Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Fully Automated Ultra-Personalized 3D Printed Prosthetic Breasts
*Corresponding author: Mario Gazziro, Federal University of ABC, Santo André, SP, Brazil
Received: April 16, 2024; Published: April 22, 2024
DOI: 10.34297/AJBSR.2024.22.002935
Abstract
There are significant scientific research showing the psychological, social and self-esteem impact on women, from the diagnosis of breast cancer to the complete removal of the breast, which makes it essential to seek beyond medical needs, aesthetic and social needs for the rehabilitation of postoperative mastectomy patients. This initiative is justified by the needs of patients and the absence of companies that offer breast prostheses which are personalized for each patient individually (ultra-personalized) using 3D technology, given the research and development challenges for their production. In the present work, software and protocols were successfully developed for acquiring and printing molds with the purpose of creating ultra-personalized external breast prostheses that meet the needs of women who underwent mastectomy and who were unable or unwilling to receive internal silicone prostheses, improving the quality of life in these patients.
Keywords: Mastectomy, Breast Implants, Additive Manufacturing, 3D Scanning, 3D printer
Abbreviations: CMOS: Complementary Metal-Oxide Semiconductor; GPU: Graphics Processing Unit; RGB-D: Red, Green, Blue and Depth
Introduction
Breast cancer is one of the most common human neoplasms, responsible for approximately a quarter of all cancers in women. According to a report from the World Health Organization, an estimated 2.3million diagnoses in 2020, which represents 11.7% of the total number of cases, breast cancer surpassed lung cancer. It has become the most common disease in the world [1]. For example, in Brazil, breast neoplasms were responsible for the highest number of new cases among women in 2020, reaching 29.7% (66,280 cases), of which 18,280 were only in the state of São Paulo (Brazil), according to the Brazilian Cancer Institute [2].
The use of external breast prostheses is a difference in the lives of women undergoing mastectomy. These artifacts help recover the body's natural shape after the surgery above. Furthermore, they help with balance, curvature of the spine, and reduction of neck pain, among other benefits. Additionally, there is significant scientific production showing the psychological, social, and self-esteem impact on women, from the diagnosis of the disease to the complete removal of the breast. Due to these factors, it is essential to seek to meet not only medical needs but also aesthetic and social needs for the rehabilitation of patients post-operatively after mastectomy [3-6].
In some countries, government or health insurance compensates for an external breast prosthesis and even its periodical replacement, given that it is considered assistive technology. Unfortunately, this does not happen in all of them, such as in Brazil, where the public system covers the surgical reconstruction procedure with the implant of the prosthesis. However, thousands of people are waiting for this surgery. Many patients will not even enter that line (because of comorbidities or chronic diseases, age, or fear of more aches than the rest of the treatment obliges to) or perhaps consider it later (when radiotherapy is needed first). For those who cannot or don’t want to receive an implant, the solution of an external prosthesis appears to be a resource for improving self-esteem [7].
However, a low rate of women who underwent mastectomy surgery had their breasts reconstructed, with many of them opting to use external breast prostheses [8] points out that this happens due to the difficulty of performing the surgery, the fear of not adapting to the internal prostheses or even the fear of adverse events.
For example, in Brazil, a country that doesn’t cover the expenses of external breast prostheses, some parameters emerge from the manufacture of breast prostheses, such as weight, shape, volume, materials, and colors. These are the most important features in the prostheses found in the Brazilian market, and this paper discusses prosthesis properties, parameters, and the notice that external breast prostheses are not compensated in Brazil [7].
It was verified that what is offered in Brazil is quite different from the available choices in the countries whose public healthcare system covers external breast prostheses. There are some industries who make products and some other offered by non-profit associations. The organizations are usually institutions created and supported by women who have breast cancer. However, the solution they found is a rudimentary handmade (Figure 1).
According to [7], the prostheses they make, one by one, are cut according to a pattern designed initially by another association of mastectomized women (Figure 1a). Sizes range from 36 to 52, only in pair numbers, because they used to correspond to the most common bra sizes in Brazil. Besides the pattern cut in foam, the weight guides the sizes (Figure 1b). A chart indicates how many grams each size should contain to provide the same weight as the corresponding breast would have. It is not a matter of volume. To get to the final volume, sometimes some more fabric is also added between the main filling, composed of lead balls (Figure 1c) glued with contact adhesive (Figure 1d) or polyethylene pellets. The result of the step-by-step shown in Figure 1 is depicted in (Figure 1e) lateral view.
Brazilian Researchers described in [9] used a method to acquire the 3D geometry of a healthy woman's left breast. The 3D modeling software Blender was used to make the prosthesis model and mold with adjustments to the model's mesh, such as smoothing, assigning thickness, and creating the walls of the prosthesis mold. Two counter-molds were created. The pieces were manufactured on a 3D printer using polylactic acid filament. Finally, the silicone was pigmented, and the mold was filled.
Then, in [9], a personalized prosthesis was developed through four phases. In the first phase, the 3D geometry of a healthy breast was acquired, processed, and modeled. In the second phase, an external breast prosthesis was digitally modeled using the 3D healthy breast image model. Subsequently, in the third phase, the prosthesis mold was manufactured using the FFF process of additive manufacturing. Within the last phase, the silicone was pigmented to fill the mold and generate the external breast prosthesis. However, all modeling is done manually, which differs from the research presented in this paper where the innovation is that this process is automated.
Clinical-quality 3D modeling of the breast surface assumes an increasingly important role in advancing treatment planning, prediction, and evaluation of breast cosmetics. However, existing 3D torso scanners are expensive, either infrastructure-heavy or subject to motion artifacts [9]. In addition to patients' needs, the absence of companies offering ultra-personalized breast prostheses personalized for each patient individually using 3D technology is notable, due to the research and development challenges for their production.
