Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
In Situ Hydration of CO2, Solvated in the Seawater; A Different, Efficient, and Ecofriendly Way to Solve the Big CO2 Challenge
*Corresponding author: Abdolkaliq Almatari, Sixth-year medical student, Faculty of medicine Umm Al-Qura University.
Received: June 10, 2024; Published: June 13, 2024
DOI: 10.34297/AJBSR.2024.22.003024
Abstract
Carbon dioxide (O=C=O) appears as a colorless odorless gas at atmospheric temperatures and pressures. Relatively nontoxic and noncombustible. Heavier than air and may asphyxiate by the displacement of air. Soluble in water. Forms carbonic acid, a mild acid. Carbon dioxide, solid appears as an odorless, white solid. Can cause damaging frostbite.
Keywords: Acidification, Carbon dioxide, Carbohydrates, Hydrogen, Glucose, Oxygen, Water acidification, Water dissociation.
Background
Carbon dioxide is commonly used as an insufflation gas for minimal invasive surgery (laparoscopy, endoscopy, and arthroscopy) to enlarge and stabilize body cavities to provide better visibility of the surgical area. It has been used also in cryotherapy and as respiratory stimulant before and after anesthesia. It could be used also in expansion of blood vessels if required, to increase carbon dioxide level after rapid breathing, and to stimulate breathing after a period of nonbreathing.
CO2 can be used to flood the surgical field during cardiac surgery. Because of its density, carbon dioxide displaces the air surrounding the open heart so that any gas bubbles trapped in the heart are carbon dioxide rather than insoluble nitrogen. Similarly, CO2 is used to de-bubble cardiopulmonary bypass and Extracorporeal Membrane Oxygenation (ECMO) circuits. It is used to adjust pH during cardiopulmonary bypass procedures when a patient is cooled [1].
Carbon dioxide is excreted by the lungs in gas form, and, in the form of bicarbonate ion, by the kidney, intestine and skin [2]. Carbon dioxide is transported in the blood in diverse forms: dissolved in the plasma, or linked to proteins independently of the PCO2. Carbone dioxide is transported by the hemoglobin back to the lungs, where it is exhaled.
Carbon dioxide is produced by metabolism at approximately the same rate as O2 is consumed. At rest, this value is about 3mL/kg per minute, but it may increase dramatically with heavy exercise. Carbon dioxide diffuses readily from the cells into the blood, where it is carried partly as bicarbonate ion (HCO3-), partly in chemical combination with hemoglobin and plasma proteins, and partly in solution at a partial pressure of about 6 kPa (46 mmHg) in mixed venous blood. CO2 is transported to the lung, where it is normally exhaled at the rate it is produced, leaving a partial pressure of about 5.2 kPa (40mmHg) in the alveoli and in arterial blood.
Carbon dioxide can be absorbed (or excreted) by the skin. About 2.7% of the carbon dioxide produced is excreted by the skin [3]. Carbon dioxide is produced by the body's metabolism and is always present in the body at about 6% concentration. An average adult human will produce more than 500g of carbon dioxide daily under resting conditions and will produce much more when active. Additional carbon dioxide has several effects on the body, and responses are immediate. It stimulates breathing, which exhales the carbon dioxide carried to the lungs from the cells by the bloodstream. An increase in carbon dioxide concentration stimulates the heart rate, increases the blood pressure, increases adrenalin flow, and relaxes the vascular smooth muscles. In addition, carbon dioxide reacts with water in the body to form carbonic acid, which dissociates to hydrogen ion and bicarbonate. An increase in carbon dioxide in the body increases acidity, and then the kidneys act to restore normal acidity [4].
CO2 is used in carbonated beverages, fire extinguishers, dry ice, and propellants. A product of fermentation; [ACGIH] A product of animal metabolism and released when organic materials burn; a constituent of the earth's atmosphere at about 0.03% by volume; is also used in the deliming stage of leather production [5].
