Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Development And Assessment of Quality Criteria of Routine Phytosomes in The Treatment of Periodontitis
*Corresponding author: Sevil Mehraliyeva Jabrail, PhD, Associate Professor, Pharmaceutical technology and management, Azerbaijan Medical University, Baku.
Received: May 29, 2024; Published: May 15, 2024
DOI: 10.34297/AJBSR.2024.22.002978
Abstract
Currently, one of the priority issues of pharmaceutical nanotechnology is to develop phytosomal gel technology, a new drug delivery system based on rutoside in the treatment and prevention of dental diseases. In this regard, it was determined that the size of rutin phytosomes prepared in the Laboratory of Pharmaceutical Technology of Azerbaijan Medical University is 60-180 nm based on SEM, TEM, and other analysis methods. Nanomedicine was prepared by incorporating rutin phytosomes into gel (a mass obtained from alginic acid and polysaccharides of plantain seeds). The innovative gel developed by us based on phytosomes has several advantages, which are significantly different from traditional gel preparations. The phytosomal gel studied at the Department of Therapeutic Dentistry of AMU provided 85-90% natural cell regeneration in the damaged tissue during the treatment of periodontitis. As a result of one-time use of phytosomal gel, it was observed that its effect is long-lasting, even lasting for several years. Also, the effect of the gel restores the barrier function of the periodontal tissue and local immunity, which helps to prevent repeated inflammation of the tissue. The conducted comparative studies once again proved that the phytosomal gel of rutin has a therapeutic effect within 120 hours, while well-known drugs in the treatment and prevention of periodontitis (Metragil, Parodium, Levomekol, Miramistin, etc.) have a therapeutic effect within 7-14 days.
Keywords: Periodontitis, Rutin, Phytosomes, Lecithin, Electron Microscopy Analysis, Anti-inflammatory effect
Introduction
Currently, periodontitis is one of the most common dental diseases and is a social problem, because it leads to disruption of the supporting function of teeth, disruption of chewing function, bleeding gums and, as a result, loss of teeth, and infection of periodontal pockets is negative affects the body as a whole. Almost every person suffers from periodontitis to one degree or another. And periodic bleeding of the gums when brushing your teeth is the first symptom of a dangerous chronic disease. The cause of the development of periodontitis can be carious teeth, overhanging edges of fillings, traumatic gingival papilla, etc., but the main reason that triggers the development of periodontitis is dental plaque. WHO claims that 10-15% of the world's population is infected with this disease [8]. In modern times, drug delivery systems are widespread. Conducting research in this direction is distinguished by its relevance. We have conducted research on the production of nanoemulsions, nanocapsules, and niosomal gel with plant and animal extracts, gold, and silver nanoparticles, and obtained appropriate results [1-7].
In addition to the above, the synthesis of various types of nanoparticles, a number of their physicochemical properties, biological activity, antiparasitic effect, application in industry, bioaccumulation in various components of a simple food chain (plants, molluscs, fish, parasites) and pathological changes caused by Transmission Electron Microscope (TEM)) studied the ultrastructural characteristics [9-16]. Phytosomes, which are one of the drug delivery systems, are distinguished by the simplicity of the preparation technology, long-term effect, and stability of the received drug form. The preparation of phytosomal gel based on rutoside is considered a priority in the treatment and prevention of dental diseases. In terms of the lack of long-term effect and side effects, the application of phytosomal gel, a new delivery system, is considered satisfactory in such diseases. Phytosomes have been selected as a new delivery system and are mainly based on plant-derived raw materials. Phytosomes, distinguished by a number of advantages, improve the absorption of biologically active substances, synergistic effect and reduce the need for their amount; increases the absorption of oil-insoluble hydrophilic compounds; increases the bioavailability of phytoconstituents orally and topically; has the ability to easily pass through the cell membrane and enter the cell; phytosomes exhibit a good stability profile due to the formation of chemical bonds between the phosphatidylcholine molecule and phytoxents [19].