Figment Face, i-Med Tech, and Vectra 3D software are among the companies and software related to this project. Figment Face, a company created by former students of the Federal University of ABC (UFABC), is a startup that also uses 3D printing as a method of producing facial prosthetics, pre-surgical bio-models, and fetal replicas but does not operate in the production of breast prostheses. The acquisition of data relating to the measurement of body parts is done using computed tomography.
The South African company i-Med Tech 2 took the initiative to produce 1000 breast prostheses and donate them to 1000 mastectomized patients. The molds of these artifacts are produced in 3D modeling software, printed via additive manufacturing, and used to model the prosthesis with biocompatible silicone. Ultra-personalized prostheses produced by i-Med Tech have pre-established sizes, meaning patients need to adapt to pre-existing sizes.
The Vectra 3D software, shown in (Figure 2), was developed by Canfield Imaging Systems. This program performs a 3D simulation of the final result of plastic surgeries: breast augmentation or elevation, body contouring, chin augmentation, rhinoplasty, rhytidoplasty, and neck elevation. These soft tissue simulations are based on the point cloud resulting from scanning the patient's body and aim to help with communication between patient and doctor and in decision-making so that the result of the surgery is as assertive as possible (Figure 2).
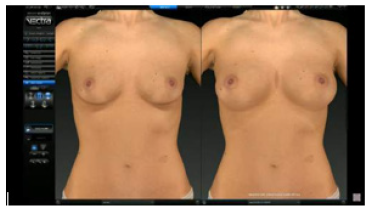
Figure 2: Screenshot of the Vectra3D software simulating the final result of breast augmentation surgery.
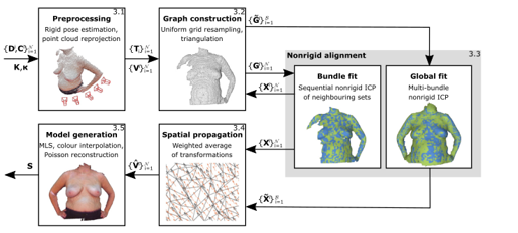
For example, [10] presented a system employing a single consumer-grade RGBD camera with an ICP-based registration approach to jointly align all points from a sequence of depth images non-rigidly. They proposed a markerless template-free nonrigid reconstruction method for accurate 3D breast surface modeling in breast cancer treatment planning and evaluation in the presence of postural sway (Figure 3). The method is based on combining the linear problem formulation for pairwise nonrigid alignment of the former with the notion of an embedded deformation graph from the latter (Figure 3).
It is believed that the hyper- or ultra-personalization of products and services (i.e., solving problems and demands in a particular and unique way) is one of the biggest challenges facing the productive and public sectors. The proposed solution consists of providing a service for the production of external and ultra-personalized breast implants for women who have undergone the mastectomy procedure. For example, the system proposed here differs from that already implemented by this company regarding data acquisition related to body part measurement, done with an RGB-D sensor (Red, Green, Blue - Depth). The difference between ultra-personalized prostheses by i-Med Tech and this project lies in personalizing the artifacts generated, which imitate the shape and weight of the removed breast. Regarding the system presented in [10], the system proposed here automatically crops this region after selecting the bust.
Therefore, our objective is to develop and automate a system for making molds for breast prostheses through breast scanning and 3D printing. Therefore, we hope to offer affordable, ultra-personalized external breast implants that meet the aesthetic and health needs of women.
Prostheses
According to the dictionary [11], a prosthesis is an artificial part that replaces an organ or part of the body with a severe deficiency, ensuring its respective functions or helping or improving a natural function.
In addition to functional prostheses, there are cosmetic prostheses, which improve the appearance of the amputated person and are also recommended in the case of deformities arising from disease or trauma, such as eye prostheses, prosthetic hands, fingers, breasts, feet, and toes [12-14]. Facial prosthetics and breast implants are the most common types of cosmetic prosthetics. Breast implants are designed for women who have had all or part of one or two breasts removed [12].
Breast Prostheses
External breast prostheses are an essential post-mastectomy product when reconstructive plastic surgery does not occur. The mastectomy prosthesis, also known as a breast prosthesis, is an artifact that mimics the artificial shape of this organ and is intended to give the patient the appearance and sensation of the removed or altered breast [15]. It is also capable of restoring pride in mastectomized women [16] and reducing depression caused by breast loss.
The prosthesis simulates the natural shape of the breast and offers many physical benefits, such as body balance [17], relief from uneven tension [18], and prevention of spinal curvature [19]. It also prevents shoulder pain [20] and chronic back and neck pain [21]. In addition to reconstructing the female body figure without additional surgery [19], breast prostheses improve body image and quality of life [22], restore mental health [18], and reduce the emotional suffering of patients who are not suitable for surgical breast reconstruction. In short, breast implants give mastectomized women a feeling of completeness. shows the types of breast implants (Figure 4).

Figure 4: Types of external breast prosthesis: a) Silicone prosthesis. b) Prosthesis made of other materials such as foam, rubber, fiber filling, cotton or beads. c) Attachable breast prosthesis. d) Prosthesis with soft post-surgical filling. e) Partial breast prosthesis.
