Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
LDLR mRNA-Loaded Exosomes Serve as Key Vehicle in Improving Familial Hypercholesterolemia
*Corresponding author: Cai-Ping Zhanga, Department of Biochemistry & Molecular Biology, Hengyang Medical School, University of South China, China.
Received: February 08, 2024; Published: April 08, 2024
DOI: 10.34297/AJBSR.2024.22.002915
Abstract
The exosomes selectively deliver their loaded bioactive substances to adjacent cells or their distal sheath target tissues, and regulate immune response, inflammation, tumor growth and infection in a local or a long-distance. The exosomes-mediated Low Density Lipoprotein Receptor (LDLR) mRNA delivering could restore LDLR expression and reversing high serum low density lipoprotein cholesterol (LDL-C) levels. Familial hypercholesterolemia (FH) which is one kind of an autosomal dominant genetic diseases, was primarily caused by loss-function mutations in the LDL receptor gene. Homozygous FH (HoFH) was characterized with extremely elevated LDL-C levels, which was hard to cure due to few effective medications available for it. LDL receptor is responsible for clearance the majority of serum LDL-C. Employing LDL receptor mRNA-loaded exosomes maybe serving as a critical vehicle for improving HoFH.
Keywords: Exosomes; Familial hypercholesterolemia; Therapeutic vehicle; Gene therapy
Introduction
Exosomes secreted by various of cells are characterized with lipid bilayer-enclosed extracellular (EVs) in a size range of 30 to 150 nm diameter, which contain various bioactive substances, such as DNA, RNA, mRNA, protein, lipid and so on. (Figure1).
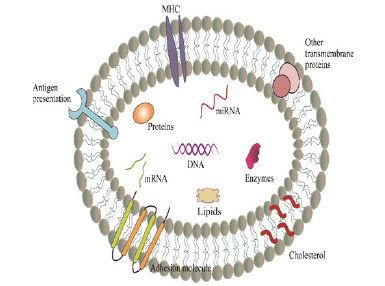
Figure 1: Composition of exosomes. Exosomes are secreted by mammalian cells and are widely distributed in cellular systems. They are composed of various proteins such as antigen presentation, adhesion molecule, other transmembrane proteins and so on. Exosomes also contain cholesterol and nucleic acids, such as mRNA, miRNA, and DNA in their lumen.
The exosomes were firstly found in the supernatant of sheep erythrocyte cultured in vitro, lately they were found to have the ability of transferring information, acting as a natural signal carrier, through selectively deliver bioactive substance to adjacent cells or their distal sheath target tissues [1,2]. More interestingly, mutation genes in diseased cells were corrected by exosomes in the way of introducing genetic material or edition tools [3], which maybe brings hopeful for previously incurable diseases, such as hereditary, cancer, and autoimmune diseases [4]. Familial Hypercholesterolemia (FH) is mainly caused by a single pathogenic mutation in the Low-Density Lipoprotein Receptor (LDLR) or its associated genes [5], which belongs to one of easily atherogenic metabolic disorders [6]. Employing LDL receptor mRNA-loaded exosomes may be employed to provide novel ideas for the clinical treatment of FH.
Familial Hypercholesterolemia: From Pathophysiology to Current Treatment
FH, also known as familial hyper-β-lipoproteinemia belonging to one kind of an autosomal dominant genetic diseases [7], is one of high risks leading to atherosclerotic cardiovascular, and can be effectively improved the survival rate by early screening and drug therapy [8]. FH is possessed with the features including familial aggregation of disease onset, significant increase in serum LDL-C level, and early occurrence and rapid progression of atherosclerotic cardiovascular disease [9-11]. FH is caused by dysfunctional mutations in the LDL receptor gene [12-14] which impairs serum Low-Density Lipoprotein (LDL) clearance. FH was primarily divided into heterozygous FH (HeFH) and homozygous FH (HoFH) [15].
Although statins [16], ezetimibe (a cholesterol absorption inhibitor) [17], and PCSK9 inhibitors [18] have some beneficial effects in HeFH, few medicinations are effective in HoFH [19]. Lipid-lowering treatment with plasma or LDL apheresis complementary is kind of an invasive and expensive treatment to FH patients, which requires maintenance therapy weekly or every 2 weeks [20]. Liver transplantation can restore LDL receptor to clearance apoB-containing lipoproteins, which requires lifelong immunosuppression for FH patients against organ rejection [21]. Therefore, transferring hepatocyte-directed LDLR gene may be a potential strategy for the treatment of this monogenic disease.