Carbon dioxide is used as a pesticide for insect control in stored grain under modified atmospheres containing approx. 60% carbon dioxide [6]. Carbon dioxide is used by the food industry, the oil industry, and the chemical industry. It is used in many consumer products that require pressurized gas. Life jackets often contain canisters of pressured carbon dioxide for quick inflation. Aluminum capsules are also sold as supplies of compressed gas for air guns, paintball markers, for inflating bicycle tires, and for making seltzer.
CO2 is used also as refrigerant; in processing of foods; preserving foods; crusting of food; cryogenic freezing of food; production of urea, sodium carbonate (Solvay process), methanol, carbonic acid, lead carbonate, potassium carbonate, potassium bicarbonate, ammonium carbonate, ammonium bicarbonate, sodium salicylate, carbonated petroleum, hydrocarbon products; provides an inert atmosphere for fire extinguishers, refinery products, petroleum products; displacing oxygen to prevent deterioration and flavor loss; in high pressure applications; oil well stimulation; in livestock slaughtering; as fertilizer; hardening of molds for metal castings.
Much of the carbon dioxide generated in the world is a byproduct of ammonia and hydrogen production, which make much more carbon dioxide than is ever recovered. Other sources are still exploited, but these are generally less efficient, and financially less attractive [7].
In the production of ammonia/ desulfurization of the hydrocarbon feedstock (e.g., natural gas, naphtha) is carried out before catalytic steam reforming of the hydrocarbon to give a gaseous mixture of hydrogen, carbon dioxide, and carbon monoxide. Air is added and further steam reforming is affected in a gaseous mixture that then also contains nitrogen. Because only hydrogen and nitrogen are required to make ammonia, the carbon oxide is removed from the gas stream. Most of the carbon monoxide is catalytically converted to carbon dioxide, and the latter is removed by dissolution under pressure.
Large quantities of carbon dioxide are generated by fermentation processes, and up to 80% of this gas may be recoverable. Before being suitable for further use, however, the carbon dioxide must be freed of the impurities inherent in this method of manufacture, i.e., hydrogen sulfide, sulfur dioxide, and various organic compounds such as aldehydes, acids, and higher alcohols and diols. Two general methods are available to purify fermentation carbon dioxide. Both use water scrubbers to remove the bulk of the entrained material. The impurities are then taken out by passing through either an activated charcoal bed or solutions of potassium permanganate and potassium dichromate. The first method relies on adsorption, whereas the second involves chemical reactions. In the second case the gas must be treated further downstream to remove oxidation products formed in the earlier stages as well as any traces of the reagents used in the purification.
The main impurities present in carbon dioxide from natural sources are methane and hydrogen sulfide [8]. 44% of carbon dioxide is used captively (42% as a chem raw material for urea production; and 2% in the production of methanol and sodium carbonate, for inverting, pressurizing in coal mining and oil recovery); 56% is used as merchant carbon dioxide (11% in refrigeration; 5% in carbonation; 3% as chem raw material; 3% in inserting; 1% for pressurizing in soft drink manufacturing, breweries, wineries, and as an aerosol propellant in some food products; 30% in oil well stimulation; and 3% in other application [9]. About 51% of the carbon dioxide used in the US is used in the food industry...it is generally used for food freezing or chilling; approximately 18% is used for beverage carbonation...both soft drinks and beer production consume the largest quantity for carbonation; about 10% is used for chemical manufacturing; other applications include metal working (4%) and oil and gas recovery (6%) [10].