Silicone prostheses have been widely commercialized in the breast prosthesis industry since the 1930s [13] due to their moldability, flexibility, durability, and ability to simulate an inert biological substance. The characteristics of silicone allow manufacturers and designers to develop different products in various shapes and sizes to meet the needs of patients [23]. Although silicone can be used for most prosthetics, this material can create thermal and sweating problems in hot weather [21]. Therefore, denture manufacturers have tried to improve the performance of silicone prostheses by using different technologies to solve these problems. Commercial and pre-molded breast implants are available in different forms to meet the needs of different patients in terms of shape, size, and the particularities of the exact location of the surgery. Their shapes can be classified into two main types: symmetrical and asymmetrical.
The symmetrical prosthesis can be used when the same shape fits on the right and left sides. It is generally made up of a breast-shaped front and a concave back. There are four different shapes of symmetrical prosthesis: triangular, oval, teardrop shape, and heart shape [19, 24, 25]. These formats can be seen in (Figure 5).

Figure 5: Breast prosthesis shapes: a) Triangular. b) Drop shape. c) Oval. d) Heart Shape. e) Asymmetric.
Asymmetrical prosthesis, shown in (Figure 2), fits only one of the breasts. Depending on the direction of the asymmetry of the prosthesis, it fits only one of the breasts and can fill a pervasive post-surgical removal area. The asymmetrical prosthesis is mainly indicated for patients undergoing radical mastectomy. It can be extended to the arm or upper chest region [15] to help balance all areas affected by the surgery.
3D Scanners
A 3D scanner is a device used for measuring elements of the physical world, creating virtual models that reproduce the shape and volume proportional to the element in the real world. To do this, such a device generates a cloud of points representing a position in 3D physical space (x, y, z coordinates) converted into a polygonal mesh that captures the geometry of physical objects.
Among the technologies used is the low-cost RGB-D sensors, which use CMOS (complementary metal-oxide semiconductor) technology to obtain information about colors and depth. The depth sensor captures the reflection of a known pattern emitted by an infrared light source. In a technique called triangulation, the sensor's internal image processor uses the relative position of the points present in the emission pattern to calculate the distance between the light plane, the camera, and the object plane.
Commercial RGB-D-based 3D scanners with this type of sensor use different techniques for acquisition, model reconstruction, and mesh processing. However, some of these devices do not create a model of the scanned person but use the information obtained to modify a generic standard model. Furthermore, until the advent of the multifunctional scanner developed in the laboratories of the Federal University of ABC (UFABC) [26], some of them could only automatically produce a user-colored mesh by manually editing the mesh to remove noise and unwanted parts, such as the floor.
This device [26] is composed of 2 RGB-D sensors working together with the ReconstructMe SDK software development kit [27] and the MeshLab software to perform postural analysis following the SAPO protocol (Postural Assessment Software).
3D Processing and Reconstruction
3D scanner systems obtain data in the form of point clouds. These equipment must work with other methods to align these clouds and use them to reconstruct a polygonal model known as a 3D mesh.
[28] surveys three-dimensional mesh reconstruction techniques. One of the most used is the Poisson reconstruction algorithm, proposed by [29]. Several software can be used to process polygonal meshes, one of which is widely recommended, the MeshLab software presented in [30]. This free software offers several advanced processing features, including the Poisson reconstruction algorithm, and is widely used due to its simplicity.
Anthropometric Measurements
An essential application of the 3D scanner is the analysis of anthropometric measurements, which can be used to personalize products such as clothing and footwear or create prosthetics. Traditionally, the dimensions of the human body are extracted manually using measuring tapes, but this process is time-consuming and requires direct contact with the object of study. To perform this type of analysis, the 3D model needs to undergo segmentation. The area of 3D mesh segmentation methods has been extensively studied since the beginning of this century. A review and analysis of existing methods can be found in [31].
However, these methods are often very complex and consume a lot of computational resources. The simpler segmentation approach [32, 33] may be more advantageous, considering that, for a medical examination, the segmentation method does not need to be invariant and will always be applied to human models.
3D Printers
3D printing creates a physical object from a three-dimensional digital model, typically stacking successive thin layers of some material to reproduce the digitally described object [34]. Here are some examples:
Fused Deposition Modeling (FDM) is among the most common and current printers on the market. It extrudes molten thermoplastic material by continuously depositing it line by line to form a 3D structure. The materials most used by these printers are ABS (acrylonitrile butadiene styrene) or PLA (polylactic acid) [35-37].
3D PolyJet is a type of printer that jets layers of a curable liquid photopolymer onto a mounting tray and uses instant ultraviolet (UV) curing on tiny droplets of the liquid photopolymer. The thin layers build up on the mounting tray to create a precise 3D model or part. With a process very similar to the precursors of 3D printers, it is stereolithography using concentrated UV rays that harden a resin, modeling it in three-dimensional form [36,34,38].
The Selective Laser Sintering (SLS) printer uses a high-power laser to fuse plastic, ceramics, glass powder, and titanium to form a 3D object. The laser selectively fuses material onto the surface of a layer of powder [39,36].
Another type of printer is the syringe extruder, which is based on a syringe connected to a robotic mechanism that can print any substance with a pasty texture. This printer prints food products such as chocolate, cheese, and cakes. A similar mechanism is also used for concrete printers, which use a concrete base and can print a house [39,36].
Finally, Bioprinters. 3D bioprinting is the automated digital fabrication of multicellular tissues through spatially defined cell deposition. The ability to spatially control deposition in the x, y, and z axes allows the creation of tissue-specific patterns or compartments with in vivo-like architecture that mimics critical aspects of native biology [40].
Even today, specific information related to 3D objects, such as color, texture, thickness, lines, and others, is stored in files with the STL extension, which refers to the original terminology, even though light is no longer part of the current deposition process. Layers. Many STL files are available on the market or can be created by appropriate software.
