Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
An Investigation of Revisional Surgery After Implantation of Device for Intervertebral Motion (DIAM): A Case Series Study
*Corresponding author: Jia Ping Wu, Department of Medical Technology, Department of Nursing, Shaoguan University, Shaoguan city, Guangdong Province, China.
Received: October 12, 2023; Published: October 25, 2023
DOI: 10.34297/AJBSR.2023.20.002711
Abstract
Background: The application of the Device for Intervertebral Assisted Motion (DIAM™) is a spinal fusion device recently and widely development for the treatment of lumbar degenerative diseases. Among the Interspinous Process Devices (IPDs) the Device for Intervertebral Assisted Motion (DIAM™) is a relatively newer fusion technology, based on the placement of a flexible IPD.
Purpose: This study was aimed to evaluate the clinical outcome of a widely used IPDs called DIAM™.
Methods: The patients (n=44) had undergone DIAM™ placement were evaluated the medical records in our hospital. The demographic data and diagnosis were recorded. Revision surgery was performed for each patient and follow-up was based on medical and radiological records.
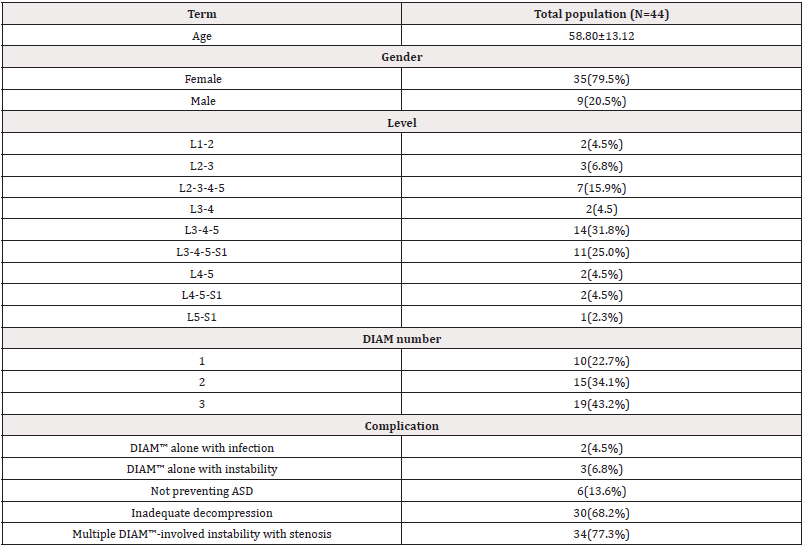
Results: Forty-four patients with a mean (±SD) age of 58.80(±13.12) years underwent the revision surgery of DIAM™. The complication of patients’ previous revision surgery, arranging from low to high was in DIAM™ alone with infection (2 cases), DIAM™ alone with instability (3 cases), preventing Adjacent Segment Disease (ASD) (6 cases), inadequate decompression (30 cases), DIAM™-involved instability with stenosis (34 cases).
Conclusions: DIAM™ may not be a comprehensive interspinous process decompression device to treat all kinds of spinal diseases due to its various postoperative complications. The predominant DIAM™ indications would be disc herniation, spinal stenosis, black disc disease, and fusion after ASD (topping Off).
Keywords: Spinal fusion device, DIAM™ Device, Revision surgery, Postoperative complications, Low back pain, Interspinous process devices.