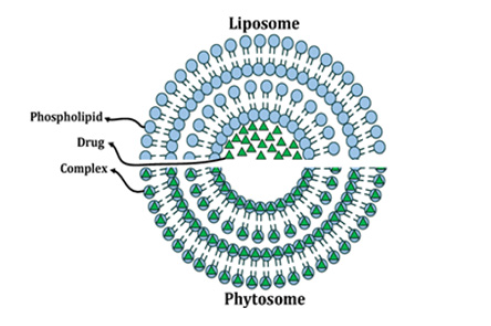
Phytosomes are not liposomes and structurally the two are very different (Figure 1). Unlike phytosomes, liposomes are formed by mixing a water-soluble substance with phosphatidylcholine. No chemical bonds are formed, and the phosphatidylcholine molecules surround the water-soluble substance. There may be hundreds or even thousands of phosphatidylcholine molecules surrounding the water-soluble compound.
Unlike the phytosome process, phosphatidyl-choline and the individual plant components are actually in a 1:1 or 2:1 ratio, depending on the substance. The liposomal drug complex is formed in the presence of water or a buffer solution, where the phytosomes are immobilized with a solvent of reduced dielectric constant.
Currently, world scientists have prepared phytosomes from plants such as curcumin, echinacea, ginkgo biloba, licorice, ginseng, silybin, grape seed, olive, green tea, ginger, and rose hips in the field of medicine and cosmetology. These phytosomes have anti-inflammatory, immunomodulatory, anti-aging, anti-cancer and antioxidant effects [17-30] (Figure 1).
Rutoside, which we have chosen as a research object for the treatment of periodontitis, as a result of the literature review, it was found that rutin prevents the accumulation of platelets, reduces capillary permeability, improves blood circulation, and has anti-inflammatory properties. It can be used in the treatment of routine hemorrhoids, varicose veins and microangiopathies. In relatively high doses, rutin increases the absorption of iodine by the thyroid gland, reduces the levels of T3 and T4 hormones. At the same time, it was determined that rutin is more powerful than quercetin, hesperidin and naringin in terms of antioxidant activity [31]. Taking into account the mentioned effective therapeutic properties of rutin, the main goal was to conduct research on the development of phytosomal gel as a transdermal drug delivery system in the treatment of periodontitis.
Material and Methods
Materials. Rutoside, which was used as a research object, was identified in laboratory conditions from the fruits of Japanese sophora, distributed in Azerbaijan. Phosphatidylcholine (soy lecithin) was obtained from Russia (TS 10.89.19.-029-13578491-2019; OOO Rostov Pharmaceutical factory). Research were carried out in the laboratories of the "Pharmaceutical technology", "Cytology, embryology and histology" and "Therapeutic dentistry" departments of the Azerbaijan Medical University, "Catalysis" and "Geology and Geophysics" Institutes of the Ministry of Science and Education of the Republic of Azerbaijan.
Preparation Technology<
The preparation of rutin phytosomes was carried out with different ratios of rutin and phosphatidylcholine.To do this, transfer the routine powder into a 150 ml flask and add acetone to it. Then phosphatidylcholine is added to a 250 ml flask and a solution of rutoside in acetone is added. The resulting mixture is heated at 65°C for 2 hours under reflux. The solution is concentrated until 10 ml of the original volume remains. After 2 hours, the flask is separated from the refrigerator, cooled to room temperature, and filtered. After evaporating the organic solvent for 1 day, the resulting phytosomes are dried in a desiccator.
Differential Scanning Calorimetry (DSC)<
Interactions in DSC can be observed by comparing transition temperatures, appearance of new peaks, disappearance of original peaks, melting points, and changes in relative peak area. Phyto-phospholipid complexes usually show radically different characteristic peaks than physical mixtures. The studied phytosome was a physical mixture of rutin and PX (1:1). DSCstudies of phytosome samples consisting of pure rutin and phosphatidylcholine-rutin mixture were conducted in a Jupiter STA 449 F3 differential scanning calorimeter manufactured by the German company NETZSCH by heating in closed metal pans in the temperature range of 100-750°C under a nitrogen gas environment at a speed of 20°C per minute.
