Opinion 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Using the Internet of Microbes to Survive the Assault on the Human Microbiome
*Corresponding author: Rodney R Dietert, Professor Emeritus of Immunotoxicology, Cornell University, Mailing address: c/o 10035 E. Tristan Dr., Hereford, AZ 85615 USA.
Received: May 05, 2023; Published: June 07, 2023
DOI: 10.34297/AJBSR.2023.19.002552
Abstract
The past several years has seen what has amounted to an assault on the human microbiome. Public health institutions tasked with protecting the public from hazardous toxic exposures have failed to either remove hazardous products or adequately alert the public to the presence of dangerous microbial toxicants in specific pharmaceuticals (e.g., proton pump inhibitors), food (e.g., emulsifiers), and household products (e.g., many household cleaners). This lack of attention to the microbiome in general (i.e., as per the need for a robust microbiome to facilitate pathogen colonization resistance) and to microbiome safety specifically was compounded by Covid-19 pandemic-related mandates.
Several Covid-related mandates further degraded the human microbiome by restricting supportive microbial exchanges involving human-human contact, human-nature emersions (e.g., closed beaches and restricted park access), and even seed-based gardening (as occurred in Michigan). In the absence of a much-needed medical and public health focus on our microbiome, this article provides examples for self-empowerment in which connecting to the internet of microbes can be both health promoting and self-supporting.
The examples described concern
a) The benefits of soil-plant-human activities such as gardening and traditional farm activities as well as
b) Exposure to animals as might occur on a farm resulting in microbiome-immune driven protection against some chronic diseases.
Keywords: Human superorganism, Holobiont, Microbiome, Internet of microbes, Food safety and security, Healthspan, Selfempowerment, Gardening, Soil-based microbes, Farm animals
Abbreviations: HMOs: Human Milk Oligosaccharides; VOCs: Volatile Organic Compounds; NSAIDs: Nonsteroidal Anti- Inflammatory Drugs
Introduction
During the past three years in the midst of the Coronavirus pandemic, medical and public health officials led much of humanity into a state of fearing microbes, isolation, and separation from other humans as well large sectors of the environment (e.g., beaches, salt air). It also ignored the power of both natural immunity and of microbiome-directed colonization resistance that protect us every day from a large range of pathobionts. Of course, the Covid-19-justified social engineering-directed behaviors included large numbers of healthy individuals and were counter to the fundamental nature of humans as superorganisms [1] who are by several measures mainly microbial [2]. We need not only our social interaction among humans but also an immersion in nature and its rich microbial life. Public health actions cut us off from activities that are critical for our body’s maintenance [3].
Ironically, over a similar period of time of the pandemic, scientific research generated new understandings about microbe-microbe, microbe-plant, microbe-animal, microbe-human interactions to the microbial elements in nature that affect us not only as individuals but also as social, microbially connected beings. At the height of heavy-handed Covid mandates that separated us not only us each other but also from the internet of microbes and health promoting environmental factors (e.g., vitamin D, salt air), state leaders in one U.S state (Michigan) went so far as to restrict the sale of (and thereby access to) seeds that might be used in home garden food production. Apart from the lack of a real health protection from the banning of seeds, the impediments to self-controlled food production, significant time outdoors, and a prolonged exposure to garden-associated soil microbes was a direct assault on the human microbiome [3].
As we will see this ban affecting self-controlled food production and environmental human microbiome enrichment is only part of a much longer trend in which microbiome-unfriendly practices have damaged the human microbiome. A real concern is the extent to which medical, public health, agricultural and/or policy decisions have degraded the human microbiome and/or the environmental microbiomes through which we network. Blaser, et al. [4] raised an alarm in his consideration of microbiome degradation in what he termed “an age of extinctions.”
Additionally, we previously illustrated how recent advanced western medicine, pharmaceutically driven therapies, and public health policies have been woefully devoid of chronic disease cures and, instead, have propagated comorbid chronic disease, polypharmacy, and shortened healthspans [5-7]. To date few, if any, foods, pharmaceuticals, and agricultural chemicals that damage the human and/or environmental microbiomes have been removed by regulatory oversight agencies. Human microbiome restoration has been an uphill battle in the face of ongoing microbiome toxicants persisting in food (e.g., emulsifiers) [8-10], common pharmaceuticals (e.g., NSAIDs, proton pump inhibitors) [11,12], and environmental and agricultural chemicals (e.g., glyphosate) [13,14].