Carbon dioxide (CO2) is the primary greenhouse gas emitted through human activities. In 2013, CO2 accounted for about 82% of all U.S. greenhouse gas emissions from human activities. Carbon dioxide is naturally present in the atmosphere as part of the Earth's carbon cycle (the natural circulation of carbon among the atmosphere, oceans, soil, plants, and animals). Human activities are altering the carbon cycle-both by adding more CO2 to the atmosphere and by influencing the ability of natural sinks, like forests, to remove CO2 from the atmosphere. While CO2 emissions come from a variety of natural sources, human-related emissions are responsible for the increase that has occurred in the atmosphere since the industrial revolution. The main human activity that emits CO2 is the combustion of fossil fuels (coal, natural gas, and oil) for energy and transportation, although certain industrial processes and land-use changes also emit CO2. The main sources of CO2 emissions in the United States are electricity, transportation, and industry [11].
Carbon dioxide is found in the products of combustion of all carbonaceous fuels, in naturally occurring gases, and as a product of animal metabolism. An average human being of 70kg is a 900g emitter of CO2 every 24 hours.
Carbon dioxide was blamed for the deaths of around 1700 people in Cameroon, west Africa, in 1986 when a massive release of gas occurred from Lake Nyos, a volcanic crater lake. The clinical findings in 845 survivors seen at or admitted to hospital were compatible with exposure to an asphyxiant gas. Rescuers noted cutaneous erythema and bullae on an unknown proportion of corpses and 161 (19%) survivors treated in hospital; though these lesions were initially believed to be burns from acidic gases, further investigation suggested that they were associated with coma states caused by exposure to carbon dioxide in air. The disaster at Lake Nyos and a similar event at Lake Monoun, Cameroon, two years previously provide new information on the possible medical effects of large-scale emissions of carbon dioxide, though the presence of other toxic factors in these gas releases cannot be excluded [12].
Ocean Acidification (OA)
The continued growth of CO2 emissions poses a grave threat to marine species, food chains and economies in the form of ocean acidification. The ocean absorbs up to 30% of annual carbon emissions, resulting in a fall in the pH value of its seawaters, thus signifying a rise in their acidity. The past 20–30 years have seen a rapid increase in ocean acidification and, unless decisive actions are taken to stem emissions, it will continue to rise. This will have detrimental impacts on the chemistry of the oceans, threatening the well-being of the marine ecosystems, coastal industries and the human communities that depend on them [13].
The world is waking up to the threat that Ocean Acidification (OA)—a rise in the acidity of seawater caused by excess carbon dioxide entering it from the atmosphere—poses to marine ecosystems and to the adjoin coastal economies. Since OA’s damaging effects on shellfish were first documented 15 years ago, research organizations have mobilized to collect, on an ongoing basis, huge volumes of OA-related data from the world’s oceans. Based on those data, as well as data gathered in coastal areas, scientists have published a wealth of studies examining the causes and effects of OA.
Ocean acidification is a growing threat to many forms of marine life and to the communities that rely on them for food, jobs and economic wellbeing. OA is a direct result of the growing carbon dioxide (CO2) emissions generated by human activity. Up to 30% of carbon released into the atmosphere each year is absorbed by the ocean, which helps to mitigate global warming. But the ocean’s ability to sequester carbon cannot keep pace with rising emission volumes. The result is a decline in the pH level of seawater and a rise in its acidity [14].
The pH level of seawater is a key indicator of ocean acidification. The pH logarithmic scale runs from 0 to 14. Over the past century, the mean surface ocean pH level has fallen from 8.2 to below 8, which is a lot in a biological system. About half of that decline has occurred in the past 40 years – a sign that acidification has been accelerating.
But pH is already lower than 8 in some waters, especially along some of our coasts, which is logical given that the coasts are where the discharge of wastewater begins. If global carbon emissions continue to increase at their current rate, by 2100, pH will be below 8 in most of the ocean.
The detrimental impacts of acidification are more than scientific speculation. It is known to have a damaging impact on marine organisms such as oysters, mussels, scallops, crabs and other shellfish. In the Pacific North-west, large-scale losses of oyster larvae in 2007-08 were found to be tied to a rise in the acidity of hatchery waters [15]. More recently, scientists have connected OA to weaker larval shells of Dungeness crab in waters along the US Pacific coast, negatively impacting the organisms’ growth and threatening a valuable source of aquaculture revenue [16]. And recent reports from the north-east Atlantic region show negative impacts from increasing OA on Atlantic cod and cold-water coral reefs, critical habitats for regional fisheries [17].