Materials and Methods
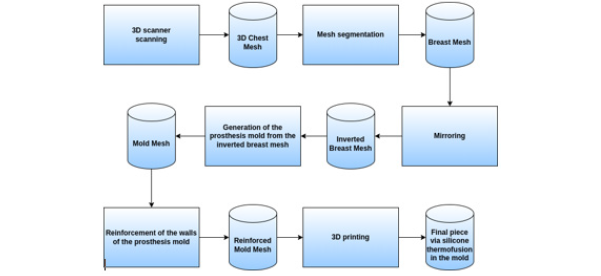
This project to create molds for manufacturing ultra-personalized breast prostheses follows the data flow shown in (Figure 6).
Digitization
The 3D scanner developed by the UFABC laboratory was used in previous projects in the postgraduate program in computer science, under the supervision of the co-supervisor of this work, to perform 3D acquisitions relating to the patients' chests. The equipment has a turntable, a vertical physical structure assembled with aluminum profiles, and two RGB-D sensors. The system structure design can be seen in (Figure 7).
The choice of two sensors for this equipment was based on the studies reviewed in the previous sections. A single sensor would require the patient to be too far away for their entire body to be scanned; otherwise, the sensor would have to be moved vertically to cover as much area as possible. This type of system is mechanically more complex and expensive. Using more sensors, however, increases the flow of data that the computer must manage, requiring a machine with better settings and, significantly, a better Graphics Processing Unit (GPU). Therefore, choosing two sensors represents the best cost-benefit ratio in the long term. The sensor is rotated 900 about its normal working position to reduce the required distance and cover a larger scanning area due to its more significant field of view in this orientation.
The center of the turntable is 1.10 meters from the vertical column with the sensors and remains motionless until the user starts the scanning process. When the process begins, the computer sends a signal to the microcontroller to activate its relay module, thus allowing power supply and operation of the turntable, which gently rotates the user 3600 for about 30 seconds while the sensors acquire depth and color information.
The scanning software is built using the ReconstructMe SDK software development kit and manages data from both sensors simultaneously, creating a single 3D model at the end of the process. However, this model contains open holes and unwanted artifacts that must be addressed.
Mesh Processing
Mesh processing uses scripts designed to run in the MeshLab software [30], automating the process. Two main steps must be performed: removing defects or unwanted parts, such as walls and floors, and rebuilding the model. In this project, only the floor will need to be removed, as the environment in which the scanning is carried out is large, and the walls are not captured by the sensors.
To remove unwanted parts from the mesh using the MeshLab software, they must be disconnected from the central part. For this removal to be allowed, the first step is to create holes in this region so that they can be considered separate objects. The easiest way to perform this step is to eliminate mesh polygons below a height threshold and with approximately vertical orientation. With this preprocessing done and the ground disconnected from the body, we can use MeshLab filters and functions to eliminate the rest of the unwanted sections. The following Cleaning and Repairing category filters can be used: Remove Duplicated Faces, Remove Duplicated Vertices, Remove Zero Area Faces, Remove Unreferenced Vertices, and Remove Isolated Pieces.
The model reconstruction uses the Poisson reconstruction function [29] present in the Remeshing, Simplification, and Reconstruction category of MeshLab filters. As an input value, defining the reconstruction resolution by modifying the Octree Depth parameter is necessary. As this parameter increases, higher resolution functions are used for reconstruction, capturing finer details. However, this increases processing time. The value of 10 showed a good mesh quality. After reconstructing the model, the result is a closed mesh (without holes) but without color. Another necessary step is the colorization of the new mesh, in which the colors associated with the vertices before reconstruction are transferred to the vertex color matrix after reconstruction using the Vertex Attribute Transfer filter from the MeshLab Sampling category.
Breast Segmentation and Mirroring
The next step will be to carry out the breast segmentation and mirroring process, taking the point cloud resulting from the scan as input. Both procedures are illustrated in the infographic in (Figure 8).
Segmentation automatically separates the data collected relating to the breast from other body parts. As can be seen in (Figure 8), data were obtained regarding the shape of the volunteer's chest as a whole, not just the breast, making it necessary to separate only the data that will be used in creating the prosthesis. This separation will be done through automatic analysis of the concavity of the mesh. Mirroring consists of adjusting the point cloud of the breast so that it reproduces the shape of the breast that was removed. This procedure only uses linear transformations applied to the mesh points.
Subtraction Operation using Vedo
With the data cloud of the isolated and mirrored breast, the next step consists of creating the 3D model of the mold (Figure 9), which will model the silicone rubber and later become the prosthesis (Figure 9).
This procedure will be done using the Vedo 3D data manipulation library, specifically the Vedo module that performs the Boolean operation of subtracting the mesh from a solid block, creating a negative 3D model of the breast, which will be used as a form for the breast prosthesis.
3D Printing and Generation of the Final Part via Thermodiffusion
Once the 3D model of the prosthesis mold has been finalized, the next step consists of sending this data to an FDM 3D printer model Ender 3 (Figure 10) available in the laboratories of the Center for Engineering, Modeling, and Applied Social Sciences at UFABC (CECS -UFABC), which uses the thermoplastic filament PLA (Polylactic Acid), which was chosen for its low cost and easy access (Figure 10).

Figure 10: The 3D printer model Ender 3 is available at the Center for Engineering, Modeling, and Applied Social Sciences at the Federal University of ABC.
Once printing is complete, the mold, thermo-moldable rubber (Figure 11), and cure must be prepared (Figure 11).
These procedures are described in the results section, referring to software prototyping. At the end of this step, the prosthesis will be ready to be placed in the mastectomy bra.
Results and Discussion
This section presents and discusses the main results obtained, specifically the prototype for creating the prosthesis mold, software development, and future improvements.