Transmission Electron Microscopy (TEM) <
Samples were dispersed in distilled water and a drop was placed on a carbon coated copper square mesh grid (Electron Microscopy Science, USA). Nanoparticles examples were examined under the Transmission Electron Microscope JEM-1400 (JEOL, Japan) at a voltage of 80-120 kV. The morphometric analysis (Min, Max, mean SD, et al.) of the electronograms was carried out in TIF format via a computer program (TEM Imaging Platform- ITEM) developed by Olympus Soft Imaging Solutions GmbH (Germany).
Scanning Electron Microscopy (SEM) <
Rutin and our prepared phytosome sample were examined by JSM-6010RV scanning electric microscope manufactured by JEOL, Japan.
X-Ray Diffraction (XRD) <
Research were carried out with rutin and phytosome samples in Cu-Kα radiation at angles 50≤20≤750 with the D2 Phaser Automatic X-ray Diffractometer of the German "Bruker" company.
Fourier Transform Infrared Spectroscopy (FTIR) <
The analysis was performed on a Nicolet IS 10, Smart OMNI TRANS-Mission device. A comparative analysis of the standard and the sample was carried out. Studies were performed with pure rutin and phytosome separately (4000-500 cm1). The obtained IR spectra were analyzed according to functional groups.
Results and Discussion
Preparation of Phytosomes<
We prepared RN-P with rutin lecithin (phosphatidylcholine) in 3 different ratios (1:1-1:3). Of these, 2 formulations were pale yellow in color and lumpy, meaning they did not flow freely. Sticky masses were felt. Phytosomes had the best fluidity in the 1:1 formula. In this regard, further studies were conducted on phytosomes prepared in a 1:1 ratio. We used acetone as a solvent. Lecithin and rutin were mixed in a ratio of 1:1, acetone was added and extracted under refrigeration. The prepared phytosomes were able to maintain their stability for 2 years.
Differential Scanning Calorimetry <
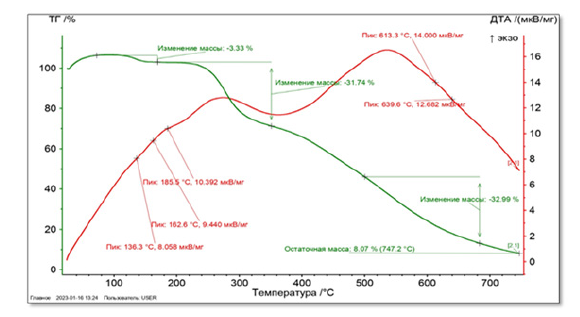
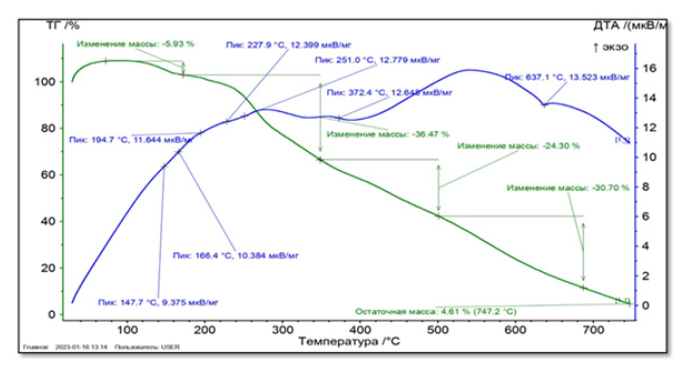
Sample weight (Phytosome IV) 5.4 mg. Starting from a temperature of 30°C, it is heated up to 750°C with a heating step of 20°C per minute. The atmosphere inside the oven is nitrogen gas. 4 main peaks were observed in this derivatogram. The first peak was observed with a minimum temperature of 147.7°C and a weight loss of 5.93% of the total mass. The second peak at 227.9°C with a weight loss of 36.47% of the total sample weight, the third peak at 372.4°C with a weight loss of 36.47%, and the fourth peak at 637.1°C with a weight loss of 30.70% has been done. As a result of the analysis, it was found that the residual mass is 4.61% of the total weight of the sample (Figures 2,3).
