-3 weeks after infection to make antibodies. We do not know yet if having antibodies to the virus can protect someone from getting infected with the virus again or how long that protection might last. To learn if you have a current infection viral tests are used. But not everyone needs this test. Most people will have mild illness and can recover at home without medical care and may not need to be tested.CDC has guidance for who should be tested but decisions about testing are made by state and local health departments or healthcare providers. If you have symptoms of COVID-19 and want to get tested call your healthcare provider first [3-5]. Although supplies of tests are increasing it may still be difficult to find a place to get tested.
An ongoing theme of the COVID-19 pandemic [6] is the need for widespread availability of accurate and efficient diagnostic testing for detection of SARS-CoV-2 and antiviral antibodies in infected individuals. This report describes various assay techniques and tests for COVID-19 diagnosis. Most tests for early detection of SARS-CoV-2 RNA rely on the reverse transcription-polymerase chain reaction (RT-PCR) but isothermal nucleic acid amplification assays, including transcription-mediated amplification and CRISPR-based methodologies, are promising alternatives. Identification of individuals who have developed antibodies to the SARS-CoV-2 virus requires serological tests, including enzyme-linked immunosorbent assay (ELISA) and lateral flow immunoassay. This report also provides an overview of current development in COVID-19 diagnostic techniques and products to facilitate future improvement and innovation. In summary, significant progress has been made in the development of diagnostic tests despite all the remaining questions and challenges [6]. Ongoing global efforts are working to communicate and facilitate new diagnostic assay development and worldwide test kit delivery [7-10].
Diagnosis of suspected [11] cases is confirmed by nucleic acid assays with real-time PCR using respiratory samples. Serology tests are comparatively easier to perform but their utility may be limited by the performance and the fact that antibodies appear later during the disease course. From a total of nine rapid detection test (RDT) kits three kits offer total antibody detection while six kits offer combination SARS-CoV-2 IgM and IgG detection in two separate test lines. All kits are based on colloidal gold-labeled immune chromatography principle and one-step method with results obtained within 15 minutes, using whole blood serum or plasma samples. The sensitivity for both IgM and IgG tests ranges between 72.7% and 100% while specificity ranges between 98.7% to 100%. Two immune chromatography using nasopharyngeal or throat swab for detection of COVID-19 specific antigen are also reviewed. The use of serology methods requires appropriate interpretations of the results and understanding the strengths and limitations of such tests [11-17].
Multiple diagnostic tests [18] are available without enough perspective on their reliability. Therefore, it is important to choose the most suitable test according to its sensitivity and specificity but also to the stage of the disease. Currently the RT-PCR detection of the viral genome in respiratory samples is the most reliable test to confirm the diagnosis of an acute SARS-CoV-2 infection. It has to be done in Class II biological safety laboratory. However, it may lack sensitivity particularly in the advanced phase of infection and depends closely on the sample’s quality. Rapid PCR by cartridge system reduces response times but is not suitable for laboratories with high throughput of requests. Detection of virus antigens on respiratory samples is a quick and easy to use technique however it has not good specificity and sensitivity and cannot be used for diagnosis and patient management. The detection of specific antibodies against SARS-CoV-2 is better used for epidemiological analyses. Research should be encouraged to overcome the limits of the currently available diagnostic tests [18].
The SARS-CoV-2 pandemic [19] has brought a new wave of challenges to health care particularly in the area of rapid diagnostic test development and implementation. Acute diagnosis of COVID-19 infection is critically dependent on detection of SARS-CoV-2 RNA from clinical specimens (e.g. nasopharyngeal swabs). While laboratory developed testing for SARS-CoV-2 is an essential component of diagnostic testing for this virus the majority of clinical microbiology laboratories are dependent on commercially available SARS-CoV-2 molecular assays. In contrast to assays approved or cleared by the Food and Drug Administration for in vitro diagnostic use assays for the detection of SARS-CoV-2 nucleic acids have Emergency Use Authorization (EUA) from the FDA. Outside of highly specialized academic and commercial laboratory settings clinical microbiology laboratories are likely unfamiliar with EUA classification and thus assay verification can be daunting. Further compounding anxiety for laboratories are major issues with supply chain that are dramatically affecting the availability of test reagents and requiring laboratories to implement multiple commercial EUA tests.