Given the lack of broad scale medical and regulatory public health actions that would protect our microbiome (and thereby promote our health), it is time that we look for self-empowering tools and practices that are proven to support food safety and security and to restore and/or enrich the human microbiome. Until proven otherwise, we can no longer count on many public institutions to reduce disease prevalence and support the integrity of the human superorganism. The integrity of the human microbiome has been and continues to be under assault when microbiome-toxic drugs, food additives, and environmental chemicals fail to be adequately labeled as hazardous and removed from our daily life. In 2015, Dietert and Silbergeld [15] raised the alarm over the need for microbiome safety assessment and regulatory action to protect the human superorganis. That warning has gone largely unheeded by public institutions funded to protect our health. This opinion article focuses on the soil-microbe component of the internet of microbes and its role in both the production of food and in the health of our own microbiome and physiological systems.
The Internet of Microbess
It is important to consider how our microbiome influences the global status of our body and connects us to the world beyond our perceived physical bodies. Part of our better understanding of human, animal, and plant interfaces to the internet of microbes stems from three factors:
a) An improved understanding of the functional ranges of microbes
b) A better understanding of how the human microbiome is connected to human consciousness and
c) The recognition that the human microbiome is not physically or chemically separated from the microbes external to our body. With the human microbiomes located at portals of entry to our body (e.g., gastrointestinal tract, skin, mouth, nose, and urogenital tract), microbe-microbe and micro-human body exchanges occur. Hence, we are connected microbially beyond our body’s three-dimensional structure. We are connected to the internet of microbes whether we know it or not and there is no subscription fee required or concern over internet outages.
The human body’s front-line interface connects our microbiome externally to Earth’s internet of microbes using communication networks that have been termed: the interactome Slijepcevic [16]. The human microbiome connects, in turn, to our internal organs (e.g., the gut-brain axis) through a form of cross-kingdom communication related to biosemiotics [17]. Slijepcevic and Wickramasinghe pointed out that while the internet of things has mainly focused on new technology and the connection between high-speed internet and human consciousness, in reality, an ancient microbial “internet” is the keystone for the internet of things [17].
In fact, the internet of microbes provides a natural extension of ourselves without evoking a need for machine “enhanced” humans. The choice-point comparisons of the nature-supported human superorganism vs. the “technologically enhanced” transhuman were recently discussed in a prior article in this journal [18]. It is our intention to discuss the entire internet of microbes in a series of articles. This current article focuses on two “internet of microbes” reservoirs that can help to supplement human microbiome damage from toxic exposures and/or a lack of early life seeding. These are: the soil microbiome and prenatal/early postnatal animal exposure (particularly farm animals). The two sources of microbes can aid human microbiome and physiological/immune development particularly during critical windows of early life.
From the Mennonites and Beyonds
Important lessons showing the ways to restore and protect the human microbiome come from a comparison of Mennonite communities using traditional farming practices vs. local non-farming families in the Rochester, NY area. Seppo, et al. [19] examined the infant microbiomes of Rochester area Mennonite farmers, the environmental exposures (e.g., soil, self-produced food, farm animals), the human milk oligosaccharides (HMOs) from nursing Mennonite mothers, and microbiota and metabolites from fecal samples vs. those from non-farming Rochester infants and mothers. Additionally, prevalence of allergy/atopy was assessed and compared among the groups after three years.
The results were striking in illustrating the marked difference in the infant microbiome, composition of breast milk (both for the HMOs and the milk microbiome), infant metabolism, and signs of allergic disease. The Mennonite infants had a fecal microbiome rich in Bifidobacterium infantis compared with the Rochester cohort. Bifidobacteria in general and B. infantis in particular are major metabolizers of HMOs Henrick et al., [20], and its presence in the newborn’s microbiome is needed to mature the immune system and shift immune system balance away from a highly proinflammatory, pro-atopy predisposition [19-21]. Specific HMO metabolites generated by Bifidobacteria are important in the immune maturation process [22].
