Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
The Porcine Experimental Model for Oocyte Cryopreservation by Vitrification
*Corresponding author: Fahiel Casillas, Department of Biology of Reproduction. Metropolitan Autonomous University- Iztapalapa campus, 09340 Mexico City, Mexico.
Received: May 26, 2020; Published: June 19, 2020
DOI: 10.34297/AJBSR.2020.09.001377
Abstract
Pigs have anatomical-physiological similarities with the human in several aspects, mainly in the reproduction function, which is of great interest in this review. However, in oocytes, there are differences between both species, impacting the vitrification success. For example, porcine oocytes have higher intracellular lipid content than women oocytes, affecting cryoprotectant permeability and increasing ice crystal formation. Despite these differences, the porcine model has been important for the development of assisted reproduction technologies in humans, as well as in the genetic improvement of livestock.
Importance of the Porcine Experimental Model
Since its domestication 7000-9000 BC, the porcine species have been historically significant as a food source for humans. In the 1950s, it began as a possible experimental model in biomedical research. Some of the most important events are the isolation of embryonic stem cells in the 1990s, the cloning of pigs in 2000, as well as the pig genome project in 2012 [1]. The latter was achieved since pigs have human-like anatomical and physiological characteristics [2]. For example, the digestive, the immune, and the reproductive systems are similar and have allowed studies to be carried out at cardiovascular, immune, metabolic, toxicological, and reproductive levels [3], which have had important applications in humans. For example, the clinical treatment of infection caused by Chlamydia and the clinical use of insulin extracted from the porcine pancreas.
The domestic pig, by its size (150-350 Kg), in comparison with other animal species such as rats, mice, and rabbits, among others, is a model of delicate handling to be used in research. However, the use of small breeds known as mini or micro-pigs has been a significant breakthrough in xenotransplantation research. One of the most commonly used breeds for research purposes in the United States of America is the Yucatan pig, also known as the hairless Mexican pig. This breed has the characteristic of weighting 70 Kg, facilitating its use in the laboratory [4]. The primary studies carried out using the Yucatan pig have had an approach as a cardiovascular and metabolic model. As mentioned above, pigs display anatomical-physiological similarities with the human in reproduction. However, in oocytes, there are differences between both species. Porcine oocytes have a higher intracellular lipid content than women, which affects the permeability of the cryoprotectants. Therefore, the porcine model is important for the development of assisted reproduction technologies (ART) in humans, as well as in the genetic improvement of livestock.
The female gamete, oogenesis in women and sows
Oogenesis takes place in the ovary and consists in the differentiation and maturation of the oocytes. In most domestic animals, the development of oogonia and oocytes is carried out during the first half of gestation. In the sow, oogenesis lasts until the first weeks after birth (24 to 25 days after birth). Also, the transformation from oogonia to oocytes is longer in sows than other species [5]. Conversion of oogonia can occur entirely up to approximately 30 days after birth. In mammals, oogenesis begins approximately three weeks after fertilization. Primordial germ cells (PGC) migrate through ameboid movements from the intestine to the genital crest, still undifferentiated, dividing into this area mitotically. These cells continue its proliferation to maintain the PGC pool while others differ in oogonia reaching in the eighth week of gestation about 600 thousand oogonia in the woman. Between 16-18 weeks of pregnancy, follicular formation is initiated, and the oogonia are surrounded by somatic epithelial cells giving origin to the primordial follicle. At this moment, the oogonia cease its mitotic activity and enter the meiosis. Once this process of cell division has begun, the oogonia give origin to the primary oocyte, which remains arrested in the prophase I of the meiosis I. During the fifth month of gestation, the fetal ovary possesses the maximum number of oocytes (8 million approximately). However, at birth, this number decreases significantly to one million, leaving less than 500 thousand at puberty [6]. Approximately 500 will grow and mature to form oocytes for fertilization. In pigs, these mechanisms are similar.