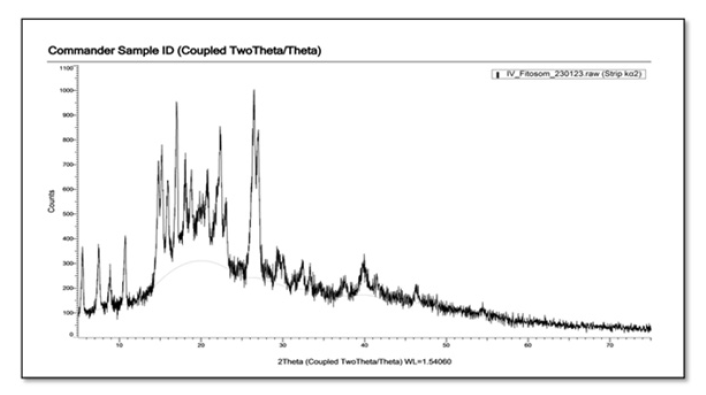
X-Ray Diffraction (XRD)<
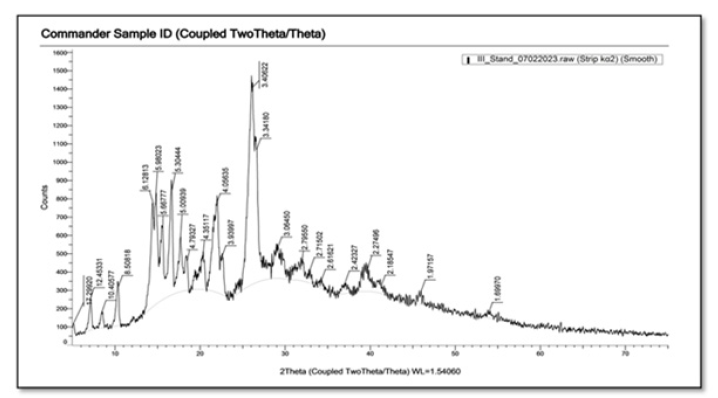
Currently, X-ray diffraction is an effective method for investigating the microstructure of both crystalline materials and some amorphous materials. X-ray diffraction is usually performed on either active components or active ingredient phyto-phospholipid complexes, PCs, and their physical mixtures. X-ray diffraction of the active ingredient and physical mixture shows dense crystalline peaks indicating a highly crystalline form. In contrast, phyto-phospholipid complexes with the active ingredient do not exhibit a crystalline peak, indicating that the components complexed with phospholipids are in molecular or amorphous form. This may explain the observation that phyto-phospholipid complexes have better lipophilicity and hydrophilicity than the active components [24]. XRD of RN shows intense crystalline peaks indicating high crystallinity of the drug (Figures 4,5). In our example, as a result of XDR analysis, it was observed that the phytosomes prepared by us have few crystal peaks. As can be seen from Figure 5, rutin phytosomes are distinguished by their high amorphousness.