In a parallel study of older Mennonite traditional farming mothers and their breast milk, the researchers found that maternal production of IgA against several potential allergens, certain cytokines and at least one milk bacterium (Streptococcus equii) was higher in the Mennonite human milk vs. that of Rochester mothers [23]. This was also associated with the lower prevalence of allergy in the Mennonite children compared with the Rochester children, and the author suggested that the differences in human milk may result in differences in immune maturation among the infants. Antibiotic use was one of the significant modifiers in milk characteristics and outcomes [23].
Other investigators have made similar findings in comparisons of farming vs. non farming communities. In a Swedish study, Lundell, et al. [24] found that dairy farm associated B cell activation was linked with protection against allergic disease. Jackson, et al. [25] provided a review of the lifestyle comparison and emphasized that B. infantis metabolism of HMOs is critical for numerous alterations to the developing postnatal immune system of infants affording protection against allergic diseases. One of the earliest studies examining lifestyle, environment, and risk of allergy/asthma was a comparison among two groups of farming families: the traditional farming Amish families and the industrialized farming Hutterite families [26].
In this comparison, the Amish families had a significantly lower prevalence of asthma but a significantly higher level of dustladen endotoxin in the homes. Dust microbe composition differed between the two groups of families. Despite a similar ancestry and farming lifestyle, the two groups of children differed significantly in innate immune profiles. Evidence that the Amish vs. Hutterite household microbes differed in innate immune effects was found using a mouse model and household dust exposures. Mice exposure to the Amish microbe-laden household dust extracts elicited suppressed airway hyperreactivity and eosinophilia compared with the innate immune pro-asthma activation seen using the Hutterite dust extracts. This was an early indicator that lifestyle/ local environment activities are critical in determining specific microbial exposures, and these early life exposures can program the immune system for risk of later life chronic diseases.
Using Microbial Reservoirs for Human Microbiome Restoration: Soil
While the microverse outside of our bodies can be a reservoir of pathobionts, several authors have discussed the importance of exposure/exchange of microbes between the environment and our bodies for our well-being. In their recent review of the environment and the human microbiome, Panthee, et al. [27] concluded that “…a closer living with nature would facilitate the diversification and balance of microbiota inside the body.” These authors illustrated that close interactions with domestic or pet animals, diverse soil, flowering plants in the yard, and close proximity of forests can facilitate the diversification and balance of human microbiota [27].
Blum, et al. [28] proposed a hypothesis that soil microbes share a particularly close historic relationship with that of the human gut microbiome. They found that the diversity of species found in the soil vs. the human gut is greater by a factor of 10 although the number of active species per gram may be similar. While our ancestors likely had more exposure to soil than we do presently, it appears to be a significant factor in our own microbial diversity. Studies in mice showed that soil was as significant a factor as diet in determining gut microbiome diversity [29], see also [28] of course part of the problem is that both the human microbiome and the soil microbes have been degraded with increased use of pharmaceuticals (e.g., antibiotics) [11] and agricultural chemicals [30], respectively. For example, Fuchs, et al. [30] found that glyphosate damages plant symbiotic microbes.
Microbial components of the environment such as soil have been shown to be particularly effective in balancing human physiology. For example, Mycobacterium vaccae is a soil borne bacterium that is particularly effective against stress, anxiety, and select psychiatric disorders [31-34]. Research by Holbrook, et al. [35] using strain NCTC 11659 demonstrated that macrophages are on the targets with shifts in cytokines facilitating a reduction in neuroinflammation. In a small sample size experiment involving healthy volunteers in soil mixing activities, those mixing soil seeding with M. vaccae vs. sterile soil, those exposed to M. vaccaeinoculated soil displayed significantly lower heart rate, elevated right occipital lobe activity and increased serotonin vs. the control group. Specific volatile organic compounds (VOCs) were released from the bacterial-laden soil [36]. Despite the small sample size, these results are consistent with neuroprotective animal studies. e.g., [31,37-39].
Beneficial physiological effects from soil microbiota exposure are not limited only to Mycobacterium vaccae. Choi, et al. [40] found that seed-sowing activities by adults using soil that had been inoculated with a common soil bacterium, Streptomyces rimosus resulted in beneficial psychophysiological alterations including improved concentration and attention. It is important to note that microbial VOCs including those from soil exert effects through both intra-kingdom and inter-kingdom interactions affecting not only other microbes but also the plant and animal kingdoms [41]. Plant-microbe and plant-plant communications utilize VOCs as a communication network [42]. Additional researchers [43] showed that VOCs in the rhizosphere could operate at a distance in producing effects.