Embryo transfer in sows
The cryopreservation of oocytes has the aim that after being fertilized, viable embryos could be stored before transferred to obtain live offspring. Embryo transfer (ET), is an ART that has been developed for approximately 60 years. In economically important species, it allows increasing the number of offspring per female, reducing the interval between generations, providing health guarantees, and creating genetic lines. For this reason, this technique has been widely used in cattle, sheep, and, in pigs, was achieved since 1951.
In sows, critical criteria have been reported for the selection of the recipient females to perform ET:
1) Donor and recipient sows must be serologically tested for diseases, mainly contagious, before selection.
2) Feeding of sows should be essential because energy deficiencies could compromise reproduction.
3) The donor sow must have a high genetic value.
4) The recipient sow must have had at least one delivery in the reproductive season.
6) Sows with infection, or vaginal fluids that may be caused by uterine inflammation (metritis) should be discarded.
Hormone induction to estrus
Hormone treatments for donor and recipient sows:
Hormonal treatment allows the induction of estrous in recipients and superovulation in donors, at a given time. This hormonal stimulation imitates the natural endocrine mechanisms that regulate the estrous cycle and sexual behavior.
Stimulation for donor sows (superovulation):
1) LH and FSH hormones are glycoproteins synthesized in adenohypophysis whose function is to promote follicular growth and maturation. FSH induces the formation of LH receptors and maintains estrogen secretion, primarily estradiol. LH, by increasing blood concentration, promotes the pre-ovulatory peak to induce ovulation. Particularly, in sows, it has been reported that the peak of estrogen promotes ovulation. Its use for superovulation is carried out in women, and analogous substances are used for domestic animals, achieving the same effect.
2) The serum gonadotropin of a pregnant mare (PMSG) or equine chorionic gonadotropin (eCG) is extracted from pregnant mares that has both LH and FSH activity. Its primary use is in sheep and goats, although it is also used in sows for the estrus synchronization.
Types of stimulation applied to recipient sows:
1) Progesterone is a steroid hormone secreted by the ovarian corpus luteum whose main function is the gestation maintenance. Its use for the estrous cycle’s stimulation allows the regression of the luteal phase in the sow so that when its concentration decreases, the follicular phase is restarted.
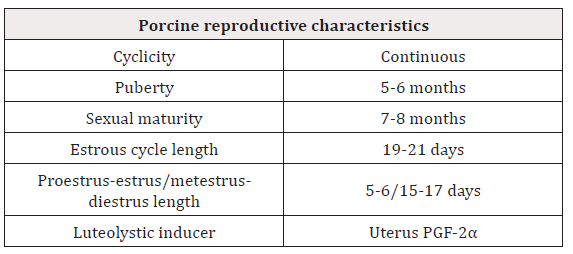
2) Stimulation with prostaglandin F2α (PGF-2α). This prostaglandin is a molecule of lipid nature, secreted mainly by the uterine endometrium in sows. Increase in its secretion induces the destruction of the corpus luteum (luteolytic), with the consequent decrease of progesterone and restart of the estrus. Knowing the parameters of the reproductive cycle of the sow allows the hormone induction to the estrus and ET to be successfully achieved [Table 1].
Principles of Cryobiology
Cryobiology, a term derived from the Greek words cryo=cold, bio=life, and logos =science, is responsible for studying the effects of low temperatures on cells and tissues to achieve their conservation. Cryopreservation processes allow the maintenance of cells, tissues, organs, and even organisms at low temperatures, generally between -80 ºC and -196 ºC, maintaining their metabolism inactivated. Biochemical reactions are stopped, allowing their development potential and viability to be preserved for long periods. Up to date, cryopreservation has been used for gamete conservation, so both cryobiology and ART have advanced together over the last 50 years. Cryopreservation of mammalian gametes originated in 1949 through demonstrating that human sperm possesses the ability to regain viability even after being kept at extremely low temperatures [7]. This prompted a revolution in the field of artificial insemination for its application in mammals, especially in humans.