Methods
The Author of this article has randomly selected 15 Articles from PUBMED Central and others sources with search word COVID-19 Test Kits. After reviewing the research articles and looking at their results it clearly shows the Importance of use of COVID-19 Test Kits. The earliest the use of COVID-19 Test Kits it prevents the whole community from getting infection from infected people. The Positive people with COVID-19 Test Kits would be Quarantined and treated in the health facilities or at home depending upon the patient condition. And then the work of Epidemiologists and other HCW started to find the Contacts and trace them to mass test in the community which comes in contact with Positive people with COVID-19 novel corona virus. A literature review or narrative review is a type of review article. A literature review is a scholarly paper that presents the current knowledge including substantive findings as well as theoretical and methodological contributions to a particular topic. The author of the article has chosen integrative literature review as a methodology for this research article [1].
This was an observational study of outpatient COVID-19 testing of HCW [5]. Prior to testing HCW were asked about the presence of 10 symptoms. Their responses were then compared to their subsequent pharyngeal swab COVID-19 (PCR) Polymerase Chain Reaction test results. These data were used to derive and evaluate a symptom-based testing criterion.961 HCW were included in the analysis of which 225 (23%) had positive test results. Loss of taste or smell was the symptom with the largest positive likelihood ratio (3.33). Dry cough regardless of the presence or absence of other symptoms was the most sensitive (74%) and the least specific (32%) symptom. The existing testing criteria consisting of any combination of one or more of three symptoms (fever, shortness of breath, dry cough) was 93% sensitive and 9% specific (AUC = 0.63, 95% CI: 0.59 - 0.67). The derived testing criteria consisting of any combination of one or more of two symptoms (fever, loss of taste or smell) was 89% sensitive and 48% specific (AUC = 0.75, 95% CI: 0.71 - 0.78) (SAEM Academic Emergency Medicine, 2020).
To compare the diagnostic efficacy [9] among three RT-PCR test kits for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleic acid detection. The throat swab samples from 40 hospitalized patients clinically diagnosed as coronavirus disease 2019 (COVID-19) and 16 hospitalized non-COVID-19 patients were recruited. The SARS-CoV-2 nucleic acid was detected in throat swab samples with RT-PCR test kits from Sansure Biotech (Sansure for short) Jiangsu Bioperfectus Technologies (Bioperfectus for short) and BGI Genomics (BGI for short). The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and Kappa value were analyzed. The viral nucleic acid was extracted from the throat swab samples by one-step cleavage and magnetic bead methods, and the efficacy of two extraction methods was also compared. The results of magnetic bead method for nucleic acid extraction by two different extractors (Sansure Natch CS S12C Fully Automated Nucleic Acid Extraction System vs. Tianlong NP968-C Nucleic Acid Extractor) were also compared. The detection efficacy for SARS-CoV-2 nucleic acid by the Sansure kit is relatively higher and the one step cleavage method has advantages of convenient operation and less time consuming (Table 1 & 2).