Prototype
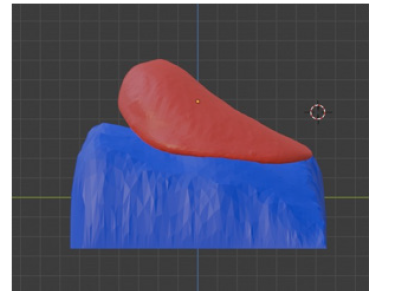
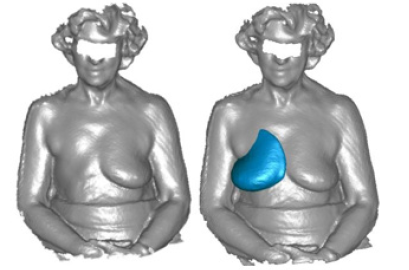
After the planning phase, the prototyping phase of creating the breast prosthesis mold is established to validate the proposed methodology. However, there is still no automation regarding breast isolation and mirroring, which are products of the software development phase. As described in the Materials and Methods section, the volunteer's chest was scanned with the 3D scanner in the UFABC laboratory, obtaining the 3D mesh of the upper front part of her body, shown in (Figures 12a and 12b), which shows the isolation of the mesh (Figure 12).
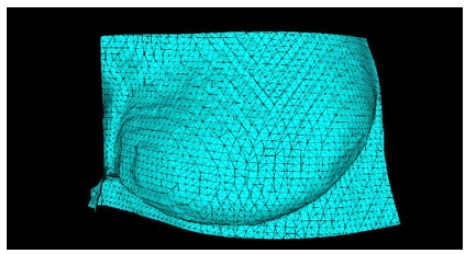
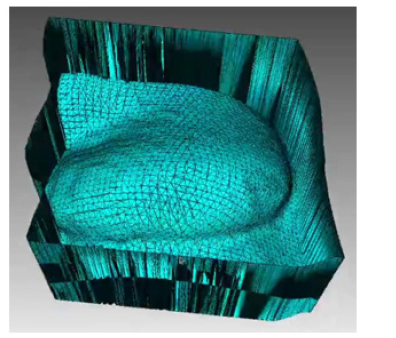
In this prototyping phase, the open-source 3D modeling software Blender [41] is used to isolate and mirror the breast mesh instead of the software to be developed. The result of this procedure can be seen in (Figure 13), which shows the healthy breast mesh isolated and mirrored using this software, in (Figure 14) a color view of a 3D model of the breast created, and in (Figure 15), which shows the breast prosthesis mold sculpted with the help of this program (Figures 13-15).
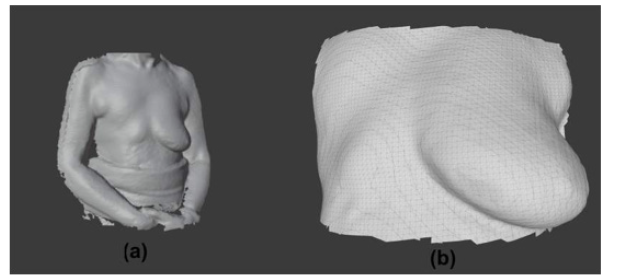
Figure 12: Meshes from scanning the volunteer’s body: (a) Complete 3D mesh generated by the scanner and (b) isolation of the chest mesh in the region close to the breasts.
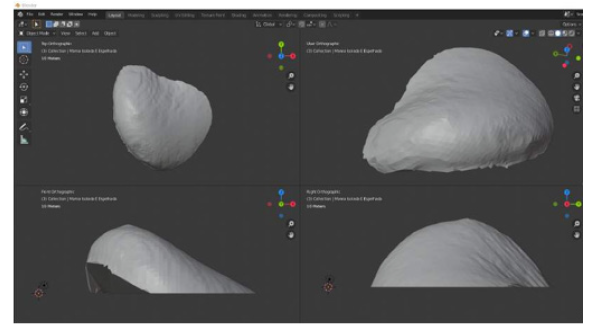
Figure 13: Views of the 3D model of the healthy breast displayed by Blender software: a) Top orthogonal view: b) Camera view: c) Front orthogonal view: and d) Right side orthogonal view.
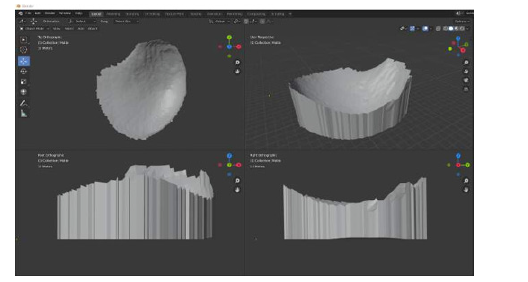
Figure 15: Views of the 3D model of the breast prosthesis mold to be printed: a) Top orthogonal view: b) Camera view: c) Front orthogonal view: and d) Right side orthogonal view.
After this step, the printing procedure began using a 3D printer model Ender 3 using 1.75mm PLA filament. We chose this printer due to its good printing results and low cost. After the impression is finished, the manual finishing and preparation of the prosthesis mold begins. Then, the residue from the sanding is cleaned with a damp cloth and, shortly after, a dry cloth. A thin layer of silicone rubber catalyst is then applied to the inside of the mold (Figure 11), which serves as a release agent; that is, it helps the silicone not to stick to the mold and smooth out some of its irregularities. With this, the mold is ready to receive the silicone rubber.