Introduction
Low back pain is a progressive degeneration of lumbar disease in the elderly [1]. However, an IPDs is one kind of spinal fusion device widely used for lumbar spinal stenosis is the reason for undergoing spinal surgery for bony decompression by DIAM™ device. Multiple studies have shown better long-term clinical outcomes of surgeries with the DIAM™ device [2]. Among the placement of flexible IPDs, DIAM™ is a relatively newer spinal fusion surgery technology on the implantation of DIAM™ spinal fixation [3]. The objective of this study was to evaluate the revisional surgeries at implantation of a DIAM™ device. However, little is known about the feasibility and efficacy of the DIAM™ device in lumbar disk degeneration patients [4]. The spinal stabilization system of DIAM™ is proclaimed to have feasibility, efficacy, and flexible support for surgical complications treatment for patients suffering from lumbar spine degeneration [5,6]. Because of LSS narrowing resulting from a degenerative change in the different ages and gender. This investigation for etiologies of revisional surgeries study assessed the efficacy of the IPDs for the DIAM™ in patients. Taylor et al. also suggested three indications for DIAM™ devices including [7,8] For discogenic disease: primary, recurrent, with, and without discectomy. For posterior disease: central, foraminal stenosis, facet disease, and ligamentous instability [9]. From junction disease: implanting a DIAM™ above the existing lumbar fusion [10,11]. However, a clinically DIAM™ has been recently applied at implantation to reduce back pain and disability. Although the DIAM™ device was generally acceptable but is accompanied by increased complications and contraindications [12]. The fixation method for the DIAM™ device to the vertebral has been used for patients unloading a pain in its early stage of degeneration with low back pain even stopping or slowing down the degenerative process. During flexion, the DIAM™ device is an example of a lumbar device that decreases surgery complication motion at the implanted levels and the adjacent [13]. The long-term surgery complications of the DIAM™ device include higher reoperation, revision surgery, and higher cost-effectiveness [14]. This retrospective study evaluated the surgical outcomes and focused on the complications resulting from the long-term elevated intra-disk pressure that has been shown to be associated with the progression of lumbar disk degeneration.
Material and Methods
Participations
Forty-four patients undergoing surgery with DIAM™ placement since 2008 and who underwent revision surgery from 2016 to 2018 were included in this study. These patients include those transferred from other hospitals or who previously underwent surgery in our hospital. Most patients underwent the first Diam operation in other hospitals, and the original medical records of each patient from those hospitals were unable to be obtained. Therefore, it is difficult to determine when the included patients first underwent Diam. Among the reasons for the agreement of revision surgery, spinal instability with residual stenosis is an important reason for patients to undergo reoperation. After evaluated physical examinations, all of these patients were assessed with dynamic X-rays and Magnetic Resonance Imaging (MRI) of the lumbar spine. The characteristic data, including these patients’ complications, were recorded, and the diagnosis and surgery outcome were also discussed.
Surgical Methods
The patients were referred for surgery after diagnosis. All patients required revision surgery under spinal anesthesia in the knee-chest position. We prepared the appropriately sized DIAM™ device waiting for use. DIAM™ device was interspaced between L4- L5-S1 spinous processes under the ligament. The operating surgery time was 35-45min. The patient was raised from bed after 24 hours.
Functional Outcomes
All patients suggest the effects of DIAM™ on low back pain.
X-ray and MRI Images
The X-ray and MRI images were performed according to standard procedures to determine the segmental instability. Injection of the disc of non-ionic contrast dye (2mL) inside the disc space by the X-ray images. The intervertebral angle was greater than 5°, we can determine the spinal segment was considered unstable. Magnetic Resonance Imaging (MRI) of the lumbar spine was used to determine implantation surgery of DIAM™ device.
Statistical Analysis
All population patients (n=44) undergoing surgery with DIAM™ placement. The demographic data and diagnosis were recorded. All methods statistical analyses were performed in SAS format. This study included 44 patients of interspinous DIAM™ spinal stabilization system population and excluded at the different levels from the index of the complication population.
Results
This study analyzed 44 patients who underwent previous DIAM™ implanting and revision surgery. Characteristics of the population of patients were observed in Table 1. The overall mean ±SD (age) of forty-four patients included in this study was 58.80±13.12 years, and 79.5% of patients were female (Table 1).
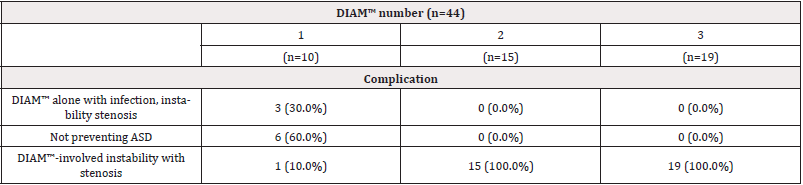
Our results showed that 19 cases (43.2%) and 15 cases (34.1%) had more DIAMs™, only 10 cases (22.7%) had one DIAM™. The complications of these 44 cases undergoing the revision surgery are shown in Table 1, and the most prevalent involved Multiple DIAM™-involved instabilities with stenosis 34 (77.3%). However, the patients were grouped according to their complications and described the treatment as listed below, respectively. Moreover, we detected the association between DIAM™ number and complications the results showed in Table 2. Table 2 presents the distribution of complications in a different number of DIAM™.