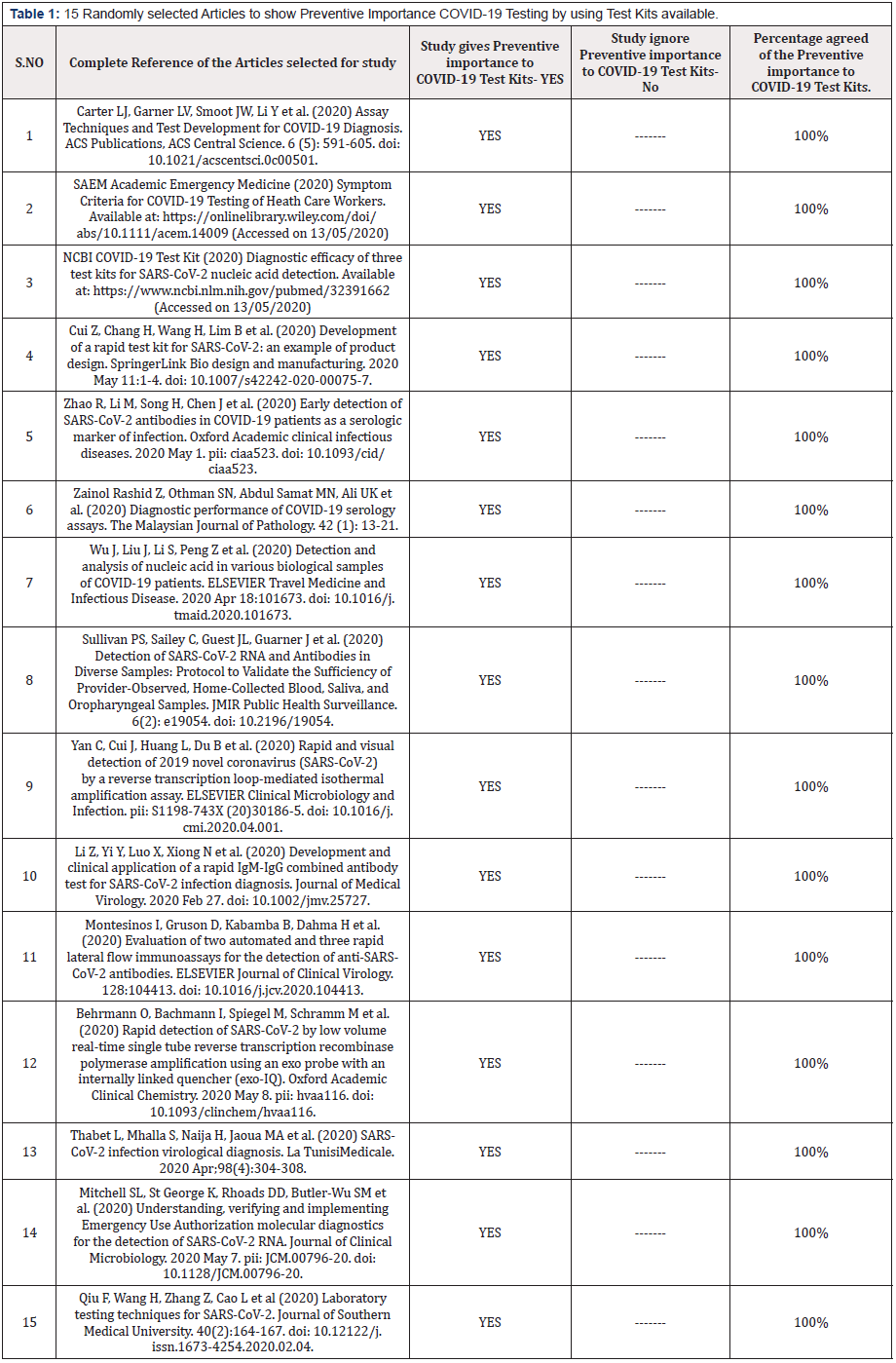
Table 1: 15 Randomly selected Articles to show Preventive Importance COVID-19 Testing by using Test Kits available.
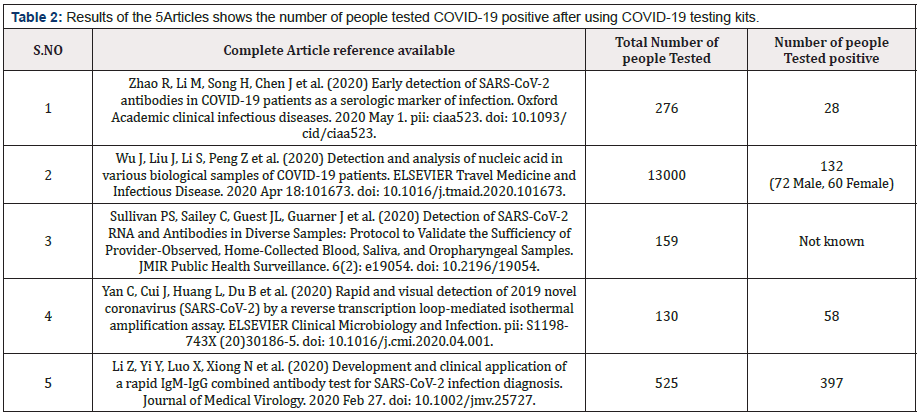
Table 2: Results of the 5Articles shows the number of people tested COVID-19 positive after using COVID-19 testing kits.
