Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
The Assessment of Purity Level of CO2 Used in Horizontal Incubators and The Effect of Additional In-Line Filters on Embryo Development
*Corresponding author:Murat Basar, PhD, Biruni University, Graduate Education Institute, Department of Histology and Embryology Address: 10. Yıl Caddesi Protokol Yolu No: 45 34010 Topkapı / İstanbul.
Received: September 24, 2022; Published: September 28, 2022
DOI: 10.34297/AJBSR.2022.17.002323
Abstract
Objectives: In this study, we aimed to investigate whether carbon dioxide (CO2) used in horizontal incubators during in
vitro fertilization (IVF) is pure enough and whether the addition of In-Line filters affects the stress on blastocysts and blastocyst
utilization rate.
Materials and Methods: To examine the effect of the purity of CO2 gas on embryo quality, two pronuclei (PN) embryos of
patients younger than 35 years old and undergoing intracytoplasmic sperm injection (ICSI) were divided into two groups. Embryos
in Group 1 were cultured in the incubator, which has an extra CO2 filter, whilst those in Group 2 (control group) were cultured
without any additional filtering. On day 3 (D3), Day 5 (D5), embryo qualities and blastocyst utilization rates between groups were
examined. Three different filters with different brands were examined regarding their effects on embryo quality within groups. For
animal studies, 10-week-old female BALB/C mice (n=10) were stimulated with Follicle Stimulating Hormone (FSH) followed by
Human Chorionic Gonadotropin (hCG) and mated with fertility-proven adult male mice. Mice with vaginal plaques after 24 hours
were sacrificed, and 1-cell embryos were collected. Embryos were pooled first and then distributed into Group 1 and Group 2
randomly. To assess the effect of gas purity on embryonic stress, Glucose-regulated Protein 78 (GRP78) level was examined using
Western blot method.
Results: CO2 gas content used in IVF laboratories was not as pure as indicated, and the following impurities were isolated: ethyl
methyl ketone, tetrahydrofuran, Fenol, 2-heptanol, and formaldehyde. We found that D3 embryo quality significantly improved
within Group 1 compared to Group 2 (p<0,001). Similarly, when the blastocyst utilization rates were assessed, a significant increase
was found in all three extra-filtered incubators compared to the ones without filters (p<0,001). Additionally, mouse embryos
cultured without an extra filter have higher GRP78 expression than those cultured with an extra CO2 filter (p<0.001).
Conclusion: It has been shown that the CO2 gasses used in the IVF laboratory are not as pure as reported. This can be eliminated
by using an additional filter, decreasing stress and increasing embryo quality. In addition, it has been shown that the UPR plays a key
role in regulating the response to ambient air/gas impurity in embryo development with long-term culture. Our study demonstrates
that additional extra CO2 filters significantly improve embryo quality and impact the UPR pathway in D3 and D5 compared with a
control group. This suggests that filtration of CO2 has a positive impact, perhaps by removing potentially harmful substances or
affecting UPR pathways.
Keywords: UPR; Grp78; In-Line Filter; IVF; Western Blot
Introduction
Infertility is defined as difficulty conceiving after one year of regular unprotected intercourse [1]. To diagnose infertility, men and women undergo many investigations. The causes of infertility can be grouped as follows: 40% of male factors, %40 of female factors, 10% of both, and 10% of idiopathic factors [2]. One of the most critical factors for successful In Vitro Fertilization (IVF) treatment is optimizing the laboratory conditions. The level of CO2 in the incubators, which is stabilized to 37°C, needs to be monitored regularly to allow the successful development of blastocysts. The most important factor is balancing the pH of the culture media, which can be impacted by inappropriate CO2 exposure. The pH level is linked to environmental CO2, and to maintain the balance of the culture conditions in IVF laboratories, CO2 measurements need to be regularly evaluated [3].
Optimizing and sustaining various culture conditions that impact the viability of embryos and pregnancy rates are crucial. Several factors such as air quality, temperature, and light can affect embryo quality [3,4]. Volatile Organic Compounds (VOCs) are highly toxic for in vitro embryo development [5]. During embryonic development, VOCs directly bind to DNA strands and impact normal development [6,7]. Due to this reason, High-Efficiency Particulate Air (HEPA) filters are used in IVF laboratories to maintain air quality and stability. To remove the toxic particles in the air, positive air pressure is also used alongside conventional filtering systems [8].