Should CO2 emissions remain at current levels, one study has found, the concomitant rise in ocean acidification would put pteropods, bivalve mollusks, finfish and warm-water corals, among other types of marine organisms, at a very high risk of damage by the end of this century [18].
The threat that OA poses to biodiversity, food chains and economies is an integral part of what the UN describes as an ocean emergency. It is the reason that the UN calls for action to combat OA as part of its Sustainable Development Goals (SDGs) [19].
The International Alliance to Combat Ocean Acidification specifies six categories of action that are needed to address the challenge [20]
a) Reduce atmospheric CO2
b) Advance scientific understanding of climate-ocean impacts.
c) Reduce local pollution that exacerbates OA.
d) Protect the environment and coastal communities from climate-ocean impacts.
e) Expand public awareness.
f) Sustain international and multi-governmental support for addressing this global problem.
Uptake of CO2 by the ocean arguably falls under the jurisdiction of UN Convention on the Law of the Sea (UNCLOS) [21]. UNCLOS has encouraged states, organizations, and institutions to urgently pursue research on OA and increase efforts to address levels of ocean acidity and its negative impact on vulnerable marine ecosystems, particularly coral reefs.
OA science and research is, in many ways, still an emerging field, particularly when it comes to characterizing local impacts and identifying effective solutions. So far, The OA Alliance is a ‘coalition of the willing”, and there are no legal mandates to create action plans. But time passes, and the problem worsens every moment without there being a light at the end of the tunnel.
But in the midst of the projection of such a bleak future, we have good news, because there is a new, very promising method to significantly improve ocean acidification, which was developed based on the biology of the human eye, by researchers from the Center for the Study of Human Photosynthesis™, S.C. located in Aguascalientes, Mexico, which, apart from being extremely efficient compared to any other current known processes, does not require electricity, nor does it require the addition of chemical substances. Below we offer a brief description of this method.
In Situ Hydration of CO2 solvated in Seawater, a New and Different Method to Solve the Challenge of Ocean Acidification
In 1990, we began an observational, descriptive study about the anatomy of the blood vessels that enter and leave the eye globe through the optic nerve in humans. This anatomical structure is very small, as it has a diameter of 1200 microns, which is equivalent to about twelve human hairs combined (Figure 1).
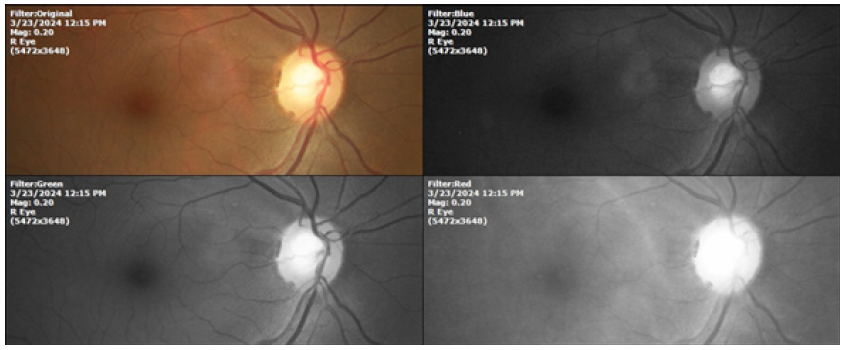
Figure 1: Digital image of the optic nerve of the right eye of a patient with glaucoma. Current technology allows images to be captured through different wavelengths, allowing greater details to be recorded. It is the equivalent of the spectrophotometric scan in CHEMISTRY laboratories.