Years ago, the main method used for the cryopreservation of gametes involved a long-lasting procedure is known as slow freezing. However, ultra-fast cryopreservation is gaining a high profile after that, it has been proven that similar or even higher results have been achieved compared to slow freezing. The ultrafast cryopreservation technique is known as vitrification. This technique is a thermodynamic process that changes a liquid state to a solid vitreous state, excluding ice crystals. It was developed in 1985 through a study in which the vitrification of mouse embryos was successfully carried out [8].
Vitrification is not a recent technique; it was first described in 1934 but was not successful until years later (1985), due to the unknown effects of cryoprotectants. Cryoprotectants are substances used to protect cells against the damage produced during freezing, vitrification, and warming processes. In 1985, Rall and Fahy resumed the application of ultra-fast cryopreservation, reducing the toxicity of the media, and reducing the cell exposure time, to improve their results [11]. In this way, the bases of cryopreservation have been extended to optimize vitrification protocols. The formation of intracellular and extracellular ice crystals, the toxicity of the use of cryoprotectants, and the osmotic changes during vitrification are important factors that should be considered to obtaining high recovery rates [9].
Osmolarity
The crystallization of water causes an increase in medium’s osmolarity, which remains in the liquid state. To maintain the osmotic balance, cells must undergo a dehydration process without exceeding the critical cell volume to avoid collapse [10]. The use of cryoprotectants allows osmotic flow not to be altered and the cellular adequate conditions during cryopreservation procedures. The plasma cell membrane is composed of lipids, proteins, and a smaller proportion of carbohydrates. This composition can vary according to the cell type and species. Cholesterol and fatty acids are the most abundant components in the plasma membrane. These determine the fluidity and resistance of the membrane during cryopreservation. The transport of molecules through the membrane is a critical point for cell survival during the heating process. The cell membrane is the structure that suffers the most significant damage during vitrification, including the loss fluidity of the lipid components, a high degree of fragility, rupture, hardening, and lipid loss. The latter is essential during the vitrification and warming process as it can generate a loss in cell expansion when returning to isotonic conditions.
During cryopreservation procedures, it is required the use of cryoprotectants whose diffusion is essential to avoid cell damage. Diffusion is the process by which the molecules of a substance are evenly distributed in free space. The low temperatures used in cryopreservation generate an increase in the rigidity of the membrane and the speed which osmotic changes must be made during vitrification and heating processes, making it difficult for ATP-dependent active transport processes to take place. Temperature from 25 ºC to 10 ºC reduces the activity of ATP pumps by up to 60%. In this way, simple diffusion processes predominate in situations of osmotic stress.
Cryopreserved samples are stored at temperatures of -133 ºC (nitrogen vapors) and -196 ºC (liquid nitrogen). Biological activities stop at these temperatures, and there are no diffusion phenomena or thermal energy [10]. The Fick law of diffusion defines the magnitude of water diffusion when a membrane is present between one zone and another. This law relates the gradient between both zones; chemical or concentration gradient and the characteristics of the membrane, thickness, area, and permeability of the solute. This is directly proportional to the surface, so that, the area of the membrane and the difference in concentration of the solute in both zones. The diffusion speed is another parameter that is determined by the properties of the solute and the membrane [10].
Cells can vary their volume in response to extracellular osmotic changes. The most studied biophysical parameters are 1) the osmotically inactive volume, which is defined as the water remaining in the cell in response to an increase in the concentration of solutes, 2) the permeability of the cell membrane to water, cryoprotectants, and solutes, and 3) the relationship between the cell surface area that differs between cell types and species. These parameters are important for the generation of optimal cryopreservation protocols because if the cell type and its biophysical profile are known, it is possible to determine the appropriate cryoprotectant concentrations to avoid cytotoxicity.