It has been shown that Unfolded Protein Response (UPR) plays a crucial role in stress response mechanisms [9]. According to a study published by Basar et al., UPR is induced by increasing ER stress during in vitro embryo development and has a detrimental effect on blastocyst formation, suggesting that activation of UPR signaling may be the cause of low blastocyst development rates [10]. Similarly, an endoplasmic reticulum chaperon protein Grp78 also occurs in early embryo development [11].
In this study, our objective was to assess the purity level of CO2 gas used in benchtop incubators in IVF laboratories and whether the usage of additional CO2 filters affects embryo development.
Material and Methods
Animals
To investigate the UPR role in embryo development, ten weeksold 25-30 grams BALB/C female (n=10) and 10-weeks-old 30-35 grams BALB/C male mice (n=4) were used for the experiments. All animal care and experimental procedures were conducted per Biruni University and Akdeniz University animal research requirements, using protocols approved by the Institutional Animal Care and Use Committee (Protocol # 2019/35-07).
Superovulation of Female Mice and Embryo Collection
10IU of human chorionic gonadotropin (hCG; Sigma, St. Louis, MO) was injected 48h hours after the PMSG (Sigma, St. Louis, MO) injection, and female mice were mated with adult male mice. A plug check was performed to verify the mating. The mice were sacrificed by cervical dislocation. The embryos were collected by puncturing the oviduct under a stereomicroscope and put in a 37°C incubator with and without additional CO2 filters in culture media (G-TL, Vitrolife, USA).
Retrospective Analysis of Human Material
This study was approved by the Ethics Committee of the Biruni Unversity Medical Faculty (No. 2020-12-9 and Date. 21.01.2020), and written informed consent was signed by each patient. Patients seeking infertility treatment at Anadolu Medical Center IVF Unit were included in the study. Inclusion criteria included being under 35 years old, having a diagnosis of tubal factor infertility, and generating more than 15 oocytes. After ICSI was performed, the two pronuclei (PN) embryos were divided into two groups: Group 1 was incubated with extra CO2 filters, and Group 2 was incubated without extra CO2 filters. Embryos were assessed on days 3 and 5 using Alpha Istanbul Consensus on Embryo assessment [12].
The Groups of Incubators and Embryo Culture
K-System G210 model incubator with BLUE; ORIGIO® Gas Line Filter was used. 2 additional in-line filters as CODA® XTRA INLINE® FILTERS – BLUE; ORIGIO® Gas Line Filter and REPROCARE GLF30 VOC Holder Filter were also attached to the incubators separately. During the experiments, all other conditions were kept the same so that the only variable remained the filtering system. The development stages of the embryos were monitored, and the blastomere size and regularity, granulation of cytoplasm, and fragmentation ratio were investigated during this assessment. Equal blastomere size and shape and absence of fragmentation were used as two parameters to define embryo quality.
Western Blot
Embryos from each group were collected in 5 mL PBS and 5 mL lysis buffer and incubated on ice for 30 minutes. Samples were mixed with 10 mL of Laemmli buffer, boiled for 5 minutes, cooled on ice, and then centrifuged at 2,000 rpm at 4oC for 5 minutes. Levels of Grp78 protein were determined using a rabbit monoclonal anti- BiP antibody (Cell Signaling Technology) in a standard Western blot protocol as described elsewhere [13].
Statistical Analysis
All experiments were repeated at least three times. Statistical data analysis used Student’s t-test and one-way analysis of variance. p<0.05 was considered statistically significant.
Results
The Purity Levels of Gasses in the Incubators
When Nova 436 Series Gas Analyzer assessed the CO2 gas content, the following impurities were isolated: ethyl methyl ketone, tetrahydrofuran, Fenol, 2-heptanol, and formaldehyde (concentrations at 0.000271%, 0.001316%, 0.001638%, 0.000012%, 0.001162%, respectively). As shown in Figure 1, it has been observed that the purity levels of substances indicated in the CO2 gas were unsuitable when the extra filter was not used. The purity increased significantly after using the extra CO2 filter.