Fourier Transform Infrared Spectroscopy (FT-IR)<
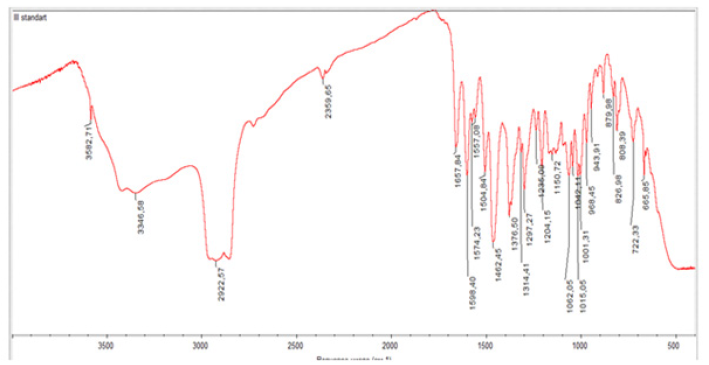
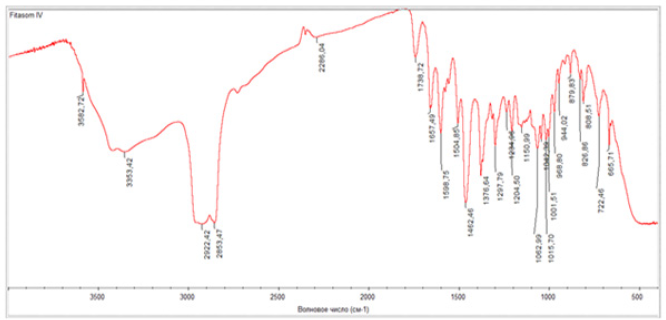
FT-IR is a powerful technique for structural analysis and yields a variety of functional groups that exhibit distinct characteristics in band number, position, shape, and intensity. The formation of phyto-phospholipid complexes can be verified by comparing the spectroscopy of phospholipid complexes with the spectroscopy of physical mixtures. Individual studies may show different results. The analysis was performed on a Nicolet IS 10, Smart OMNI TRANS-Mission device. A comparative analysis of the standard and the sample was carried out. As a result of the analysis, the IR spectrum of standard rutin is 1657 cm1 (C=0), 3582.71 cm1 and 3446.58 cm1 (OH), 2992.57 cm1 (-CH2), 1598.40, 1574.29, 1557.08 cm (aromatic ring), 1235, 1297.27, 1204.15 cm1 (C-O-C) absorption band (Picture 1), and IR spectra of Phytosome 1482.46; 1504.85 cm-1 1598.75; 1657.49 cm1 (aromatic ring), 1738.72 cm1 (-C=Ogroup), 2922.42-2853.47 cm1 (-CH2), 3582.72, 3353.42 cm1 (OH), 1297.79; It was formed in the absorption band of 1376.64 cm1 (C-O-C). (Figures 6,7).
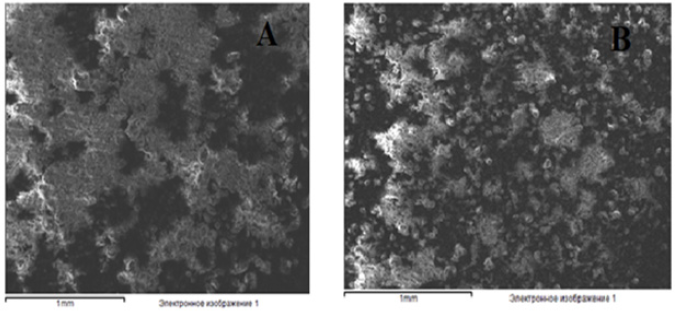
SEM Analysis<
While the high crystalline state of rutin was observed in image (A) taken with rutin during the analysis, in image (B) it was observed that rutin had already turned into phytosomes (Figure 8).
Transmission Electron Microscopy (TEM) <
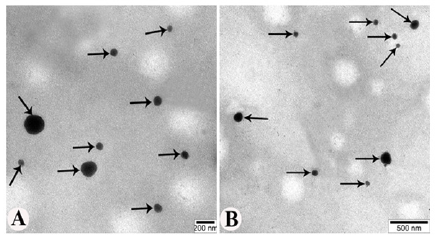
TEM studies reveal the formation of a dark discrete structure that appears as uneven spheres. When dispersed in water by gentle shaking, the phytosomes self-arranged in response to surface tension. As a result of the analysis of electrograms obtained from the large magnifications (40000-60000) of TEM, images of phytosomes up to the size of 60-180 nm (103±1,12 nm) were observed (Figure 9A and 9B).