LDLR in the way of endocytosis cycle clearances most excess LDL-C from the serum, playing a critical role for LDL-C clearance from the circulation by the liver [22-24]. Overexpression LDLR which is gained by through retroviruses [25], adenoviruses [26] and Adeno-Associated Viruses (AAV) [27] transfecting the liver tissue, is effective in lowering serum levels of total cholesterol, which was limited in the clinical application due to their inherent carcinogenicity, cytotoxicity and immunogenicity [28].
Exosomes-Based Gene Transfer
The effects of exosomes on lipid metabolism. The pathway of reverse cholesterol transport (RCT) can transport the vast majority of cholesterol content from peripheral tissue to liver tissue, maintaining intracellular cholesterol homeostasis. ATP-binding cassette transporter A1(ABCA1) and ATP-binding cassette transporter G1(ABCG1) mediate intracellular cholesterol efflux during the process of RCT [29]. Circulating miRNAs in exosomes, such as miR-30e and miR-92a, display inhibitory effects on ABCA1 and ABCG1, which arouse the accumulation of intracellular cholesterol [30]. Platelet-derived exosomes reduced scavenger receptor CD36 expression of macrophage cells, and consequently reduced the uptake of harmful cholesterol [31]. In addition, hepatocytes-derived exosomes could transfer sphingosine kinase 2 to form sphingosine-1-phosphate in hepatocytes, leading to cell proliferation and liver regeneration [32]. These findings indicated that exosomes may play a role in lipid metabolic diseases.
LDLR mRNA-loaded exosomes in the role of therapy FH. In the FH model (LDLR-/- mice), the LDLR mRNA in exosomes was effectively transported to the liver where the LDLR mRNA was translated into corresponding protein in hepatocytes [33-35] with stable function in the recipient cells (Figure 2), which showed that exosome-mediated delivery of LDLR mRNA effectively restored the expression of LDLR protein, reduced the deposition of lipid and lowered the level of serum LDL-C in LDLR-/- mice, providing a novel treatment for patients with FH.

Figure 2: A new strategy for the treatment of familial hypercholesterolemia. (1) The encoding sequence of LDLR was cloned into plasmid vector and transfected into packaging cells. (2) The exosomes rich in LDLR mRNA (ExoLdlr) were harvested, and the encapsulation effect of the exosome was examined. (3) To study whether the exosome can be effectively transferred, and the mRNA of exosomes would be translated into functional proteins of receptor cells. At last, the distribution of exosome in vivo and the efficacy of exosome-based LDLR gene therapy will be evaluated.
Large producing exosomes carrying functional mRNA. Exosomes showed more superiority than viruses in carrying hepatocyte mRNA into liver tissue, and the integrity of inner membrane of exosomes would protect the mRNA from digestion [36]. Since original exosomes which were inserted with exogenous large size of mRNA into were secreted in lowing-yields, Yang, et al. developed a new method to break through this limitation. They transfected various of cells with plasmid DNAs through a focal and transient electrical ways to elevate yields of exosomes carrying mRNAs or peptides, which actually leaded to up to more 50-fold exosomes secretion and more than 103-fold mRNA transcription of exosome [37]. Above all provide us with a new way to make exosomes as convincing clinical treatment strategy [38].
Advantages of Exosome-Based Gene Therapy
Spanning biofilm. Exosomes can be attached to the surface of targeting cells or tissues through specific molecular recognition [39], therefore, exosomes loaded with unique RNA and protein substances will play their various of biological functions. The exosome was started with endocytosis or membranous invagination of cells, which firstly is formed in the way of the early endosome, then further developed into a polycystic endosome (MVE) [40]. After fusion with the cell membrane, the exosome in the MVE is released into extracellular. Since the exosome-coated structure was similar to the cellular membrane, the exosome was born with the ability of going through the biofilm during the information exchange [41], which made themselves transfer intercellular communication and signal transduction of phenotypic traits of parent cells.