Quality of Embryos Cultured in Filtered and Unfiltered Incubators
When the embryo quality was examined on the third day, a statistically significant increase was observed in Group 1 compared to Group 2 (47%, 48%, 51%, and 52%; for Group 1, CODA, ORIGIO, and REPROCARE, respectively; p<0,001; n=30/group) (Figure 2). The rate of blastocyst utilization rate as a result of culture was examined in all three extra CO2 filter groups, and a significant increase was observed compared to Group 1 (26%, 36%, 38%, and 42%; for Group 1, CODA, ORIGIO, and REPROCARE respectively; p<0,001; n=30/group) (Figure 3). When the embryo quality on the third and fifth day was compared between the extra-filtered groups, no statistically significant difference was found (p>0.05) (Table 1)

Table 1. The comparisons of D3 and D5 embryos between Group 1 and Group 2. The quality of embryos on the third and fifth days was significantly higher in Group1 (*p<0.001).
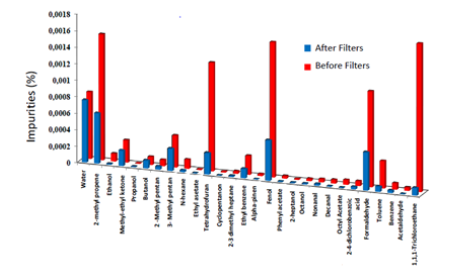
Figure 1: Percentage of impurities relative to the total amount of gas. It has been shown that the impurity percentages of the examined gasses are reduced when the extra filter is used in the incubator. (Red column: pre-filter; blue filter: post-filter).
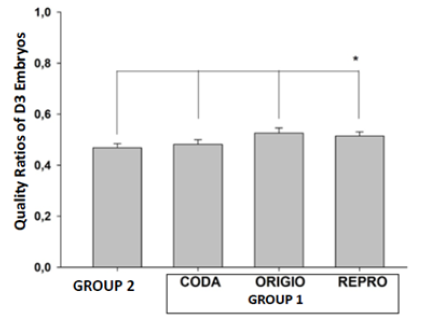
Figure 2: The ratios of Day 3 embryo qualities according to the groups with no extra filter (Group 2) and those with extra-filter (Group 1). It has been shown that the quality of embryos cultured in incubators with filters used for Group 1, CODA, ORIGIO and REPRO, is significantly better than Group 2 (*p<0.001).
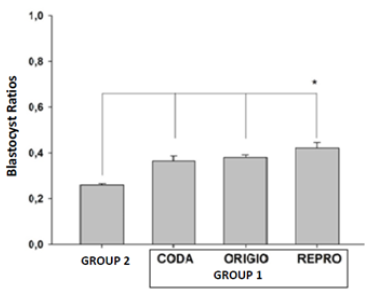
Figure 3:Blastocyst utilization rates between Group 1 and Group 2. It was shown that the quality of embryos cultured in incubators with filters used for Group 1, CODA, ORIGIO and REPRO was significantly better than Group 2 (*p<0.001).
Effect of Grp78 Protein Expression Level on Blastocyst Formation
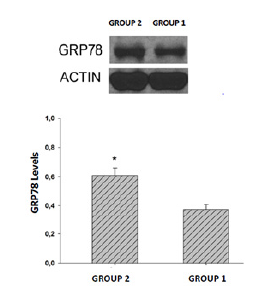
To investigate whether the difference in embryo quality and blastocyst retrieval on the third day may depend on the UPR signaling pathway, the GRP78 protein level was examined by Western Blot method. It was found to be higher in Group 2 (37%) compared to Group 1 (61%) (p<0.001) (Figure 4).
Discussion
IVF includes complex treatment processes tailored to the individual and can result in unique conditions and different outcomes. One of the most important factors known to reduce pregnancy is the air’s cleanliness in the laboratory environment. The presence of substances such as VOCs in the environment is extremely harmful to in vitro embryo development6. Volatile organic compounds, which are constantly produced by laboratory materials and cleaning agents, react with indoor ozone to produce submicron-sized particles and harmful by-products that directly affect pregnancy rates [14,15]. Air quality control within the laboratory is one of the most critical determinants of assisted reproductive success. It has been shown to increase parameters such as live birth rates significantly [16,17] Various studies have shown that gametes and human embryos are sensitive to contaminants found in clinical and laboratory settings [7,17-19]. Many studies have investigated the effect of improved air quality on embryo quality [20,21].