Solubilization of Phytosomes<
For this purpose, the solubility of phytosomes was checked in weak alkaline environment (pH-6.7-7.4) and acidic environment (pH-1.5-1.8). As a result, it was observed that phytosomes are better dissolved in a weak alkaline environment, which provides the basis for ensuring the local effect of the drug in the oral cavity.
Productivity of Phytophospholipid Complexes (Degree of Complexation)<
The formula is as follows:
Yield(%)= [(a-b)/a]x100%
While “a” is the weight or composition of the primary active component, “b” denotes the weight or composition of the free active component, and “(a - b)” denotes the weight or composition of phospholipid complexes [22]. As a result of the calculations, the surface coverage of rutin with phosphatidylcholine was 99.83-100%.
Application of Phytosomal Gel in the Treatment of Periodontitis <
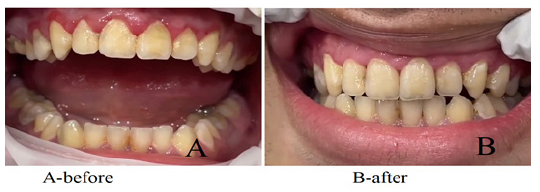
Patient F.A., 30 years old. clinic periodontal pocket. The PMA index (papillary-marginal-alveolar) was 19.4%, The Papillary Bleeding İndex (PBI) was 2.5 points, and the Periodontal İndex (PI) was 1.69 points. An application of phytosomal gel (experiment) in the amount of 0.5 ml was applied to the affected gum twice with an interval of 2 days. After 3 days, a decrease in gum inflammation was determined: PBI index - 0 points (no bleeding gums); PMA index - 0; PI - 0 points. The treatment period was 3-5 days.
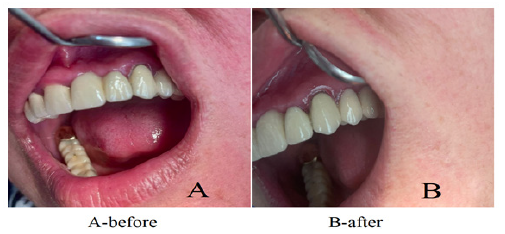
Patient N.Sh. 37 years. The indicators of the PMA index were 35.4%, the PBI index - 0.75 points, and the PI index - 1.67 points. An antimicrobial gel (metragil denta) in an amount of 0.5 ml was applied to the affected gum twice with an interval of 2 days. After 5 days, a decrease in gum inflammation was observed, a decrease in the PMA index was noted to 10.5%, the PBI index to 0.12 points, and PI to 0.15 points. The gel was reapplied. After 7 days, a persistent decrease in gum inflammation was determined (PMA index - 2%; PBI index - 0.05 points; PI index - 0 points).
Conslusions
Phytosomes are of interest to researchers as a widespread innovative delivery system in modern times. Coating the surface of many polyphenol compounds with soy and egg lecithin ensures their local, long-term effect. Since the absorption of rutin is difficult, the development of its transdermal form is considered one of the priority issues in pharmaceutical nanotechnology. Taking this into account, we developed a dental delivery system in our research. As a result of the conducted experiments, it was determined that rutin phytosomes have high bioavailability in the treatment of periodontitis and have an effective anti-inflammatory effect.
For this purpose, phytosomes based on rutin and lecithin were first prepared. X-Ray Diffraction (XRD), Fourier Transform Infrared Spectroscopy (FT-IR), Differential scanning calorimetry, SEM analysis, solubility test were performed to determine the vesicular size. TEM micrograph confirmed the structure of phytosomes (60-180 nm). Gel of rutin phytosomes is prepared on the basis of alginic acid and plantain polysaccharides. A gel made with rutin phytosomes was tested for better penetration. Phytosome gel has been found to be a good carrier for topical delivery of rutin. This fact has been proven once again when applied in the initial stage of periodontitis. It is considered appropriate to conduct extensive research in this direction.