Exosomes have the ability to cross biological barriers. Cell-derived exosomes also possessed the ability of going through the blood-brain barrier, participating in a variety of activities of the central nervous system, such as inducing the outward growth of synapses and neuronal survival, mediating neuronal development, and regulating synaptic activities [42]. In zebrafish, neurons can remotely regulate the integrity of blood-brain barrier through secretion of exosomes delivering miR-132 [43], which would provide a potential solution for the delivery of neuro pharmaceuticals. Exosomes also can survive from extreme conditions, such as gastric acid, digestive enzymes [44], which offers the possibility for exosomes through oral administration. Exosomes can escape the clearance of the mononuclear macrophage system. CD47 (the ligand of signal regulatory protein α) is one of composition proteins of exosomes, and the combination of CD47 and signal regulatory protein α (SIRPα) will inhibit the phagocytosis of immune cells [45]. The human miRNA-carrying exosomes inhibit the initiation of immune response [46] and are circulated in the extracellular environment [47]. Above all indicated that exosomes as therapeutic vectors provided a novel therapy methods for various diseases [48].
Summary
Amelioration high levels of LDL-C in HoFH still remains medical challenge [49] due to no effective medications available for it. Since LDLR is responsible for clearance most of serum LDL-C levels through binding and recognition of its ligand--LDL-C [24], the exosome which was loaden with LDLR mRNA possesses the features of immune evasion and mobile gene correction therapy, maybe serving as a critical vehicle for improving HoFH in the future.
Acknowledgments
We would like to express our gratitude to all those who helped us during the writing of this manuscript.
Funding
This review article was funded by the National Natural Science Foundation of China (Grant No. 81600291).
Compliance with Ethical Standards
This article does not contain any studies involving animals performed by any of the authors.
Conflict of Interest
The authors declare no conflict of interest.
References
- Kalluri R and VS LeBleu (2020) The biology, function, and biomedical applications of exosomes. Science 367(6478): 6977.
- Sangiliyandi Gurunathan, Min Hee Kang, Muniyandi Jeyaraj, Muhammad Qasim, Jin Hoi Kim (2019) Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells 8(4): 307.
- Ophir Shalem, Neville E Sanjana, Ella Hartenian, Xi Shi, David A Scott, et al. (2014) Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343(6166): 84-87.
- Li Duan, Limei Xu, Xiao Xu, Zhuan Qin, Xiaoying Zhou, et al. (2021) Exosome-mediated delivery of gene vectors for gene therapy. Nanoscale 13(3): 387-1397.
- Tada H, M Takamura, Kawashiri MA (2022) Individualized Treatment for Patients With Familial Hypercholesterolemia. J Lipid Atheroscler 11(1): 39-54.
- Georgios Polychronopoulos, Marios Tzavelas, Konstantinos Tziomalos (2021) Heterozygous familial hypercholesterolemia: prevalence and control rates. Expert Rev Endocrinol Metab 16(: 175-179.
- Asier Benito Vicente, Kepa B Uribe, Shifa Jebari, Unai Galicia Garcia, Helena Ostolaza, et al. (2018) Familial Hypercholesterolemia: The Most Frequent Cholesterol Metabolism Disorder Caused Disease. Int J Mol Sci 19(11): 3426.
- Chen Wang, Zhelong Li, Yunnan Liu, Lijun Yuan (2021) Exosomes in atherosclerosis: performers, bystanders, biomarkers, and therapeutic targets. Theranostics 11(8): 3996-4010.
- Bouhairie VE, Goldberg AC (2015) Familial hypercholesterolemia. Cardiol Clin 33(2): 169-179.
- Rodrigo Alonso, Leopoldo Perez de Isla, Ovidio Muñiz Grijalvo, Pedro Mata (2020) Barriers to Early Diagnosis and Treatment of Familial Hypercholesterolemia: Current Perspectives on Improving Patient Care. Vasc Health Risk Manag 16: 11-25.
- Mariko Harada Shiba, Junya Ako, Hidenori Arai, Atsushi Hirayama, Yoshitaka Murakami, et al. (2018) Prevalence of familial hypercholesterolemia in patients with acute coronary syndrome in Japan: Results of the EXPLORE-J study. Atherosclerosis 277: 362-368.
- Singh S, V Bittner (2015) Familial hypercholesterolemia--epidemiology, diagnosis, and screening. Curr Atheroscler Rep 17(2): 482.
- Mabuchi H (2017) Half a Century Tales of Familial Hypercholesterolemia (FH) in Japan. J Atheroscler Thromb 24(3): 189-207.