In this study, the purity of CO2 gas used in horizontal incubators with and without filters was investigated to evaluate whether this impacts embryo development. Heitmann et al. observed in their study that when they increased the air quality of the laboratory, the embryo implantation rate increased from 24.3% to 32%, and the live birth rate increased from 31.8% to 39.3% [22]. In another study by Bento and Esteves in 2013, pregnancy outcomes were compared after ICSI between 1999 and 2010 after the implementation of Brazilian national cleanroom standards [23]. They showed that the rate of spontaneous abortion decreased from 28.7% to 20% between the two study periods, while live birth rates increased from 25.8% to 35.6%. However, due to the wide time span of the study period, it suggests that other advances in laboratory and assisted reproductive techniques may also impact embryo development.
Contrary to other studies, in this study, since filtered and unfiltered comparisons were evaluated simultaneously, it is thought that more promising results were obtained to increase embryo quality. Khoudja et al. observed an increase in embryo quality and birth rate, as well as a decrease in spontaneous abortions, by using a new filter system for air purification in addition to the HEPA filters they used in the laboratory [5] and stated that there would be an increase in embryo quality with the reduction of volatile organic compounds. They also verified that the main reason for the change in embryo quality was the effect of the excess formaldehyde in the CO2 gas. In this study, when we examined the CO2 gas used in the laboratories, it was determined that the impurities of Methyl Ethyl ketone, Tetrahydrofuran, Phenol, 2-heptanol, and Formaldehyde substances were not suitable (Figure 1). When the extra filters were used, it was observed that these impurity rates were greatly reduced, but the amount of purity remaining after any extra filter was not sufficient due to the high amount of some species in the CO2 gas (Figure 1).
Lin et al. investigated the effects of Endoplasmic Reticulum (ER) stress and Unfolded Protein Response (UPR) on mammalian oocyte maturation and preimplantation embryo development. ER stress generally plays a negative role in oocyte maturation and/or early embryo development [23,24]. They showed that understanding the mechanistic relationships between ER stress and in vitro development during in vitro human embryo production may help the development of assisted reproductive technology. In a cohort study conducted between 1993 and 1997, Boone et al. investigated the effect of volatile organic compounds on pregnancy rate and observed an increase in oocyte and embryo quality by reducing volatile organic compounds [20]. However, they concluded that there is still a need to investigate what the effect of air quality is directly related to. Swain made a comparative analysis of embryo culture incubators in study [25]. It has been shown that the choice of the incubator is one of the most critical decisions for the IVF laboratory as the devices regulate various environmental variables that can affect embryo development. In addition to functional aspects of the incubator such as gas capacity and sensor type, temperature control and size/patient capacity should also be considered.
In this study, it has been shown that the gasses used in IVF laboratories are not as clean as thought, this impurity adversely affects embryo development, and this can be addressed and prevented by attaching an extra filter to the incubators used. In addition, it has been shown that UPR pathways play a role in the negative effect of ambient air/gas impurity on embryo development in long-term culture (5 or 6 days). As a result of this study, adding extra filters to the incubators used in IVF laboratories will reduce the stress on the embryos and provide better embryo development and, accordingly, higher pregnancy results
References
- World Health Organization (WHO) (2018) International Classification of Diseases, 11th Revision (ICD-11) Geneva: WHO 2018.
- Tanoomand A, Hajibemani A, Abouhamzeh B (2019) Investigation of the association of idiopathic male infertility with polymorphisms in the methionine synthase (MTR) gene. Clin Exp Reprod Med 46(3): 107-111.
- Tarahomi M, Melker A, Wely M, Hamer G, Repping S, et al. (2018) pH stability of human preimplantation embryo culture media: effects of culture and batches. Reproductive biomedicine online 37(4): 409-414.
- Chansel Debordeaux L, Dagorne V, Mercier M, Soula V, Chauzit E (2018) Method validation of a critical parameter in human embryos culture: culture media pH. Ann Biol Clin (Paris) 76(3): 251-258.