In a small but important proof of concept study, Brown, et al. [44] compared fecal and soil microbiota composition among gardening and non-gardening families. During the course of the gardening season, the fecal samples of gardening families acquired soil-endemic microbiota that were absent among the nongardening families. Additionally, there was a greater diversity in the fecal microbiota among those who were gardening vs, the nongardeners. Because soil microbiota are among the most diverse sources of environmental microbes, regular active exposure to this source is a ready opportunity to diversify the human microbiome (while also enjoying personally propagated, self-sourced produce.).
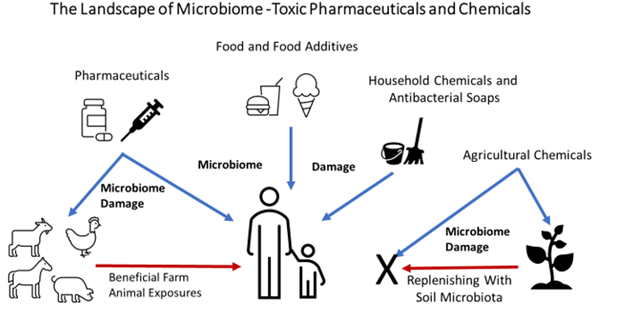
The 2022 study result from Brown, et al. [44] was presaged among several microbiome-themed books. Despite the fact that all soils are not necessarily equal (e.g., some contain unacceptable levels of heavy metals), the importance of human exposure to soil microbes is reflected in prior microbiome book titles such as “Dirt Is Good: The Advantage of Germs for Your Child’s Developing Immune System” [45] and “Let Them Eat Dirt” [46]. Figure 1 illustrates the sources of toxic drugs and chemicals that inflict damage on the human microbiome. It also shows the roles of soil microbiota and animals in possible repair of the dysbiotic human microbiome. However, even the soil and animal reservoirs are themselves vulnerable to drug and chemical toxic exposures. As a result, human and environmental microbiome protection is still needed from the oversight sources that have, thus far, allowed the problematic exposures to persist.
Conclusions
Self-protection and empowerment of individuals regarding their own microbiome has become a necessity as western medical and public health establishments have failed to adequately protect what constitutes a majority of the genes and cells of the human body. The recent Covid-19 pandemic provided a perfect example of the ease with which the fundamental nature of humans as superorganisms can be ignored when dealing with periodic health challenges. No one would approach the health of a coral reef in the manner that has been used most recently for humans.
This article describes the utility of bringing forward practices and activities that serve to better connect us with Earth’s internet of microbes. These include exposure to beneficial soil microbes and traditional farming experiences including the presence of farm animals particularly early in life. There are many other media and sources of useful microbes beyond soil that also help us sustain and/or rebiose our own microbiome. We hope to engage these media in the future. Rebalancing immune function and other physiology is difficult to achieve in the presence of a dysbiotic microbiome. For this reason, solutions for human superorganism should begin with the human microbiome and ideally, with support from beneficial microbes that surround us outside of the human body.
Acknowledgements
We are grateful to Canadian acquaintances who encouraged us to develop this paper.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- Dietert R (2016) The Human Superorganism. Dutton Penguin Random House 341.
- Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV et al. (2018) Current understanding of the human microbiome. Nat Med 24(4): 392-400.
- Dietert RR (2021) The Microbiological Basis of Human Superorganism Freedom. Am J Biomed Sci & Res 13(6): 653-661.
- Blaser MJ (2018) The Past and Future Biology of the Human Microbiome in an Age of Extinctions. Cell 172(6): 1173-1177.
- Dietert RR (2021) Microbiome First Medicine in Health and Safety. Biomedicines 9(9): 1099.
- Dietert RR (2021) Microbiome First Approaches to Rescue Public Health and Reduce Human Suffering. Biomedicines 9(11): 1581.
- Dietert RR, Dietert JM (2022) Using Microbiome-Based Approaches to Deprogram Chronic Disorders and Extend the Healthspan following Adverse Childhood Experiences. Microorganisms 10(2): 229.
- Zhao S, Jang C, Liu J, Uehara K, Gilbert M, et al. (2020) Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature 579(7800): 586-591.