Measure and statistical Analysis: (IBM (2006) IBM SPSS Software.)
Figure 2-7
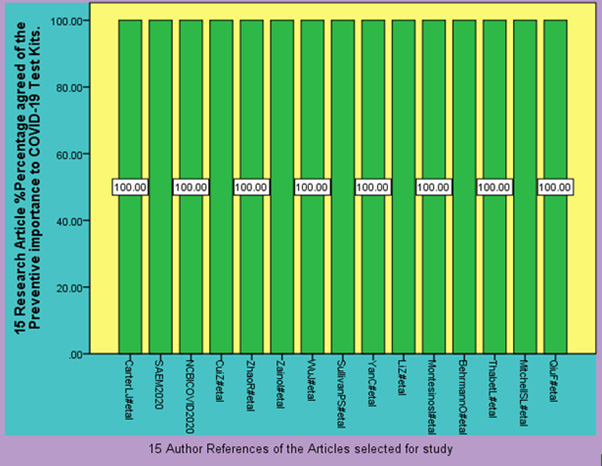
Figure 2: 15 Randomly selected Articles to show Preventive Importance COVID-19 Testing by using Test Kits available.
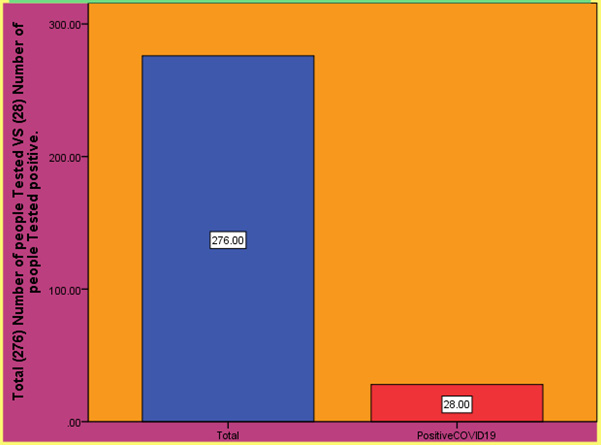
Figure 3: Results of the (Zhao R, Li M, Song H, Chen J et al, 2020) shows the number of people tested COVID-19 positive after using COVID-19 testing kits.
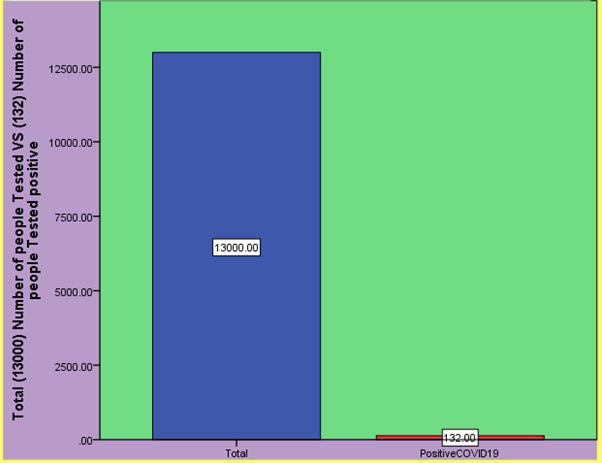
Figure 4: Results of the (Wu J, Liu J, Li S, Peng Z et al, 2020) shows the number of people tested COVID-19 positive after using COVID-19 testing kits.
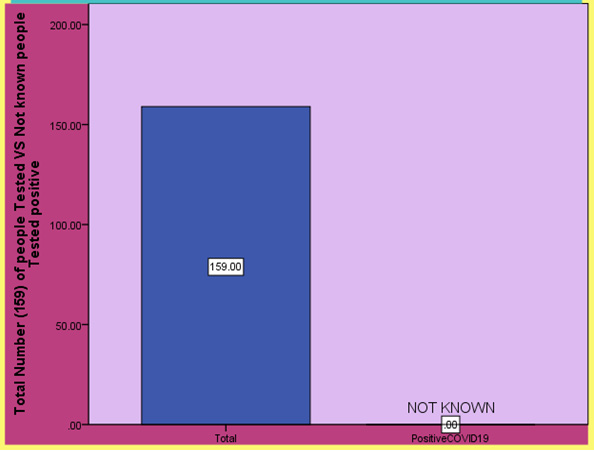
Figure 5: Results of the (Sullivan PS, Sailey C, Guest JL, Guarner J et al, 2020) shows the number of people tested COVID-19 positive after using COVID-19 testing kits.