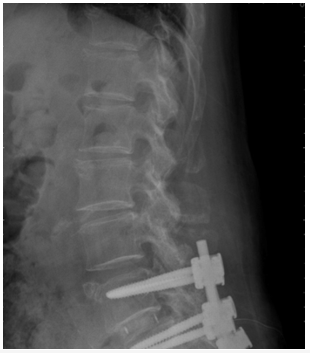
In patients only use one DIAM™ had the complication for infection, instability, or stenosis (n=3, 30.0%), but not preventing Adjacent Segment Disease (ASD) (n=6, 60.0%), and DIAM™-involved instability with stenosis (n=1,10%). However, in patients with 2 numbers and 3 numbers DIAMs™, we did not observe the complication for infection, instability, or stenosis, and not preventing Adjacent Segment Disease (ASD), but we observed 2 numbers and 3 numbers DIAMs™-involved instability with stenosis. DIAM™-involved instability with stenosis was noted in all the patients. From X-ray and MRI sagittal and axial plane results showed from Figure 1 and Figure 2, we found low back pain caused by lumbar disk degeneration disc diseases at L4-L5 and L5-S1 disc. This chronic low back pain has been found to be the most prevalent internal disk disruption. Most cases of degenerative disc disease can be managed by X-ray and MRI methods. DIAMs™ device implantation surgical treatment is an option in cases of severe to surpassing facet joint and sacroiliac joint pain. However, the standard placement of DIAM™ surgical treatment for lumbar degenerative disc disease is fusion surgery. This fusion surgery has been found to reduce pain by eliminating motion at the spinal segment. The result has been shown that a patient underwent revision surgery and internal fixation for L4-5-S1 with stainless steel after nerve decompression by DIAMs™ device (Figure 3). Moreover, Figure 4 result showed that we can observe a DIAM™ device with a foreign body reaction in which two vertebrae are grafted together, and for effective pain relief (Figure 1-4).
Discussion
The DIAM™ device implant has the development of dynamic stabilization techniques, but the clinical relation to lumbar fusion instrumented is still uncertain. It is a posterior interspinal dynamic stabilization or balancing device14. The DIAM™ device is thought to work by reducing loading of the disc, restoring the posterior tension band, realigning the facet joint line, and increasing foraminal height [15,16]. For this reason, the DIAM™ implant was developed for low back pain. Installation of DIAM™ is not preferred in the L5- S1 space [17-19] as the spine process of S1 is too small to support DIAM™ appropriately. In revision surgery patients, DIAM™ was installed in L5-S1and obviously, instability was reported. Accordingly, the installation of DIAM™, in elderly patients with poor bones, the spinal process is very fragile and cannot withstand the opening force of DIAM™. Any accident can cause a fracture of the spinal process (Table 1). In patients with relatively unstable spine receiving spinal decompression surgery with DIAM™ alone, greater spinal instability is noted. Even if DIAM™ is used again, the spine might not be stabilized, leading to further complications from DIAM™ alone (Table 2). Facet joint osteoarthritis occurs only in cases of disc degeneration [20]. Therefore, DIAM™ might not be suitable for patients with facet joint arthritis. Otherwise, it might further cause more damage to spinal instability [21,22]. However, as the follow-up time increases, DIAM’s™ effectiveness in preventing ASD will become less effective, and more patients will need revision surgery to treat junctional level instability. When choosing DIAM™ to prevent ASD of patients after long fusion, the patient’s disc space is suggested to be no less than 1/2, and without arthritis in the facet joint, otherwise, the effect of DIAM™ in preventing ASD is not obvious by multi-DIAMs™. This study focused on the L4-5-S1 disc herniation, spinal stenosis, and back disc disease by x-ray and MRI images (Figure 1 and Figure 2). The x-ray and MRI results of these patients all showed spinal stenosis. Moreover, this study observed that most revision surgery patients in our hospital have received multiple DIAM™ devices, which caused persistent back pain, lower leg numbness, and spinal instability leading to neural tube stenosis (Figure 3). Besides, these kinds of revision surgery are not difficult to perform because adhesions are found only in lamina decompression and are still the virgin site near the nerve root [23,24]. DIAM™ being a foreign body can cause infection in a few cases. If rubbed with Dura for a long time, it might cause granulomas and possibly infection (Figure 4). The Borderline indication is suggested for DIAM™ which includes stable degenerative spondylolisthesis and osteoporotic cases. The contraindications of DIAM™ are suggested to be the use of multiple inter-spinal process devices, inappropriate to pars fracture cases, unstable spine, and prohibited use for L5-S1 site [25,26]. If the facetectomy is performed, adequate decompression can be achieved.