- Long Jiang, Lu Ya Wang, Xiao Shu Cheng (2018) Novel Approaches for the Treatment of Familial Hypercholesterolemia: Current Status and Future Challenges. J Atheroscler Thromb 25(8): 665-673.
- Raul D Santos, Evan A Stein, G Kees Hovingh, Dirk J Blom, Handrean Soran, et al. (2020) Long-Term Evolocumab in Patients With Familial Hypercholesterolemia. J Am Coll Cardiol 75(6): 565-574.
- Andrew Neil, Jackie Cooper, John Betteridge, Nigel Capps, Ian McDowell, et al. (2008) Reductions in all-cause, cancer, and coronary mortality in statin-treated patients with heterozygous familial hypercholesterolaemia: a prospective registry study. Eur Heart J 29(21): 2625-2633.
- Claude Gagné, Daniel Gaudet, Eric Bruckert; Ezetimibe Study Group (2002) Efficacy and safety of ezetimibe coadministered with atorvastatin or simvastatin in patients with homozygous familial hypercholesterolemia. Circulation 105(21): 2469-2475.
- Frederick J Raal, Narimon Honarpour, Dirk J Blom, G Kees Hovingh, Feng Xu, et al. (2015) Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet 385(9965: 341-50.
- Saeideh Hajighasemi, Armita Mahdavi Gorabi, Vanessa Bianconi, Matteo Pirro, Maciej Banach, et al. (2019) A review of gene- and cell-based therapies for familial hypercholesterolemia. Pharmacol Res 143: 119-132.
- Atsushi Nohara, Hayato Tada, Masatsune Ogura, Sachiko Okazaki, Koh Ono, et al. (2021) Homozygous Familial Hypercholesterolemia. Periodical Homozygous Familial Hypercholesterolemia. J Atheroscler Thromb 28(7): 665-678.
- Eline Van Craeyveld, Frank Jacobs, Stephanie C Gordts, Bart De Geest (2011) Gene therapy for familial hypercholesterolemia. Curr Pharm17(24): 2575-2591.
- Liwei Ren, Yuan Sun, Hong Lu, Dien Ye, Lijuan Han, et al. (2018) (Pro)renin Receptor Inhibition Reprograms Hepatic Lipid Metabolism and Protects Mice From Diet-Induced Obesity and Hepatosteatosis. Circ Res 122(5): 730-741.
- Thomas A Lagace, David E Curtis, Rita Garuti, Markey C McNutt, Sahng Wook Park, et al. (2006) Secreted PCSK9 decreases the number of LDL receptors in hepatocytes and in livers of parabiotic mice. J Clin Invest 116(11): 2995-3005.
- Debapriya Basu, Yunying Hu, Lesley Ann Huggins, Adam E Mullick, Mark J Graham, et al. (2018) Novel Reversible Model of Atherosclerosis and Regression Using Oligonucleotide Regulation of the LDL Receptor. Circ Res 122(4): 560-567.
- M Grossman, D J Rader, D W Muller, D M Kolansky, K Kozarsky, et al. (1995) A pilot study of ex vivo gene therapy for homozygous familial hypercholesterolaemia. Nat Med (1)11: 1148-1154.
- J Li, B Fang, R C Eisensmith, X H Li, I Nasonkin, et al. (1995) In vivo gene therapy for hyperlipidemia: phenotypic correction in Watanabe rabbits by hepatic delivery of the rabbit LDL receptor gene. J clin Invest 95(2): 768-773.
- Anton P McCaffrey, Paul Fawcett, Hiroyuki Nakai, Ramona L McCaffrey, Anja Ehrhardt, et al. (2008) The host response to adenovirus, helper-dependent adenovirus, and adeno-associated virus in mouse liver. Mol Ther 16(5: 931-941.
- Frank Jacobs, Eline Van Craeyveld, Yingmei Feng, Jan Snoeys, Bart De Geest (2008) Adenoviral low density lipoprotein receptor attenuates progression of atherosclerosis and decreases tissue cholesterol levels in a murine model of familial hypercholesterolemia. Atherosclerosis 201(2): 289-297.
- Wei Wang, Neng Zhu, Tao Yan, Ya-Ning Shi, Jing Chen, et al. (2020) The crosstalk: exosomes and lipid metabolism. Cell Commun signal 18(1): 119.