First, it is necessary to measure how many grams of rubber fit in the mold. In this case, the value was 255g. The weight of the catalyst to be used is then calculated, which must be 4% of the weight of the silicone rubber. In this case, the value was 10.2g. Apply the silicone rubber and catalyst mixture to the mold,and wait 6 hours until the rubber cures completely. Once this material has cured, it will be ready to be placed in the mastectomy bra (Figure 16) shows the finished prosthesis (Figure 16).
Software
The Python language was chosen for developing this software due to its versatility and scientific analysis. This language's 3D object visualization module, called Vedo, was responsible for showing and modifying meshes. Together, the Pygmsh module [42] was also used, as well as a 3D mesh generation module using finite elements, which is responsible for the solid meshes used to increase the thickness of the mold walls. The source code for this software will be detailed below.
Initially, the software loads the mesh in the PLY (Polygon File Format) file into memory. This file is the product of scanning the upper front part of the volunteer's body using the RGB-D sensor. Such loading is done through the Mesh method of the Vedo module. Once this is done, the program opens a window showing the newly loaded mesh, as seen in (Figures 12 and 13), whose visualization is generated by the Show method of this same module (Figure 17).
Next, the software invokes the addCutterTool method from the Vedo module, which isolates the mesh from the chest region containing the breasts. A parallelepiped is then presented in the same previously opened window, whose function is to select the part of the mesh to be segmented. Along with this geometric shape, six white spheres are also presented, which are positioned on each face of the parallelepiped, and an additional one is positioned in the center, as shown in (Figure 18a). These spheres are manipulators for resizing the parallelepiped so that they change their shape when moved by the user with the mouse cursor.
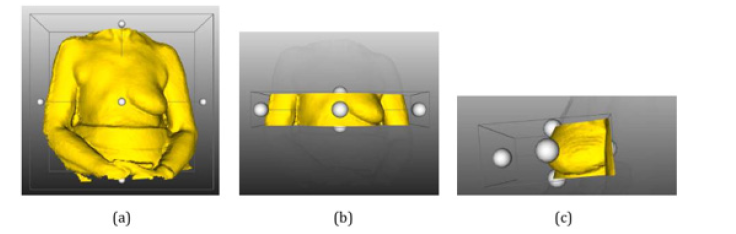
Figure 18: Detail the segmentation process of the chest mesh around the breasts: a) Add the Cutter Tool tool immediately after it is started. b) Parallelepiped of the add CutterTooltool after resizing on the z-axis. c) Resized and rotated parallelepiped, selecting the region of the chest close to the breasts.
Then, the user must use them to modify the parallelepiped so that it only wraps the chest mesh around the breasts. Such parallelepiped resizing can be seen in (Figure 18b), in which this geometric solid's dimension changed only on the z-axis. In addition to resizing, the user can rotate the mesh and geometric solid around the central white sphere, making the cut more precise and improving its final appearance. In (Figure 18c), the final aspect of the prism above can be seen so that a precise cut can be made of the chest in the breast region (Figure 18).
Once the mesh selection is complete, the user must press the “x” key on the keyboard and close the window the software opens to perform the cutting. Once this is done, it will start post-processing the cut region, which consists of using and defining the following methods and parameters of the Vedo module: fill holes, search for holes and repair them; Clean method, searches for and removes duplicate points; parameter law (line width), defines the line thickness of the resulting mesh and parameter c (color), which defines its color. Once the chest mesh has been processed, it will be displayed in a new window, as shown in (Figure 19).
Once the chest mesh is around the breasts, the next step is to isolate the mesh relative to the healthy breast. For this, the software first makes a copy of the chest mesh using the clone method and mirrors it in relation to the x-axis using the mirror method, both of which belong to the Vedo module. After this step, the cloned mesh will be cut using the cutWithPlane method so that the resulting mesh only represents the healthy breast.
This method requires two parameters: the cutting plane's origin coordinates and the average plane's direction, which defines which part of the mesh will be maintained. Thus, the x coordinate corresponding to half of the mesh is calculated and passed on as the origin of the cutting plane. Normal is defined as (-1,0,0) so that the part of the mesh relative to the healthy breast is maintained. As a final adjustment in this step, the resulting mesh will be rotated at an angle of 45° around the y-axis so that the mesh is parallel to the xy plane. The result of this processing can be seen in (Figure 20).
Once the breast mesh has been isolated, the following process consists of constructing the mesh for the prosthesis mold. For this, it is necessary to have the breast mesh wrap and, from there, generate the walls of the mold. For this purpose, the Vedo method called boundaries is used, which generates a mesh corresponding to the contour of another provided as a parameter. Thus, from this envelope, extrusion is made in the negative direction of the z-axis, whose height is defined by the software adjustment variable called “extrusionHeight,” which is predefined with a value of 100mm.
The next step is to fuse the mesh of the mold walls with that of the already isolated healthy breast. This procedure is done using the merge method of the Vedo module. After this, it is necessary to trim the excess walls at the bottom of the resulting mesh using the cut With Planes method, not modifying the part of the breast mesh. For this, a cutting plane was configured with an origin defined by the variable “moldBaseHeight” as a safety margin, subtracted from the smallest z coordinate of the points of this mesh. The direction of the normal is pointed in the negative direction of the z-axis. The mesh resulting from this processing can be seen in (Figure 21).
As seen in the figure referenced above, the walls of the mold mesh have become thin, resulting in a fragile and complex mold for an additive manufacturing process. An algorithm is then added to this software to increase the thickness of the mold walls using the Pygmsh module, which generates solid 3D meshes using the finite element method. For this, a rectangle is defined parallel to the XY plane, with a coordinate equal to the smallest coordinate of the mold mesh points and with the same centroid as this so that its edges are distanced from the mold walls by an amount equal to the variable “wallThicKness,” which controls the thickness of the generated walls.