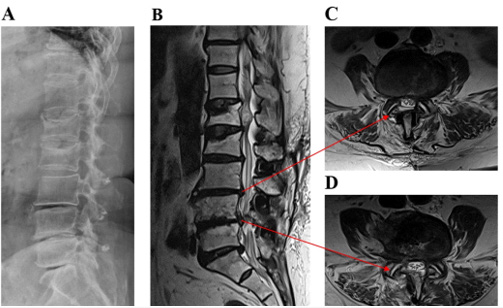
Figure 1: A case with persistent back pain caused by lumbar disk degeneration disc disease at L4-L5 without multiple DIAMs™.
(A) X-ray
(B) MRI sagittal plane
(C, D) MRI axial plane

Figure 2: A case with severe back pain caused by lumbar disk degeneration disc disease at L5-S1 without multiple DIAMs™.
(A) X-ray
(B) MRI sagittal plane
(C, D) MRI axial plane
Conclusion
This study is to determine inserting DIAM™ implants to sufficient decompression during the operation unstable spine. It usually requires further revision surgery entire intervertebral process DIAMs™ to fully decompress, and fuse with the implant. The DIAMs™ has interspinous devices available known the effects of these devices on the treated segment and on the adjacent segments of the spine.
Acknowledgements
None.
Conflict of Interest
None.
References
- Fan W, Zhang C, Wang QD, Guo LX, Zhang M (2023) The effects of topping-off instrumentation on biomechanics of sacroiliac joint after lumbosacral fusion. Comput Biol Med 164: 107357.
- Han Y, Ren X, Liang Y, Ma X, Wang X (2023) Biomechanical effects of transverse connectors on total en bloc spondylectomy of the lumbar spine: a finite element analysis. J Orthop Surg Res 18(1): 484.
- Fonseca G, Vakiel P, Cripton PA (2023) UBC Neck C4-C5: An Anatomically and Biomechanically Accurate Surrogate C4-C5 Functional Spinal Unit. Ann Biomed Eng 51(8): 1802-1815.
- Sun B, Han Q, Sui F, Zhang A, Liu Y, et al. (2023) Biomechanical analysis of customized cage conforming to the endplate morphology in anterior cervical discectomy fusion: A finite element analysis. Heliyon 9(1): e12923.
- Xu YK, Weng PW, Chen SH, Lin SC (2023) Biomechanical comparisons of dynamic fixators with rod-rod and screw-spacer joints on lumbar hybrid fixation. Clin Biomech (Bristol, Avon) 104: 105943.
- Vasquez Alvarez M, Zapata U, Casado FL (2022) Development of an Intervertebral Disc for Cervical Spondylosis Composed of Seeded Biomaterials. Annu Int Conf IEEE Eng Med Biol Soc 3931-3934.
- Dong J, Liang B, Sun Y, Li X, Han P, et al. (2022) Biomechanics of a novel artificial cervical vertebra from an in vivo caprine cervical spine non-fusion model. J Orthop Translat 37: 61-68.
- Zhong Y, Wang Y, Zhou H, Wang Y, Gan Z, et al. (2023) Biomechanical study of two-level oblique lumbar interbody fusion with different types of lateral instrumentation: a finite element analysis. Front Med (Lausanne) 10: 1183683.
- Moore AC, Holder DA, Elliott DM (2023) Off-Axis Loading Fixture for Spine Biomechanics: Combined Compression and Bending. J Biomech Eng 145(10): 105001.