Acknowledgments
None.
Conflict of Interest
None.
References
- Sanko Nguyen, Marianne Hiorth (2015) Advanced drug delivery systems for local treatment of the oral cavity. Ther Deliv 6(5): 595-608.
- Mehraliyeva Sevil Jabrayil (2023) Procedure for taking nanocapsules. Patent I 0075.
- Sevil Mehraliyeva (2023) Preparation of Nano Medicines from Deer Antones in Azerbaijan and Study of Quality Criteria. Biomed J Sci Tech Res 40379-40385.
- Sevil Mehraliyeva, Elmar Babayev (2022) Preparation of nanoemulsion from deer antlers (Cervus elaphus Sibiricus) fed in Azerbaijan and assessment of some quality indicators. Sci Collection «InterConf+» 20(105): 267-275.
- Sevil Mehraliyeva, Mahbuba Valiyeva, Nijat Abbaslı et al. (2021) Development of novel antibacterial gel using clove and calendula extracts with colloidal silver nanoparticles. Eurasian Chem Com 3.3: 170-179.
- Sevil Mehraliyeva, Nigar İsmayılova (2021) Devolopment of optimal phytoextract and niosom for acne vulgares treatment , determination of some quality indicators. Scientific Collection «InterConf» 50: 462-476.
- Sevil Mehraliyeva, Bahruz Mammadov, Mahbuba Valiyeva (2022) Preparation of nanoemulsion and nanogels from Hybiscus extracted by effective extraction, study of features. InterConf.
- Bahruz Mammadov Samad (2022) Study rheological properties of regenerating ointment made on the basis of chitosan. İnterConf 20(105).
- Evgeni Yaroslavovna Haruk. A remedy for the treatment of periodontal diseases and how to obtain it.
- Ismat S Ahmadov, Mahammadali A Ramazanov, Eldar K Gasimov, Fuad H Rzayev, Solmaz B Veliyeva (2020) The Migration Study of Nanoparticles from Soil to the Leaves of Plants. Biointerface Res Applied Chem 10(5): 6101-6111.
- Nargiz J. Agayeva, Fuad H. Rzayev, Eldar K.Gasimov, Chingiz A. Mamedov, Ismat S. Ahmadov, et al. (2020) Exposure of rainbow trout (Oncorhynchus mykiss) to magnetite (Fe3O4) nanoparticles in simplified food chain: Study on ultra structural characterization. Saudi J Biolo Sci 27(12): 3258-3266.
- Ilaha Hasanova, Eldar Gasimov, Fuad Rzayev, Elman Hajiyev, Goncha Eyvazova, et al. (2021) PEG-assisted controlled precipitation of calcium hydroxide and calcium carbonate nanostructures for cement reinforcement. Materials Chem Phy 271: 124865.
- Rzayev F.H, Gasimov E.K, Agayeva N.J, Manafov A.A, Mamedov C.A, et al. (2022) Microscopic characterization of bioaccumulated aluminium nanoparticles in simplified food chain of aquatic ecosystem. J King Saud Uni Sci 101666.
- Huseynzada A, Aghayev M, Hajiyeva S, Israyilova A, Sayin K, at al. (2023) Synthesis, nanostructuring and in silico studies of a new imine bond containing macroheterocycle as a promising PBP-2a non-β-lactam inhibitor. J Mater Chem B 11(34): 8271-8280.
- Nasirov,A, Rzayev F, Seyidli Y, Gasimov E, Bunyatova K, et al. (2024) The Effect of ZnO Nanoparticles to Paradilepis scolicina Rudolphi, 1819 (Cyclophyllidea: Dilepididae) Cestode Observed First in Common Carp (Cyprinus carpio L., 1758) in Azerbaijan. Egyptian J Veter Sci 55(1): 83-99.