The study began in 1990 and ended in 2002 and included digital images of ophthalmological studies from the records of 6,000 patients. Digital magnifications allow the observation of minute morphological details, both in the anatomical region of interest and in adjacent areas. And the systematization of the anatomical details observed allowed us, after 12 years and six thousand patients, to understand that our body does not take oxygen from the air that surrounds it, but from the water that the cells that make up us have inside, like in the plants [22].
Our observation resolves a 200-year-old heated discussion, since the 19th century there were researchers who questioned Lavoisier's hypothesis, arguing that the atmosphere contains little oxygen, and our body has almost five times as much [23].
And since current biology is based on living beings taking oxygen from the air or water that surrounds them (except plants), it turns out that it is necessary to rewrite all books on biology and related sciences, including study plans. of the different careers in this regard.
The impact of our observation about the unsuspected capacity of our body to take or extract oxygen from water, dissociating it like plants, opens new horizons in numerous areas of human knowledge, but in this article, we will refer specifically to the serious problem of acidification of the oceans.
CO2 and Seawater
Carbon dioxide in solution can react with water to form carbonic acid (H2CO3), which then dissociates to form bicarbonate (NaHCO3 or CaHCO3) and hydrogen ions. The first of these reactions is slow, and in biological systems is facilitated by the enzyme carbonic anhydrase, a zinc-containing enzyme and one of the fastest enzymes known.
Current Concepts of CO2 Environmental Fate and Behavior
The CO2 cycle is part of carbon cycle in the full ecosystem. CO2 cycles in the environment (atmospheric air and surface water) through respiration (aerobic and anaerobic), photosynthesis, decomposition, and release from earth's carbon sinks (fossil fuels—coal, petroleum, methane; and calcium carbonate rocks). During combustion CO2 dissolved in water reacts with calcium to form calcium carbonate and precipitates to the ocean floor. Most common reactions in the CO2 and carbon cycles in animals, plants, and the environment using or producing energy are presented below [24]:
Aerobic Metabolism
Glucose (C6H12O6) + Oxygen (O2) ↔ Carbon Dioxide (CO2) + Water (H2O).
Reaction in the Water (including body fluids)
Carbon Dioxide (CO2) + Water (H2O) ↔ Carbonic Acid (H2CO3) and Carbonic Acid (H2CO3) ↔ Proton (H+) + Bicarbonate (HCO3−).
Reaction in Water in Oceans
Calcium Carbonate + Carbon Dioxide (CO2) + Water (H2O) ↔ Calcium ion (Ca2+) + Bicarbonate (HCO3−).
Anaerobic Decomposition
Carbon Dioxide (CO2) + Hydrogen (H2) ↔ Methane (CH4) + Water (H2O).
Combustion
Methane (CH4) + Oxygen (O2) ↔ Carbon Dioxide (CO2) + Water (H2O).
They are very established, widely accepted concepts. But our discovery of the unsuspected ability of the human body to dissociate the water molecule [25], through several molecules [26], derails the first reaction. Namely
Aerobic Metabolism
Glucose (C6H12O6) + Oxygen (O2) ↔ Carbon Dioxide (CO2) + Water (H2O).
Since we now know that several molecules in our body can dissociate the water molecule, and that the oxygen that our body contains does not come from the air that surrounds us, then this reaction is ordered differently. It is not a cause for concern to rule out the first reaction about glucose, since the already 7000 intracell published reactions about metabolism of the human eukaryotic cell, more than 97% is entirely theoretical [27].
The right description of the process is as follows:
Water dissociation by intracell molecules impelled by Light (visible and invisible) ………..
Water (H2O) → Oxygen (O2) → Carbon Dioxide (CO2) + Water (H2O) + Oxygen (O2) → Glucose (C6H12O6) + Oxygen (O2)
This is, like in plants.
---ooo---
Thereby, our body can reduce CO2 to carbohydrates, hydrating it, like plants [28]. The very first requirement is high levels of dissolved oxygen, like in the vitreous body. These high levels must be maintained constantly, night and day, all the time.