Results
Table 1 shows the review of 15 distinguished articles resulting all of the articles agrees 100 percent for the Preventive importance of COVID-19 Test Kits. Table 2 shows the results of the 5 Articles about the number of people tested COVID-19 positive after using COVID-19 testing kits. Author of this article has used SPSS 19 software diagrammatic presentation of the data and results in this article.
Efforts to test have been hampered [13] by limited reagents limitations in the availability of swabs used for the collection of nasopharyngeal swabs (NPS) specimens’ limitations in personal protective equipment (PPE) for health care providers collecting the NPS specimens and limitations in viral transport media for transporting the specimens. More flexible options for screening for SARS-CoV-2 RNA and serologic responses are critical to inform clinical and public health responses. Patient self-collection of samples will be done with observation by a health care provider during a telemedicine session. Participants will be mailed a specimen collection kit engage in a telehealth session with a provider through a HIPPA (Health Insurance Portability and Accountability Act of 1996) compliant video meeting and collect specimens while being observed by the provider. Providers will record whether they are confident in the suitability of the specimen for laboratory testing that would inform clinical decision making. The protocol was approved by the Emory University USA Institutional Review Board (IRB) on March 30, 2020 (Protocol number 371). To date we have enrolled 159 participants tested.
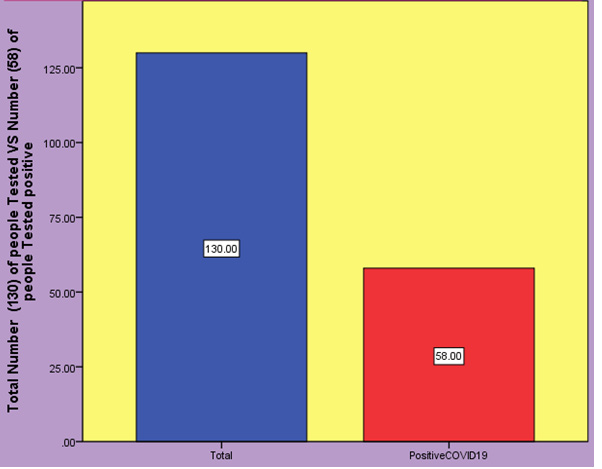
Here is evaluation of reverse transcription loop-mediated isothermal amplification [14] (RT-LAMP) assay for detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and compare it with RT-PCR. The primer sets orf1ab-4 and S-123 amplified the genes in the shortest times the mean (±SD) times were 18 ± 1.32 min and 20 ± 1.80 min respectively, and 63°C was the optimum reaction temperature. The sensitivities were 2×101 copies and 2×102 copies per reaction with primer sets orf1ab-4 and S-123 respectively. This assay showed no cross-reactivity with 60 other respiratory pathogens. To describe the availability of this method in clinical diagnosis we collected 130 specimens from patients with clinically suspected SARS-CoV-2 infection. Among them 58 were confirmed to be positive and 72 were negative by RT-LAMP. The sensitivity was 100% (95% CI 92.3%-100%), specificity 100% (95% CI 93.7%-100%). This assay detected SARS-CoV-2 in a mean (±SD) time of 26.28 ± 4.48 min and the results can be identified with visual observation [14].