After this, the extrusion of this rectangle is defined, with the height defined by the sum of the z-dimension of the mold with the variable that controls the thickness, subtracted from the “safety margin” variable. Finally, it is necessary to define the minimum and maximum sizes of the generated polygons, knowing that the smaller the polygons, the greater the computational complexity to generate them, as well as the size of the resulting files. For computers with up to 8GB of RAM (Random Access Memory), it is suggested that the minimum size of polygons is not smaller than 0.1 and that the maximum size is not smaller than 1.
The following algorithm command calls the generate_mesh method, which generates the solid mesh itself, resulting in a parallelepiped of dimensions
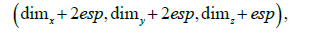
where  are the mold dimensions in each direction, and esp is the wall thickness.
are the mold dimensions in each direction, and esp is the wall thickness.
Before carrying out the subsequent processing, it is necessary to change the variable's object type that stores the mesh, converting it from meshio mesh object to vtkFollower [43]. To do this, this solid is broken down into its constituent components: lines, triangles, tetrahedrons, and vertices, and stored separately in different variables. Then, the TetMesh method from the Vedo module is used to recreate it, which only uses data relating to points and tetrahedra.
Moreover, the tomesh method ensures no holes in the mesh. Once the block is obtained, creating a mold for the prosthesis with reinforced walls is possible. For this, a subtraction operation is carried out between the block and the prosthesis mold with thin walls using the cutWithMesh command in the Vedo module. Moreover, for the mold to have the part of the opening that will receive the material to be molded, it is necessary to rotate it 1800 around the x-axis. The final mold of the breast prosthesis can be seen in (Figure 22) on the left.
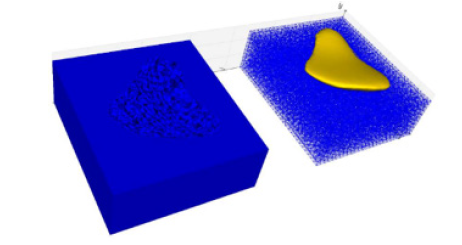
Figure 22: Finalization of the breast prosthesis mold: On the left is the solid mesh subtracted from the thin-walled prosthesis mold mesh. On the right is a preview of how the mold will accommodate the prosthesis.
As future improvements to the software, it is planned to modify the configuration of the breast prosthesis mold generated by the software so that it is composed of two parts that fit together, are approximately symmetrical, and are divided parallel to the xz-axis. Additionally, this mold would have two holes at the top: one where the silicone rubber would be placed, and the other would serve as an air outlet. A preview of the mold modeled by the Blender software, which was also based on the volunteer's healthy breast and chest, can be seen in (Figure 23a and Figure 23b) (Figure 23).
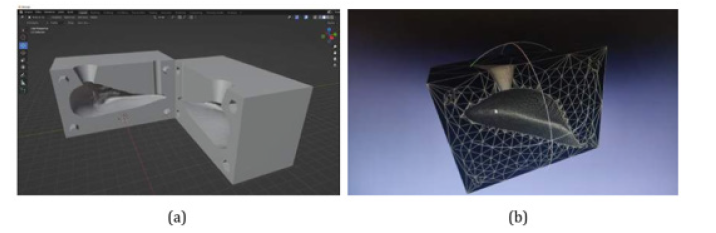
Figure 23: A new mold configuration will be developed in future software versions: a) The two mold parts are opened in the Blender software. b) Provide Details of one of the mold halves in the Meshlab software.
Compared to the current one, the advantages of this new mold are greater practicality, as there will be no need to worry about the mold lid, as the shape of the chest is already included in the bottom part of the mold; that is, it is a closed mold. Another advantage would be the non-formation of burrs on the prosthesis since, in the current process, it is still necessary to remove them manually at the end.
It was also decided to print from a new mold generated manually to validate the new format. Such a printed molds can be seen in (Figures 24a and 24b) (Figure 24).
Conclusion
This paper presented one software and protocols associated with the 3D printing of molds used for modeling ultra-personalized external breast prostheses, that is, that attempt to reproduce breasts that were removed during mastectomy surgery, meeting the needs of women who have been undergoing this type of surgery and who were unable or unwilling to receive internal silicone prostheses, and improving the quality of life of these patients.
For this purpose, software was developed that received as input a mesh resulting from a 3D scanning process carried out by a 3D scanner developed by the Federal University of ABC. This developed program automatically isolates the mesh relative to the patient's remaining breast, mirrors it, and subtracts it from a solid block, generating a mold ready to be materialized in PLA via additive manufacturing.
As proposed improvements, we saw the need to change the final configuration of the breast prosthesis mold generated by the software so that it is composed of two parts that fit together, are approximatel symmetrical, and divided parallel to the xz axis, also adding a hole for insertion of silicone rubber and another for air exit.
Acknowledgements
The authors thank Julino Soares.
Conflict of interest
The authors declare no conflict of interest.
References
- Oms Globocan (2019) Estimated cancer incidence, mortality and prevalence worldwide in 2020.
- Inca Brasil (2020) Ministério da Saú Instituto Nacional do Câncer. Estatı́sticas do câncer no Brasil.
- Inocenti A (2016) Impact of the effects of the reconstructive surgery in the life of women with breast cancer. Texto & Contexto-Enfermagem SciELO Brasil 25(2).
- Pereira G (2017) Impacto do tratamento do câncer de mama na autoimagem e nos relacionamentos afetivos de mulheres mastectomizadas. Life Style 4(1): 99-118.