At the Human Photosynthesis™ Study Center, S.C, we managed to reproduce the process that happens inside the human eye in 2007 (Figure 2).

Figure 2: The material that reproduces the dissociation of water, like what happens inside the human eye, is observed at the bottom of the flask. The oxygen bubbles that are constantly released are visible to the naked eye.
Elevated levels of dissolved oxygen are associated with better water quality [29], but it is necessary to raise them so that the physicochemical properties of molecular oxygen come into play and disassemble organic molecules into simple molecules, as happens in nature (Figure 3).
The ordered dissociation of water, in chemistry, is considered the opposite of catalysis for synthesis, because through synthesis we can obtain increasingly complex organic molecules, but with the ordered dissociation of water, the opposite happens, since complex organic molecules tend to disassemble, forming simple molecules [30] (Figure 4).

Figure 3: The material developed at the Center for the Study of Human Photosynthesis™, S.C. marketed under the name QBLOCK™, it efficiently clarifies, and without the need for electricity, frequent polluting elements, such as detergents, with little formation of toxic sludge. The quality of the water obtained favors the formation of organic carbon (green zones).
Comment
The observation that our body contains molecules capable of dissociating the water molecule derivates of Protoporphyrin IX (PTP IX) (Figure 5) marks a before and after in biology and related sciences. Textbooks will have to be rewritten since our body does not take oxygen from the air but from the water that the cells that make up us contain inside.
Correcting Lavoisier’s hypothesis, from the 18th century, will allow the advancement of knowledge. In addition, we have identified a process that allows the various bodies of water to be continuously oxygenated without requiring electricity or added chemicals. In addition, the average life of our material (QBLOCK TM) is a minimum of 25 years.
Conclusion
The growing problem of ocean acidification threatens all forms of life, and we are already seeing its effects in the loss of biodiversity, which is considered somewhat irreversible. Circumstantially, a discovery in relation to the biology of the human eye offers a new way to face the problem of acidification by CO2. Nobody would have thought that the constant elevation of dissolved oxygen levels in bodies of water due to acidification by CO2 results in the hydration of the solvated CO2 molecules present in seawater. Said in situ hydration results in the formation of carbohydrates, of which glucose is the most soluble molecule among them.
Acknowledgments
We appreciate the unrestricted support of the Center for Studies of Human Photosynthesis (TM) S.C. placed in Aguascalientes, México; for the preparation of this work.
Conflict of Interest
There are no conflicts of interest to report.
References
- Brunton L, Chabner B, Knollman B (2011) Goodman and Gillman's The Pharmaceutical Basis of Therapeutics, Twelfth Edition, McGraw Hill Medical, New York, NY: p. 558.
- Osol A, R Pratt (1973) (). The United States Dispensatory. 27th ed. Philadelphia: J.B. Lippincott: p. 231.
- Hayes WJ Jr, ER Laws Jr (1991) (eds.). Handbook of Pesticide Toxicology Volume 1. General Principles. New York, NY: Academic Press, Inc: p. 139.
- (2009) SEPA/Office of Pesticide Programs, Reregistration Eligibility Decision Document - Carbon and Carbon Dioxide EPA-4019 (September 1991): pp.8-9.
- (2020) ACGIH - Documentation of the TLVs and BEIs, 7th Cincinnati: ACGIH Worldwide.
- (2009) USEPA/Office of Pesticide Programs; Reregistration Eligibility Decision Document - Carbon and Carbon Dioxide EPA-4019 (September 1991): p.5.
- Topham S (2014) Carbon Dioxide. Ullmann's Encyclopedia of Industrial Chemistry 7th NY, NY: John Wiley & Sons. Online.
- Pierantozzi R (2003) Kirk-Othmer Encyclopedia of Chemical Technology. NY, NY: John Wiley & Sons; Carbon Dioxide. Online.