Cryopreservation methods
For the development of cryopreservation, different procedures have been used. These procedures are as follows:
Slow freezing:
In this technique, cells are exposed before cooling to a cryoprotective solution with a concentration of 1-2 M, which is considered hyperosmotic (>300 mOsm). Cells respond to the dehydrated osmotic change while slowly incorporating the cryoprotectants. For cell survival, it is extremely important to determine the appropriate cooling rate allowing precise cellular dehydration without intracellular ice formation. It is determined by the cell type and the nature of the cryoprotectants used. This technique is performed by gradually subjecting the cells to low temperatures with specialized equipment. These temperatures range from -4 ºC, and -80ºC to -196ºC. However, it is known that for the vitrification of oocytes, this technique cannot prevent intra and extracellular ice crystals [11].
Ultra-fast cryopreservation or vitrification:
This procedure consists of aqueous solutions from a liquid state to a solid vitreous state without ice crystals forming due to the fast decrease in temperature. To achieve the vitreous state, the viscosity of the sample increases until the molecules remain motionless. This increase in viscosity requires fast cooling speeds and high concentrations of cryoprotectants. The main strategy of vitrification is to pass the critical temperature range (-30 ºC to -80 ºC) as quickly as possible to reduce the risk of damage and bring the cell to -196 ºC immediately [12].
The rationale for current vitrification procedures involves the exposure of the cell to a reduced volume of cryoprotectants at high concentrations, followed by its rapid introduction into liquid nitrogen at a temperature of approximately - 196 ºC [12]. Unlike slow freezing, vitrification induces a high degree of cellular dehydration before cooling by exposure to solutions with high cryoprotective concentrations (4-8 M). The main disadvantage is the cytotoxic effect that cryoprotectants can produce if exposure to cryoprotectants is not properly performed. It is possible to limit the toxicity of vitrification media using the combination of two intracellular cryoprotectants. Thus, it is possible to increase their permeability to cells, allowing them to recover to lower temperatures and reduce toxicity [11].
Cell conservation systems
Oocytes are placed and stored in a series of containers specially designed to maintain the optimum preservation temperature. These systems involve the following:
Liquid nitrogen containers:
The storage of samples at temperatures below -120 °C guarantees the absence of chemical reactions. The only reaction that could occur is the slow accumulation of ionizing radiation, but this is only significant after storage for hundreds of years, which is involved with the decline in viability. Containers must ensure the maintenance of the samples at the appropriate temperature and are made with a double insulating jacket with a space that protects its interior [11]. It is important that for storage, samples destined for a container are of the same cell type exclusively, for example, gametes. This would control sample contamination.
Liquid nitrogen:
It is a potentially dangerous product due to its chemical characteristics, it is extremely cold, at atmospheric pressure boils at -196 ºC, and produces large amounts of nitrogen vapors, causing a decrease of oxygen in the environment. However, its use is essential for vitrification.
Cell devices:
To optimize the cryopreservation processes, several cell devices have been developed, such as Super Open Pulled Straws [12], microdrops, solid surfaces [13], Cryoloop, Cryotop, Cryolock, among others. Unlike others, the Cryolock container handles a minimum solution volume of 0.1 μL, which benefits the vitrification process. It is currently used as a tool in ART and animal biotechnology, which has been designed and developed to facilitate the handling of existing techniques. Its use has been especially for the conservation of oocytes and embryos whose characteristics allow proper and safe maintenance since it has a lid that protects the sample from contamination by contact with liquid nitrogen during storage [14,15 and 16].
In summary, most studies that are carried out to evaluate porcine oocyte recovery and development after vitrification, have reported better rates when cryopreservation is carried out in embryo development stages or zygotes, but not in oocytes. For this reason, many studies have been performed to improve porcine immature oocyte vitrification protocols [16], testing the use of different cryoprotectant concentrations, cooling devices, and incubation times. However, only a few of them have reached higher blastocysts production and live birth. Therefore, understanding the specific species characteristics may improve cryopreservation by vitrification protocols efficiency [17,18].