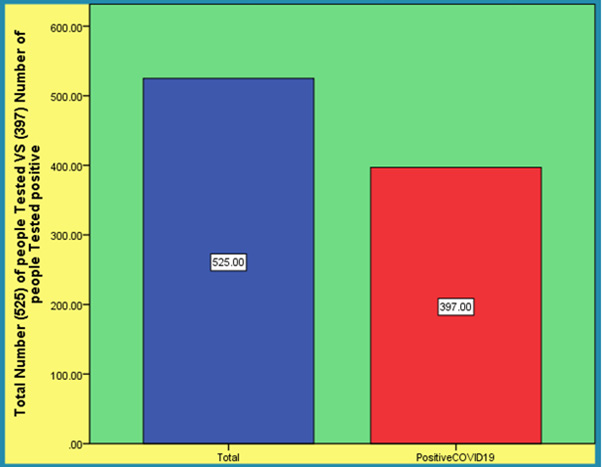
Although the virus (SARS coronavirus SARS-Cov-2) [15] nucleic acid real-time polymerase chain reaction (PCR) test has become the standard method for diagnosis of SARS-CoV-2 infection these real-time PCR test kits have many limitations. In addition, high false-negative rates were reported. We have developed a rapid and simple point-of-care lateral flow immunoassay that can detect immunoglobulin M (IgM) and IgG antibodies simultaneously against SARS-CoV-2 virus in human blood within 15 minutes which can detect patients at different infection stages. With this test kit we carried out clinical studies to validate its clinical efficacy uses. The clinical detection sensitivity and specificity of this test were measured using blood samples collected from 397 PCR confirmed COVID-19 patients and 128 negative patients at eight different clinical sites. The overall testing sensitivity was 88.66% and specificity was 90.63%. In addition, we evaluated clinical diagnosis results obtained from different types of venous and fingerstick blood samples. The results indicated great detection consistency among samples from fingerstick blood serum and plasma of venous blood. The IgM-IgG combined assay has better utility and sensitivity compared with a single IgM or IgG test. It can be used for the rapid screening of SARS-CoV-2 carriers symptomatic or asymptomatic in hospitals clinics and test laboratories [15].
Rapid first line testing protocols are needed for outbreak control and surveillance [17]. We used computational and manual design to generate a suitable set of reverse transcription recombinase polymerase amplification (RT-RPA) primer and exonuclease probeinternally quenched (exo-IQ) probe sequences targeting the SARS-CoV-2 N gene. RT-RPA sensitivity was determined by amplification of in vitro transcribed RNA standards. Assay selectivity was demonstrated with a selectivity panel of 32 nucleic acid samples derived from common respiratory viruses. To validate the assay against full-length SARS-CoV-2 RNA total viral RNA derived from cell culture supernatant and 19 nasopharyngeal swab samples (8 positive and 11 negatives for SARS-CoV-2) were screened. All results were compared to established RT-qPCR assays. The 95% detection probability of the RT-RPA assay was determined to be 7.74 (95% CI: 2.87 - 27.39) RNA copies per reaction. The assay showed no cross-reactivity to any other screened coronaviruses or respiratory viruses of clinical significance [17].
Discussion
Expanded molecular testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [7] is urgently needed to enable identification of infected individuals tracing and quarantining of their contacts and clearing of healthy people to return to work. Unfortunately test kits continue to be in short supply. If an assay is sensitive enough to perform well even when a positive sample is diluted by a factor of k then specimens from k people can be combined and tested together. Those individuals would then be separately tested only if their pool tested positive. If the pool was negative the individuals who were included in it would be presumed to be negative. Specimen pooling has been further developed for other applications in epidemiology such as for retrospective case-control studies and for in silico protection of personal data in meta-analyses. However, the original value of Dorfman’s idea as a powerful method for efficient screening is often overlooked. Despite the fact that pooled testing is an old idea, it has apparently rarely been implemented for coronavirus disease 19 (COVID-19) (Oxford Academic American Journal of Epidemiology, 2020).
We present [8] an example of applying need driven product design principle to the development of a rapid test kit to detect SARS-COV-2 (COVID-19). The tests are intended for use in the field and, longer term for home use. They detect whether a subject is currently infected with the virus and is infectious. The urgent need for large numbers of tests in field setting imposes constraints such as short test time and lack of access to specialist equipment laboratories and skilled technicians to perform the test and interpret results. To meet these needs an antigen test based on RTLAMP with colorimetric readout was chosen. Direct use of swab sample with no RNA extraction was explored. After extensive experimental study (reported elsewhere) a rapid test kit has been fabricated to satisfy all design criteria.