- (1983) Chemical Products Synopsis: Carbon Dioxide.
- (1991) Kirk-Othmer Encyclopedia of Chemical Technology. 4th Volumes 1: New York, NY. John Wiley and SonsV5: p. 49.
- (2015) USEPA. Climate Change. Overview of Greenhouse Gases. Carbon Dioxide Emissions.
- Baxter PJ, Kapila M, Mfonfu D (1989) Lake Nyos disaster, Cameroon, 1986: the medical effects of large scale emission of carbon dioxide? BMJ 298(6685):1437-1441.
- Guinotte JM, Fabry VJ (2008) Ocean acidification and its potential effects on marine ecosystems. Ann N Y Acad Sci 1134: 320-342.
- (2022) With the exception of 2020, global CO2 emissions have grown consistently over time, reaching an estimated 37.5 billion tonnes in 2022. Global Carbon Project, Global Carbon Budget.
- (2015) The hatchery losses and the successful measures taken by scientists to eliminate them by reducing seawater acidity are documented in A Barton, G Waldbusser, R Feely et al, Impacts of Coastal Acidification on the Pacific Northwest Shellfish Industry and Adaptation Strategies Implemented in Response.
- N Bednaršek (2020) Exoskeleton dissolution with mechanoreceptor damage in larval Dungeness crab related to severity of present-day ocean acidification vertical gradients. Science Direct: 716.
- (2023) E McGovern et al, OSPAR, The 2023 Quality Status Report for the North-East Atlantic.
- JF Gattuso, A Magnan, R Billé, WWL Cheung, EL Howes, et al (2015) Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios. Science 349(6243): aac4722.
- SDG target 14.3 reads: Minimize and address the impacts of ocean acidification, including through enhanced scientific cooperation at all levels.
- OA Alliance, Action Plan Toolkit.
- The United Nations Convention on the Law of the Sea (UNCLOS). Commentary. InforMEA.
- Arturo Solís Herrera, María del Carmen Arias Esparza (2022) Oxygen from the Atmosphere Cannot Pass Through the Lung Tissues and Reach the Bloodstream. The Unexpected Capacity of Human Body to Dissociate the Water Molecule. Journal of Pulmonology Research & Reports 4(1): 1-4.
- Albert Gjedde (2010) Diffusive insights: on the disagreement of Christian Bohr and August Krogh at the Centennial of the Seven Little Devils. Adv Physiol Educ 34(4):174-185.
- Iva Srdanovic, Sidhartha D Ray (2024) in Encyclopedia of Toxicology (Fourth Edition).
- Herrera AS, Del C A Esparza M, Md Ashraf G, Zamyatnin AA, Aliev G (2015) Beyond mitochondria, what would be the energy source of the cell? Cent Nerv Syst Agents Med Chem 15(1): 32-41.
- Herrera AS (2015) The Biological Pigments in Plants Physiology. Agricultural Sciences 6(10): 1262-1271.
- Stobbe, Miranda D (2012) The road to knowledge: from biology to databases and back again. University of Amsterdam. Faculty of Medicine. Doctoral dissertation: 168.
- Arturo Solís Herrera, María del Carmen Arias Esparza, and Martha Patricia Solís Arias (2023) The Unexpected Role of Dissolved Oxygen Levels in Wastewater Treatment. Advanced Research in Biological Science 3: 114-133.
- Rudolph A, Ahumada R, Pérez C (2002) Dissolved oxygen content as an index of water quality in San Vicente Bay, Chile (36 degrees 45'S). Environ Monit Assess. 78(1): 89-100.
- Lee GR, Kim J, Hong D, Ye Ji Kim, Hanhwi Jang, et al. (2023) Efficient and sustainable water electrolysis achieved by excess electron reservoir enabling charge replenishment to catalysts. Nat Commun 14, 5402.





 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.