- Zhisheng Wang, Jiayun Zhang, Songlan Zhang, Shifang Yan, Zihui Wang, et al. (2019) MiR30e and miR92a are related to atherosclerosis by targeting ABCA1. 19(4): 3298-3304.
- S Srikanthan, W Li, R L Silverstein, T M McIntyre (2014) Exosome poly-ubiquitin inhibits platelet activation, downregulates CD36 and inhibits pro-atherothombotic cellular functions. J Thromb Haemost 12(11): 1906-1917.
- Hiroyuki Nojima, Christopher M Freeman, Rebecca M Schuster, Lukasz Japtok, Burkhard Kleuser, et al. (2016) Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J Hepatol 64(1): 60-68.
- Lotvall J, Valadi H (2007) Cell to cell signalling via exosomes through esRNA. Cell Adh Migr 1(3): 156-188.
- Zhelong Li, Ping Zhao, Yajun Zhang, Jia Wang, Chen Wang, et al. (2021) Exosome-based Ldlr gene therapy for familial hypercholesterolemia in a mouse model. Theranostics 11(6): 2953-2965.
- Rafael Escate, Teresa Padró, Rosa Suades, Sandra Camino, Ovidio Muñiz, et al. (2021) High miR-133a levels in the circulation anticipates presentation of clinical events in familial hypercholesterolaemia patients Cardiovasc Res 117(1): 109-122.
- Mathiyalagan P, S Sahoo (2017) Exosomes-Based Gene Therapy for MicroRNA Delivery Methods. Mol Biol 1521: 139-152.
- Zhaogang Yang, Junfeng Shi, Jing Xie, Yifan Wang, Jingyao Sun, et al. (2020) Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat Biomed Eng 4 (1): 69-83.
- Hyosuk Kim, Eun Hye Kim, Gijung Kwak, Sung Gil Chi, Sun Hwa Kim, et al. (2020) Exosomes: Cell-Derived Nanoplatforms for the Delivery of Cancer Therapeutics. Int J Mol Sci 22 (1): 14.
- Yujie Liang, Xiao Xu, Xingfu Li, Jianyi Xiong, Biquan Li, et al. (2020) Chondrocyte-Targeted MicroRNA Delivery by Engineered Exosomes toward a Cell-Free Osteoarthritis Therapy. ACS Appl Mater Interfaces 12(33): 36938-36947.
- Colombo M, G Raposo, C Théry (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30: 255-289.
- Askenase PW (2021) Ancient Evolutionary Origin and Properties of Universally Produced Natural Exosomes Contribute to Their Therapeutic Superiority Compared to Artificial Nanoparticles. Int J Mol Sci 22(3): 1429.
- Frühbeis C, D Fröhlich, EM Krämer Albers (2012) Emerging roles of exosomes in neuron-glia communication. Front Physiol 3: 119.
- Xu B, Y Zhang, XF Du, et al. (2017) Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity. Nature 27(7): 882-897.
- Abderrahim Benmoussa, Chan Ho C Lee, Benoit Laffont, Patricia Savard, Jonathan Laugier, et al. (2016) Commercial Dairy Cow Milk microRNAs Resist Digestion under Simulated Gastrointestinal Tract Conditions. J Nutr 146(11): 2206-2215.
- Chao MP, IL Weissman, R Majeti (2012) The CD47-SIRPα pathway in cancer immune evasion and potential therapeutic implications. Curr Opin Immunol 24(2): 225-232.
- Alireza Hejrati, Bahare Hasani, Mozhgan Esmaili, Davood Bashash, Naeimeh Tavakolinia, et al. (2021) Role of exosome in autoimmunity, with a particular emphasis on rheumatoid arthritis. Int J Rheum Dis 24(2): 159-169.
- Sushrut Kamerkar, Valerie S LeBleu, Hikaru Sugimoto, Sujuan Yang, Carolina F Ruivo, et al. (2017) Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546(7659): 498-503.
- Yamashita T, Y Takahashi, Y Takakura (2018) Possibility of Exosome-Based Therapeutics and Challenges in Production of Exosomes Eligible for Therapeutic Application. Biol Pharm Bull 41(6): 835-842.
- Børge G Nordestgaard, M John Chapman, Steve E Humphries, Henry N Ginsberg, Luis Masana, et al. (2013) Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J 34(45): 3478-90a.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.