Thousands of medical staff had been infected with SARS-CoV-2 virus with hundreds of deaths reported [10]. Using CHO cell expressed full length SARS-CoV-2 S1 protein as capturing antigen a COVID-19/SARS-CoV-2 S1 serology ELISA kit was developed and validated with negative samples collected prior to the outbreaks or during the outbreak and positive samples from patients confirmed with COVID-19. The specificity of the ELISA kit was 97.5%, as examined against total 412 normal human samples. The sensitivity was 97.1% by testing against 69 samples from hospitalized and/or recovered COVID-19 patients. The overall accuracy rate reached 97.3%. The assay was able to detect SARS-CoV-2 antibody on day one after the onset of COVID-19 disease. The average antibody levels increased during the hospitalization and after been discharged for two weeks. SARS-CoV-2 antibodies were detected in 28 out of 276 asymptomatic medical staff and one out of five nucleic acid test-negative "Close contacts" of COVID-19 patient [10].
Reverse Transcriptase polymerase chain reaction (RT-PCR) [12] and viral gene sequencing are the gold standard for the diagnosis of COVID-19. This observation study included 132 patients diagnosed with COVID-19 in the infectious disease areas of the East Section of Renmin Hospital of Wuhan University. The RT-PCR test kits Bio-Germ were recommended by the Chinese Center for Disease Control and Prevention. Results comes as 132 the results of 2019-nCoV nucleic acid test of various biological samples during the treatment of confirmed COVID-19 cases are as follows: the positive rate of 2019-nCoV nucleic acid test of nasopharyngeal swab is 38.13% (180/472 times), the positive rate of 2019-nCoV nucleic acid test of sputum is 48.68% (148/304 times), the positive rate of blood 2019-nCoV nucleic acid test is 3.03% (4/132 times), and the positive rate of 2019-nCoV nucleic acid test of feces is 9.83% (24/244 times). The positive rate of 2019-nCoV nucleic acid detection in anal swabs is 10.00% (12/120 times). Simple detection of nasopharyngeal swab 2019-nCoV nucleic acid detection positive rate is not high, multi-sample 2019-nCoV nucleic acid detection can improve the accuracy, [12] reduce the false negative rate, better guide clinical treatment and evaluate the therapeutic effect.
The purpose of this study [16] was to assess the performance of five immunoassays for the detection of SARS-CoV-2 antibodies. Two quantitative automated immunoassays (Maglumi™2019-n-Cov IgG and IgM and Euroimmun Anti-SARS-CoV-2 IgG and IgA assays) and three lateral flow rapid tests were performed. This retrospective study included 200 residual sera from patients and healthy volunteers. Case serum samples (n =128) were obtained from COVID-19 patients confirmed by RT-qPCR and CT-scan. Days since onset of symptoms was collected from their medical records. Control non-SARS-CoV-2 samples (n = 72) were obtained from anonymous stored residual serum samples. Maglumi™ IgG/IgM tests showed overall less sensitivity than Euroimmun IgG/IgA test (84.4 % versus 64.3 %). Both tests showed similar specificities of IgG at 99 % and 100 %, respectively. The two tests showed similar specificity for IgG at 99 % and 100 %, respectively. The results from the lateral flow assays were easily interpretable with unambiguous colored reading bands. The overall sensitivity of the three tests was similar (around 7%) without any significant differences [16].
The Coronavirus SARS-CoV-2 [20] is highly infectious and transmitted mainly through droplets and contacts and is associated with a high risk of pneumonia. A small number of patients may present with acute respiratory distress syndrome with severe respiratory complications which can lead even to death. The selection of appropriate detection techniques and methods for accurate and rapid identification of pathogens therefore plays a key role in improving the diagnosis and treatment of the patients and containing the outbreak. In this review the authors give an overview of the virus laboratory detection technology including virus isolation and culture real-time fluorescent PCR, gene sequencing, serological antibody detection, and the gene editing technology based on CRISPR/Cas13 system. These techniques are expected to provide valuable assistance in controlling the epidemic and new ideas for future researches [20-21].
Conclusion
As we all know that current Pandemic of COVID-19 novel corona virus is deadly and without vaccine and proven Treatment. More than quarter million people recently has died and more than four million people suffering from COVID-19 all over the world till May 2020. World health organization has issued the warning in December 2019 for the Novel corona virus in Wuhan China. Governments of the countries including most developed countries such as the USA, the UK and EU Countries act slowly with ignoring the facts about COVID-19 severity. The lesson learned from the Pandemic COVID-19 is that our health systems and health agencies do not have abilities to save their citizens and they have to work hard to improve their abilities to save their citizens.