- Aysel Hajiyeva, Chingiz Mamedov, Eldar Gasimov, Fuad Rzayev, Rovshan Khalilov, et al. (2023) Ultrastructural characteristics of the accumulation of iron nanoparticles in the intestine of Cyprinus carpio (Linnaeus, 1758) under aquaculture. Ecotoxicol Environ Saf 1(264): 115477.
- Kumar P, Yadav S, Agarwal A, Kumar N (2010) Phytosomes a novel phytophospholipid carriers: an overview. Int J Pharm Res Dev 2(6): 1-7.
- Zhilkina V Yu, Marakhova AI, Kezimana P, Blynskaya EV (2015) PHYTOSOMES ARE AN INNOVATIVE TECHNOLOGY FOR THE DELIVERY OF PLANT COMPONENTS. Uspekhi sovremennogo estestovoznaniya 11(1): 31-34.
- Mariana Deleanu, Laura Toma, Gabriela Maria Sanda, Teodora Barbălată, et al. (2023) Formulation of Phytosomes with Extracts of Ginger Rhizomes and Rosehips with Improved Bioavailability, Antioxidant and Anti-Inflammatory Effects In Vivo. Pharmaceutics 15(4): 1066.
- Jagruti Patel, Rakesh Patel, et al. (2008) An overview of phytosomes as an advanced herbal drug delivery system. Asian J Pharmaceutical Sci 4(6): 363-371.
- Nayyer Karimi, et al. (2015) Phytosome and liposome: the beneficial encapsulation systems in drug delivery and food application. Applied food biotechnology 2(3): 17-27.
- Lu Mei, Qiu Qiujun, Luo Xiang, Liu Xinrong, Sun Jing, rt al. (2019) Phyto-phospholipid complexes (phytosomes): A novel strategy to improve the bioavailability of active constituents. Asian J Pharm Sci 14(3): 265-274.
- Bhattacharya S (2009) Phytosomes: emerging strategy in delivery of herbal drugs and nutraceuticals. Pharma Times 41(3): 9-12.
- Das MK, Bhupen Kalita (2014) Design and Evaluation of Phyto-Phospholipid Complexes (Phytosomes) of Rutin for Transdermal Application. J Applied Pharmaceutical Sci 4(10): 051-057.
- Saharkhiz M, Ayadilord M, Emadian Razavi F, Naseri M (2022) Effects of phytosomal curcumin treatment on modulationof immunomodulatory and pulp regeneration genes in dental pulpmesenchymal stem cells. Odontology 110(2): 287-295.
- Ezlo B, Ripamonti V (1992) Pharmaceutical and cosmetic compositions containing complexes of flavanolignans with phospholipids. European Patent 0300282.
- Pawar HA, Bhagyashree Dilip Bhangale (2015) Phytosome as a novel biomedicine: a microencapsulated drug delivery system. J Bioanal Biomed 7(1): 6-12.
- Kalita B, Kalita B, Das MK (2015) Resveratrol-phospholipid complexes (phytosome) with improved physicochemical properties favorable for drug delivery via skin. World J Pharmaceutical Res 4(5): 1497-1517.
- Joshua J, et al. (2018) Formulation and evaluation of antiaging phytosomal gel. Asian J Pharmaceutical Clinl Res 11(3): 409.
- Burjwal G, Singh A, Jain SK, Jain NK (2023) Preparation & Characterization of Phytosome of Morinda citrifolia Extract. Chem Rese J 8(3): 15-22.
- Bahareh Abd Nikfarjam, et al. (2017) Treatment with Rutin - A Therapeutic Strategy for Neutrophil-Mediated Inflammatory and Autoimmune Diseases. J Pharmacopuncture. 20(1): 52-56.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.