- Khoudja RY, Xu Y, Li T, Zhou C (2012) Better IVF outcomes following improvements in laboratory air quality. Journal of assisted reproduction and genetics 30(1): 69-76.
- Ritz B, Wilhelm M (2008) Ambient air pollution and adverse birth outcomes: methodologic issues in an emerging. Field Basic Clin Pharmacol Toxicol 102(2): 182-190.
- Morbeck DE (2015) Air quality in the assisted reproduction laboratory: a mini-review. J Assist Reprod Genet 32(7): 1019-1024.
- Dickey RP, Potts A, Wortham JWE, Welch A (2010) Effect of IVF laboratory air quality on pregnancy success. Fertility and Sterilty 94(4): 151.
- Wang T, Babayev E, Jiang Z, Li G, Zhang M, et al. (2018) Mitochondrial unfolded protein response gene Clpp is required to maintain ovarian follicular reserve during aging, for oocyte competence, and development of pre-implantation embryos. Aging Cell 17(4): e12784.
- Basar M, Bozkurt I, Guzeloglu-Kayisli O, Sozen B, Tekmen I, et al. (2014) Unfolded protein response prevents blastocyst formation during preimplantation embryo development in vitro. Fertil Steril 102(6): 1777-1784.
- Wang M, Wey S, Zhang Y, Ye R, Lee AS (2009) Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal 11(9): 2307-2316.
- Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology (2011) The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod 26(6): 1270-1283.
- Seval Y, Cakmak H, Kayisli UA, Arici A (2006) Estrogen-mediated regulation of p38 mitogen-activated protein kinase in human endometrium. J Clin Endocrinol Metab 91(6): 2349-2357.
- Cecchi M, Esposito B, Gilligan A, Garrisi GJ, Schimmel T (1997) Removal of volatile organic compounds from incubators used for gamete and embryo culture. Fertility and Sterilty 68(1): 165.
- Munch EM, Sparks AE, Duran HE, Van Voorhis BJ (2015) Lack of carbon air filtration impacts early embryo development. Journal of Assisted Reproduction and Genetics. 32(7): 1009-1017.
- Esteves SC, Bento FC (2016) Air quality control in the ART laboratory is a major determinant of IVF success. Asian Journal of Andrology 18(4): 596-599.
- Esteves SC, Bento FC (2016) Implementation of cleanroom technology in reproductive laboratories: the question is not why but how. Reproductive Biomedicine Online. 32(1): 9-11.
- Palter S, DiPaola K, Sparks AE, Degelos S, Koulianos GT (2016) Multi-center study: innovative control of ambient air quality in multiple IVF laboratories is associated with statistically significant improvements in clinical outcomes - analysis of 5319 cycles. Fertil Steril 106: e27-e28.
- Munch EM, Sparks AE, Van Voorhis BJ, Duran HE (2014) Poor laboratory air quality and its impact on early embryo development. Fertil Steril 101(2): e14.
- Boone WR, Crane MM, Johnson JE, Locke AJ, Price TM (1999) Control of air quality in an assisted reproductive technology laboratory. Fertility and Steril 71(1): 150-154.
- Johnson JE, Boone WR, Bernard RS (2016) The effects of Volatile Compounds (V.C.) on the outcome of in vitro mouse embryo culture. Fertil Steril (Suppl 1): S98-S99.
- Heitmann RJ, Hill MJ, James AN, Schimmel T, Segars JH (2015) Live births achieved via IVF are increased by improvements in air quality and laboratory environment. Reproductive Biomedicine Online 31(3): 364-371.
- Esteves SC, Bento FC (2013) Implementation of air quality control in reproductive laboratories in full compliance with the Brazilian Cells and Germinative Tissue Directive. Reproductive Biomedicine Online 26(1): 9-21.
- Lin T, Lee JE, Kang JW, Shin HY, Lee JB (2019) Endoplasmic Reticulum (E.R.) Stress and Unfolded Protein Response (UPR) in Mammalian Oocyte Maturation and Preimplantation Embryo Development. International Journal of Molecular Sciences. 20(2): 409.
- Swain JE (2014) Decisions for the IVF laboratory: comparative analysis of embryo culture incubators. Reproductive biomedicine online 28(5): 535-547.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.