- Gislaine Ferreira, Keyla Dourado, Anne Jacob, Tânia Christiane, Gleide Regina, et al. (2018) Mulheres submetidas á mastectomia: aspectos sentimentais e emocionais. Revista Enfermagem Contemporânea 7(1): 72-80.
- Monteiro Crav, Paiva Arb (2018) Qualidade de vida em mulheres mastectomizadas. Revista de Investigação Biomédica 10(1): 30-37.
- Branco LR, Foggiatto JA (2018) External Breast Prostheses: Brazilian Performance Parameters. Advances in Ergonomics in Design 588: 498-505.
- Howard J (2019) Worldwide recall issued for textured breast implants tied to rare cancer.
- Juliane Cristina Leme, Rodrigo Martins de Oliveira Spinosa, Sandy Oliveira Leal, Anna Beatriz Barreto Hirsch, Angélica Lodovico, et al. (2023) Development of low-cost and personalized external silicone breast prosthesis produced by additive manufacturing for women who have undergone mastectomy: A pilot study. Clinical Biomechanics 110.
- RM Lacher, F Vasconcelos, NR Williams, G Rindermann, J Hipwell, et al. (2019) Nonrigid reconstruction of 3D breast surfaces with a low-cost RGBD camera for surgical planning and aesthetic evaluation. Medical Image Analysis 53: 11-25.
- Michaelis (2020) Moderno Dicionário da Lı́ngua Portuguesa.
- Ams (2013) American Cancer Society. Prostheses.
- Disabled W (2015) Prostheses-Prosthetics: Artificial Limb Information.
- Bailey A (2015) What are the different types of prosthetics.
- Yip CHB (2009) reast cancer in asia. Cancer Epidemiology 51-64.
- Gardner KE (2000) Hiding the scars: A history of post-mastectomy breast prostheses, 1945–2000. Enterprise & Society Cambridge University Press 1(3): 565-590.
- Reaby LL (1998) Reasons why women who have mastectomy decide to have or not to have breast reconstruction. Plastic and reconstructive surgery 101(7): 1810-1818.
- Ching S (2010) Coping of chinese women with breast cancer: psychological adjustment through reframing.
- Acs (2020) American Cancer Society. Mastectomy Products.
- (2010) Breast cancer facts in Hong Kong report 2.
- P Gallagher, A Buckmaster, S O'Carroll, G Kiernan, J Geraghty, et al. (2010) External breast prostheses in post-mastectomy care: women’s qualitative accounts. European journal of cancer care 19(1): 61-71.
- Young Ran Chae, Kumin Seo (2010) Health-related quality of life in women with breast cancer in korea: do sociodemographic characteristics and time since diagnosis make a difference? Oncology nursing forum 37(4).
- Susan Roberts, Patricia Livingston, Victoria White, Anne Gibbs (2003) External breast prosthesis use: Experiences and views of women with breast cancer, breast care nurses, and prosthesis fitters. Cancer nursing 26(3): 179-186.
- Anita (2020) Breast Care.
- Jodee (2020) Breast Forms.
- Gazziro M (2019) Multi-sensor 3d scanner for postural analysis. Porto Alegre RS Brasil: SBC 10-21.
- Heindl C, Bauer H, Ankerl M, Pichler A (2015) Reconstructme sdk: a c api for real-time 3d scanning. 6th International Conference and Exhibition on 3D Body Scanning Technologies pp.27-28.
- Berger Matthew, Tagliasacchi Andrea, Seversky Lee, Alliez Pierre, Levine Joshua, et al (2014) State of the art in surface reconstruction from point clouds. EUROGRAPHICS start reports 1(1): 161-185.
- Michael Kazhdan, Matthew Bolitho, Hugues Hopp (2006) Poisson surface reconstruction. Proceedings of the Fourth Eurographics Symposium on Geometry Processing 7.
- Cignoni P, Corsini M, Ranzuglia G (2008) Meshlab: An open source 3d mesh processing system. Ercim news 73(45-46): 129-136.
- Xiaobai Chen, Aleksey Golovinskiy, Thomas Funkhouser (2009) A benchmark for 3d mesh segmentation. ACM Transactions on Graphics 28(3): 1-12.
- Zhong Y, Xu B (2006) Automatic segmenting and measurement on scanned human body. International Journal of Clothing Science and Technology 18(1): 19-30.
- Han H, Nam Y (2011) Automatic body landmark identification for various body figures. International Journal of Industrial Ergonomics 41(6): 592-606.
- (2017) 3d Printing Industry. The Free Beginner’s Guide.
- B Utiyama, C Hernandes, T Senra, M Gospos, J Leme, et al. (2014) Construção de biomodelos por impressão 3D para uso na prática clínica: Experiência do instituto dante pazzanese de cardiologia. XXIV Congresso Brasileiro de Engenharia Biomédica 316-319.
- Carl Schubert, Mark C van Langeveld, Larry A Donoso (2014) Innovations in 3d printing: a 3d overview from optics to organs. British Journal of Ophthalmology 98(2): 159-161.
- Berman B (2012) 3-d printing: The new industrial revolution. Business horizons 55(2): 155-162.
- Prince JD (2014) 3d printing: an industrial revolution. Journal of electronic resources in medical libraries 11(1): 39-45.
- (2020) Stratasys Direct Manufaturing. Laser Sintering Materials.
- Organovo (2020) 3D Bioprinted Human Tissue.
- Community BO (2018) Blender - a 3D modelling and rendering package.
- Schlömer N (2022) pygmsh: A Python frontend for Gmsh. Zenodo.
- Schroeder W, Martin KM, Lorensen WE (1998) The visualization toolkit an object-oriented approach to 3D graphics. Prentice-Hall Inc.











 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.