- Gerasimidis K, Bryden K, Chen X, Papachristou E, Verney A, et al. (2020) The impact of food additives, artificial sweeteners and domestic hygiene products on the human gut microbiome and its fibre fermentation capacity. Eur J Nutr 59(7): 3213-3230.
- Bancil AS, Sandall AM, Rossi M, Chassaing B, Lindsay JO, et al. (2021) Food Additive Emulsifiers and Their Impact on Gut Microbiome, Permeability, and Inflammation: Mechanistic Insights in Inflammatory Bowel Disease. J Crohns Colitis 15(6): 1068-1079.
- Weersma RK, Zhernakova A, Fu J (2020) Interaction between drugs and the gut microbiome. Gut 69(8): 1510-1519.
- Levy EI, Hoang DM, Vandenplas Y (2020) The effects of proton pump inhibitors on the microbiome in young children. Acta Paediatr 109(8): 1531-1538.
- Chiu K, Warner G, Nowak RA, Flaws JA, Mei W, et al. (2020) The Impact of Environmental Chemicals on the Gut Microbiome. Toxicol Sci 176(2): 253-284.
- Ruuskanen S, Fuchs B, Nissinen R, Puigbò P, Rainio M, et al. (2023) Ecosystem consequences of herbicides: the role of microbiome. Trends Ecol Evol 38(1): 35-43.
- Dietert R, Silbergeld EK (2015) Biomarkers for the 21st century: listening to the Microbiome. Toxicol Sci 144(2): 208-216.
- Slijepcevic P (2021) Principles of Information Processing and Natural Learning in Biological Systems. J Gen Philos Sci 52: 227–245.
- Slijepcevic P, Wickramasinghe NC (2020) An internet of microbes straddling the cosmos. Adv Genet 106: 109-117.
- Dietert RR (2022) The Earth-connected human superorganism vs. the machine. Am J Biomed Sci & Res 15(6): 653-662.
- Seppo AE, Bu K, Jumabaeva M, Thakar J, Choudhury RA, et al. (2021) Infant gut microbiome is enriched with Bifidobacterium longum ssp. infantis in Old Order Mennonites with traditional farming lifestyle. Allergy 76(11): 3489-3503.
- Henrick BM, Rodriguez L, Lakshmikanth T, Pou C, Henckel, E et al. (2021) Bifidobacteria-mediated immune system imprinting early in life. Cell 184(15): 3884-3898.e11.
- Lin C, Lin Y, Zhang H, Wang G, Zhao J, et al. (2022) Intestinal 'Infant-Type' Bifidobacteria Mediate Immune System Development in the First 1000 Days of Life. Nutrients 14(7): 1498.
- Stewart CJ (2021) Breastfeeding promotes bifidobacterial immunomodulatory metabolites. Nat Microbiol 6(11):1335-1336.
- Seppo AE, Choudhury R, Pizzarello C, Palli R, Fridy S, et al. (2021) Traditional Farming Lifestyle in Old Older Mennonites Modulates Human Milk Composition. Front Immunol 12: 741513.
- Lundell AC, Hesselmar B, Nordström I, Adlerberth I, Wold AE, et al. (2015) Higher B-cell activating factor levels at birth are positively associated with maternal dairy farm exposure and negatively related to allergy development. J Allergy Clin Immunol 136(4): 1074-1082.e3.
- Jackson CM, Mahmood MM, Järvinen KM (2022) Farming lifestyle and human milk: Modulation of the infant microbiome and protection against allergy. Acta Paediatr 111(1): 54-58.
- Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, et al. (2016) Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children. N Engl J Med 375(5): 411-421.
- Panthee B, Gyawali S, Panthee P, Techato K (2022) Environmental and Human Microbiome for Health. Life (Basel) 12(3): 456.
- Blum WEH, Zechmeister Boltenstern S, Keiblinger KM (2019) Does Soil Contribute to the Human Gut Microbiome? Microorganisms 7(9): 287
- Zhou D, Bai Z, Zhang H, Li N, Bai Z, et al. (2018) Soil is a key factor influencing gut microbiota and its effect is comparable to that exerted by diet for mice. F1000Research 7 (2018): 1588.
- Fuchs B, Saikkonen K, Damerau A, Yang B, Helander M, et al. (2023) Herbicide residues in soil decrease microbe-mediated plant protection. Plant Biol (Stuttg) 25(4):571-578.