References
- Wikipedia Article (2020) COVID-19 Testing can identify the SARS-CoV-2 virus.
- Google COVID-19 Tests (2020) Images COVID-19 Tests.
- CDC Centre of Disease control and prevention (2020) Coronavirus Disease 2019 (COVID-19), Testing for COVID-19.
- IBM (2006) IBM SPSS Software.
- SAEM Academic Emergency Medicine (2020) Symptom Criteria for COVID‐19 Testing of Heath Care Workers.
- Carter LJ, Garner LV, Smoot JW, Li Y, Zhou Q, et al. (2020) Assay Techniques and Test Development for COVID-19 Diagnosis. ACS Publications, ACS Cent Sci 6(5): 591-605.
- Oxford Academic American Journal of Epidemiology (2020) Editorial: Making the Best Use of Test Kits for COVID-19.
- Cui Z, Chang H, Wang H, Lim B, Hsu CC, et al. (2020) Development of a rapid test kit for SARS-CoV-2: an example of product design. Biodes Manuf 1-4.
- NCBI COVID-19 Test Kit (2020) Diagnostic efficacy of three test kits for SARS-CoV-2 nucleic acid detection. 49(2):185-190.
- Zhao R, Li M, Song H, Chen J, Ren W, et al. (2020) Early detection of SARS-CoV-2 antibodies in COVID-19 patients as a serologic marker of infection. Clin infect Dis pii: ciaa523.
- Zainol Rashid Z, Othman SN, Abdul Samat MN, Ali UK, Wong KK (2020) Diagnostic performance of COVID-19 serology assays. Malays J Pathol 42(1): 13-21.
- Wu J, Liu J, Li S, Peng Z, Xioa Z, et al. (2020) Detection and analysis of nucleic acid in various biological samples of COVID-19 patients. Travel Med Infect Dis : 101673.
- Sullivan PS, Sailey C, Guest JL, Guarner J, Kelley C, et al. (2020) Detection of SARS-CoV-2 RNA and Antibodies in Diverse Samples: Protocol to Validate the Sufficiency of Provider-Observed, Home-Collected Blood, Saliva, and Oropharyngeal Samples. JMIR Public Health Surveill 6(2): e19054.
- Yan C, Cui J, Huang L, Du B, Chen L, et al. (2020) Rapid and visual detection of 2019 novel coronavirus (SARS-CoV-2) by a reverse transcription loop-mediated isothermal amplification assay. Clin Microbiol Infect 26(6): 773-779.
- Li Z, Yi Y, Luo X, Xiong N, Liu Y, et al. (2020) Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol.
- Montesinos I, Gruson D, Kabamba B, Dahma H, Wijngaert SV, et al. (2020) Evaluation of two automated and three rapid lateral flow immunoassays for the detection of anti-SARS-CoV-2 antibodies. J Clin Virol 128: 104413.
- Behrmann O, Bachmann I, Spiegel M, Schramm M, Wahed AAE, et al. (2020) Rapid detection of SARS-CoV-2 by low volume real-time single tube reverse transcription recombinase polymerase amplification using an exo probe with an internally linked quencher (exo-IQ). Clin Chem pii: hvaa116.
- Thabet L, Mhalla S, Naija H, Jaoua MA, Hannachi N, et al. (2020) SARS-CoV-2 infection virological diagnosis. Tunis Medi 98(4): 304-308.
- Mitchell SL, St George K, Rhoads DD, Butler-Wu SM, Dharmarha V, et al. (2020) Understanding, verifying and implementing Emergency Use Authorization molecular diagnostics for the detection of SARS-CoV-2 RNA. J Clin Microbiol pii: JCM.00796-20.
- Qiu F, Wang H, Zhang Z, Cao L, Wang C, et al (2020) Laboratory testing techniques for SARS-CoV-2. Journal of Southern Medical University 40(2): 164-167.
- Wikipedia Article (2020) Literature review, for a focused scientific review with pre-defined methodology see Systematic review.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.