- Xu Y, Zhang X, You J, Wang H, Zheng R, et al. (2023) Analysis of the Cause of Cartilage Warping in the Rhinoplasty of Costal Cartilage and Application of Embed-In Graft in Revisional Surgery. Aesthet Surg J 43(6): 646-654.
- Park YS (2022) Revisional Surgery After Adjustable Gastric Banding: Sleeve Gastrectomy or Gastric Bypass? J Metab Bariatr Surg. 11(2): 49-53.
- Vahibe A, Aizpuru MJ, Sarr MG, Mundi MS, Vierkant RA, et al. (2022) Safety and Efficacy of Revisional Surgery as a Treatment for Malnutrition after Bariatric Surgery. J Am Coll Surg 236(1): 156-166.
- Monfared S, Weis JJ, Shah SK, Scott DJ, Felinski MM, et al. (2023) The rising tide of revisional surgery: tracking changes in index cases among bariatric-accredited fellowships. Surg Endosc 37(6): 4824-4828.
- Cho YJ, Park JB, Chang DG, Kim HJ (2021) 15-year survivorship analysis of an interspinous device in surgery for single-level lumbar disc herniation. BMC Musculoskelet Disord 22(1): 1030.
- Hsiao CK, Tsai YJ, Yen CY, Li YC, Hsiao HY, et al. (2023) Biomechanical Effect of Hybrid Dynamic Stabilization Implant on the Segmental Motion and Intradiscal Pressure in Human Lumbar Spine. Bioengineering (Basel) 10(1): 31.
- Li CY, Chen MY, Chang CN, Yan JL (2020) Three-Dimensional Volumetric Changes and Clinical Outcomes after Decompression with DIAM™ Implantation in Patients with Degenerative Lumbar Spine Diseases. Medicina (Kaunas) 56(12): 723.
- Kim KR, Lee CK, Kim IS (2020) Efficacy of interspinous device on adjacent segment degeneration after single level posterior lumbar interbody fusion: a minimum 2-year follow-up. Br J Neurosurg 35(6): 757-765.
- Onggo JR, Nambiar M, Maingard JT, Phan K, Marcia S, et al. (2021) The use of minimally invasive interspinous process devices for the treatment of lumbar canal stenosis: a narrative literature review. J Spine Surg 7(3): 394-412.
- Lo HJ, Chen HM, Kuo YJ, Yang SW (2020) Effect of different designs of interspinous process devices on the instrumented and adjacent levels after double-level lumbar decompression surgery: A finite element analysis. PLoS One 15(12): e0244571.
- Toth JM, Bric JD (2019) An evaluation of the host response to an interspinous process device based on a series of spine explants: Device for Intervertebral Assisted Motion (DIAM®). J Spine Surg 5(4): 483-495.
- Lo HJ, Chen CS, Chen HM, Yang SW (2019) Application of an interspinous process device after minimally invasive lumbar decompression could lead to stress redistribution at the pars interarticularis: a finite element analysis. BMC Musculoskelet Disord 20(1): 213.
- Boody BS, Smucker JD, Sasso W, Miller JW, Snowden R, et al. (2020) Evaluation of DIAM™ Spinal Stabilization System for lower lumbar disc degenerative disease: A randomized, prospective, single-site study. J Orthop 21: 171-177.
- Lewandrowski KU, Abraham I, Ramírez León JF, Cantú Leal R, Longoria RC, et al. (2022) A Differential Clinical Benefit Examination of Full Lumbar Endoscopy vs Interspinous Process Spacers in the Treatment of Spinal Stenosis: An Effect Size Meta-Analysis of Clinical Outcomes. Int J Spine Surg 16(1): 102-123.
- Li YC, Feng XF, Pang XD, Tan J, Peng BG (2020) Lumbar disc rehydration in the bridged segment using the BioFlex dynamic stabilization system: A case report and literature review. World J Clin Cases 8(10): 1958-1965.
- Seo JY, Ha KY, Kim YH, Ahn JH (2016) Foreign Body Reaction after Implantation of a Device for Intervertebral Assisted Motion. J Korean Neurosurg Soc 59(6): 647-649.
- Lu K, Liliang PC, Wang HK, Chen JS, Chen TY, et al. (2016) Clinical outcome following DIAM implantation for symptomatic lumbar internal disk disruption: a 3-year retrospective analysis. J Pain Res 9: 917-924.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.