- Siebler PH, Heinze JD, Kienzle DM, Hale MW, Lukkes JL, et al. (2018) Acute Administration of the Nonpathogenic, Saprophytic Bacterium, Mycobacterium vaccae, Induces Activation of Serotonergic Neurons in the Dorsal Raphe Nucleus and Antidepressant-Like Behavior in Association with Mild Hypothermia. Cell Mol Neurobiol 38(1): 289-304.
- Frank MG, Fonken LK, Dolzani SD, Annis JL, Siebler PH, et al. (2018) Immunization with Mycobacterium vaccae induces an anti-inflammatory milieu in the CNS: Attenuation of stress-induced microglial priming, alarmins and anxiety-like behavior. Brain Behav Immun 73: 352-363.
- Foxx CL, Heinze JD, González A, Vargas F, Baratta MV, et al. (2021) Effects of Immunizationwithh the Soil-Derived Bacterium Mycobacterium vaccae on Stress Coping Behaviors and Cognitive Performance in a "Two Hit" Stressor Model. Front Physiol 11:524833.
- Hassell JE, Baratta MV, Fallon IP, Siebler PH, Karns BL et al. (2023) Nguyen KT, Gates CA, Fonken LK, Frank MG, Maier SF, Lowry CA. Immunization with a heat-killed preparation of Mycobacterium vaccae NCTC 11659 enhances auditory-cued fear extinction in a stress-dependent manner. Brain Behav Immun 107: 1-15.
- Holbrook EM, Zambrano CA, Wright CTO, Dubé EM, Stewart JR, et al. (2023) Mycobacterium vaccae NCTC 11659, a Soil-Derived Bacterium with Stress Resilience Properties, Modulates the Proinflammatory Effects of LPS in Macrophages. Int J Mol Sci 24(6): 5176.
- Kim S, Son SY, Kim MJ, Lee CH, Park S, et al. (2022) Physiological Responses of Adults during Soil-mixing Activities Based on the Presence of Soil Microorganisms: A Metabolomics Approach. J Amer Soc Horticul Sci 147(3): 135-144.
- Reber SO, Siebler PH, Donner NC, Morton JT, Smith DG, et al. (2016) Immunization with a heat-killed preparation of the environmental bacterium Mycobacterium vaccae promotes stress resilience in mice. Proc Natl Acad Sci U S A 113(22): E3130-9.
- Fonken LK, Frank MG, D'Angelo HM, Heinze JD, Watkins LR, et al. (2018) Mycobacterium vaccae immunization protects aged rats from surgery-elicited neuroinflammation and cognitive dysfunction. Neurobiol Aging 71: 105-114.
- Amoroso M, Böttcher A, Lowry CA, Langgartner D, Reber SO, et al. (2020) Subcutaneous Mycobacterium vaccae promotes resilience in a mouse model of chronic psychosocial stress when administered prior to or during psychosocial stress. Brain Behav Immun 87: 309-317.
- Choi NY, Park SA, Lee YR, Lee CH (2022) Psychophysiological Responses of Humans during Seed-Sowing Activity Using Soil Inoculated with Streptomyces rimosus. Int J Environ Res Public Health 19(23): 16275.
- Weisskopf L, Schulz S, Garbeva P (2021) Microbial volatile organic compounds in intra-kingdom and inter-kingdom interactions. Nat Rev Microbiol 19(6): 391-404.
- Bouwmeester H, Schuurink RC, Bleeker PM, Schiestl F (2019) The role of volatiles in plant communication. The Plant Journal 100(5): 892-907.
- de la Porte A, Schmidt R, Yergeau É, Constant P (2020) A Gaseous Milieu: Extending the Boundaries of the Rhizosphere. Trends Microbiol 28(7): 536-542.
- Brown MD, Shinn LM, Reeser G, Browning M, Schwingel A, et al. (2022) Fecal and soil microbiota composition of gardening and non-gardening families. Sci Rep 12(1): 1595.
- Gilbert J, Knight R, Blakeslee S (2017) Dirt Is Good: The Advantage of Germs for Your Child's Developing Immune System. St Martin’s Press 175 Fifth Avenue New York, NY 10010 USA pp. 272.
- Findlay BB, Arrieta M-C (2016) Let Them Eat Dirt. Algonquin Books Chapel Hill, USA pp. 322.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.