Acknowledgements
The authors thank and dedicate this work to Dr. Yvonne Ducolomb.
References
- Gutiérre K, Dicks N, Glanzner WG, Agellon LB, Bordignon V, et al. (2015) Efficacy of the porcine species in biomedical research. Front Genet 16(6): Pp. 293.
- Kobayashi E, Hishikawa S, Teratani T, Lefor AT (2012) The pig as a model for translational research: overview of porcine animal models at Jichi Medical University. Transplant Res 1(1): p. 8.
- Swindle MM, Makin A, Herron AJ, Clubb FJ, Frazier KS, et al. (2012) Swine as models in biomedical research and toxicology testing. Vet Pathol 49(2): 344-356.
- Gutiérrez Ruiz EJ, Aranda Cirerol FJ, Rodríguez Vivas RI, Bolio González ME, Ramírez González S, et al. (2012) Factores sociales de la crianza de animals de traspatio en Yucatán, México. Bioagrociencias, sistemas de producción 5(1): 20-28.
- Black JL, Erickson BH (1968) Oogenesis and ovarian development in the prenatal pig. Anat Rec 161(1): 45-55.
- Palma GA, Argañaraz ME, Barrera AD, Rodler D, Mutto AA, et al. (2012) Biology and biotechnology of follicle development. Scientific World Journal 2012: p14.
- Polge CA, Smith AV, Parkes AS (1949) Revival the spermatozoa after vitrification and dehydratation at low temperature. Nature 164(4172): Pp666.
- Rall WF, Fahy GM (1985) Ice-free cryopreservation of mouse embryos at - 196°C by vitrification. Nature 313(6003): 573-575.
- Rojas C, Palomo JM, Albarracin JL, Mogas T (2004) Vitrification of immature pig oocytes: Study of distribution of chromosomes, microtubules and actin microfilaments. Cryobiology 49(3): 211-220.
- Avila Portillo LM, Madero JI, Lopez C, León MF, Acosta L, et al. (2006) Fundamentos de criopreservacion. Rev Colomb Obstet Gineco 57(4): 291-300.
- Bajo J (2009) Fundamentos de reproducción. Editorial Médica Panamericana. Madrid España 269-280.
- Vajta G (2000) Vitrification of the oocytes and embryos of domestic animals. Anim Rep Sci 60(61): 357-364.
- Somfai T, Ozawa M, Noguchi J, Kaneko H, Nakai M, et al. (2008) Live piglets derived from in vitro produced zygotes vitrified at the pronuclear stage. Biol Reprod 80(1): 42-49.
- Casillas F, Teteltitla Silvestre M, Ducolomb Y, Lemus AE, Salazar Z, et al. (2014) Co-culture with granulosa cells improve the in vitro maturation ability of porcine immature oocytes vitrified with cryolock. Cryobiology 69(2): 299-304.
- Casillas F, Ducolomb Y, Lemus AE, Cuello C, Betancourt M, et al. (2015) Porcine embryo production following in vitro fertilization and intracytoplasmic sperm injection from vitrified immature oocytes matured with a granulosa cell co-culture system. Cryobiology 71(2): 299-305.
- Casillas F, Betancourt M, Cuello C, Ducolomb Y, Lopez A, et al. (2018) An efficiency comprarison of different in vitro fertilization methods: IVF, ICSI, and PICSI for embryo development to the blastocyst stage from vitrified porcine immature oocytes. Porcine Health Manag 4(1): 16.
- Albarracin J, Morato R, Izquierdo D, Mogas T (2005) Vitrification of calf oocytes: effects of maturation stage and prematuration treatment on the nuclear and cytoskeletal components of oocytes and their subsequent development. Mol Reprod Dev 72(2): 239-249.
- Portillo B, Madero J, Bacter C, Bacter M, Acosta L, et al. (2006) Fundamentos de criopreservación. Rev Colom Obstet Ginecol 57(4): 291-300.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.