Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Prediction of Fetal Brain and Heart abnormalities using Artificial Intelligence Algorithms: A Review
*Corresponding author: Ashish Shiwlani, Illinois Institute of Technology, 10 W 35th St, Chicago, IL 60616, USA
Received: May 04, 2024; Published: May 09, 2024
DOI: 10.34297/AJBSR.2024.22.002970
Abstract
In fetal medicine, artificial intelligence plays a crucial role in preventing congenital fetal abnormalities. Anomalies of heart and brain in fetal ultrasonography and MRI have been shown to be recognizable, detectable, and localizable by ML algorithms and CNNs. Artificial Intelligence (AI) systems are capable of carrying out intricate analyses of aberrant image patterns in order to categorize and predict malformations in fetuses. The role of Artificial Intelligence (AI) in the prediction and risk stratification of congenital anomalies is explored in this narrative review. Fetal imaging (ultrasonography and MRI) examination may be optimized by ML and DL algorithms to reduce examination time, lighten the doctor’s workload, and increase diagnostic precision for fetal anomalies. The current study’s objective is to evaluate the algorithms being utilized to automate screening for fetal brain and heart anomalies. It also compares ML and DL algorithms in terms of efficiency and quality of the brain and heart anomaly detection in the fetus. The review highlights the importance of integrating multiple data sources, analyzing longitudinal data, and creating larger, more varied datasets for predicting congenital anomalies. The significance of human clinical expertise, interpretability, and prospective validation in real-world clinical settings are also emphasized.
Keywords: Deep learning, Machine learning, Artificial lntelligence, Congenital heart disease, Fetal anomaly
Introduction
Anything that deviates from the norm or what is anticipated is considered an anomaly. Unusual or unexpected circumstances in fetus development during pregnancy are referred to as fetal anomalies [1,2]. Congenital anomalies or birth defects are other names for fetal anomalies [1-3]. Fetal anomalies fall into two categories: a developing fetus’ body parts, including heart, lungs, kidneys, limbs, and facial features, are affected by structural anomalies leading to conditions like spina bifida, cleft lip, congenital heart and brain abnormalities, and missing toes [1]. A body system or part, such as the brain, CNS, or sensory perception, can experience functional abnormalities that impact its operation [4]. Functional birth defects encompass a variety of conditions, such as Down syndrome, muscular dystrophy, developmental disabilities, seizures, and blindness [5].
Fetal heart diseases refer to structural anomalies of the heart that develop prior to birth [6]. During pregnancy, these defects manifest in the developing fetus inside the uterus. According to WHO, approximately 0.5M adults in the USA suffer from congenital heart disease [6]. Heart defects resulting from genetic or chromosomal abnormalities, like Down syndrome, affect one out of every 100 children. The risk factors for fetal heart disease in children include excessive alcohol consumption during pregnancy, medication use, maternal viral infection during the first trimester of pregnancy, and an increased risk if a parent or sibling has a congenital heart defect [7].
Similarly, birth abnormalities known as congenital brain malformations can be caused by developmental disruptions at different stages of embryonic or fetal development [8]. The nonspecific clinical presentation may involve hypotonia, epilepsy, or developmental delay. Early and accurate diagnosis and management planning are made possible by advanced imaging [9]. Usually between weeks 18 and 23, as part of antenatal care, an ultrasound scan is performed to look for fetal anomalies [10]. The anomaly scan looks for the development and structure and function of the head, brain, and facial features of the infant, development of hands, feet, limbs etc. Although, fetal Magnetic Resonance Imaging (MRI) has been widely used in the clinical setting for screening fetal brain abnormalities over the past ten years [11].
The role of artificial intelligence can also be seen in fetal medicine in order to avoid congenital fetal abnormalities. The computer’s ability to carry out tasks associated with intelligent beings, such as learning, reasoning, and interacting, is known as Artificial Intelligence (AI) [12,13]. Machine Learning (ML) and Deep Learning (DL) are the two subsets of AI [14,15]. One of the primary forms of deep learning algorithms is Convolutional Neural Networks (CNNs), and recent work has shown that they can conduct image recognition tasks with remarkable progress [16,17]. ML algorithms and CNNs have demonstrated the ability to recognize [18], detect [19], and localize [20] standard planes in fetal ultrasonography and MRI. But very few studies have developed AI algorithms that could perform detailed analyses of abnormal patterns in fetus images to classify and predict congenital malformations; instead, nearly all recent studies using AI in fetal imaging have concentrated on the identification of normal fetal structures [21-23].
This paper highlights the ability of ML and DL algorithms to identify patterns and analyze large datasets to identify fetal brain and heart abnormalities. Predicting the onset and course of fetal abnormalities like congenital brain and heart malformations makes early interventions and preventive measures possible. Although, a number of articles have been published to review the studies done for fetal heart and brain analysis using AI algorithms [24-29]. However, this is the first state-of-the-art review that discusses the heart and brain abnormalities prediction using automated diagnosis on the basis of ultrasound and MRI. The studies that have utilized ML and DL algorithms for the prediction of fetal heart and brain abnormalities have been reviewed in this survey. This review contributes to fetal healthcare by answering the following research questions:
RQ1: Which imaging techniques are being utilized for fetal anomaly detection of brain and heart?
RQ2: How well does Artificial Intelligence (AI) detect both normal and abnormal features of the most common congenital defects affecting the fetal CNS and cardiovascular system?
RQ3: Which algorithms are most commonly utilized for the prediction of fetal brain and heart anomalies?
RQ4: What are the current applications of AI-based detection systems in diagnosing abnormalities of fetal central nervous system and heart?
Methodology
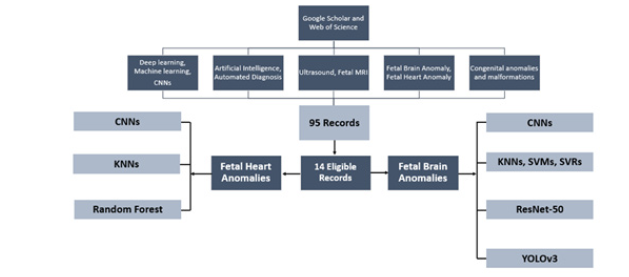
We did a thorough search of research articles on Google Scholar and Web of Science databases considering the PRISMA guidelines. The key terms that we utilized were deep learning, machine learning, CNNs, artificial intelligence, ultrasound, fetal MRI, fetal brain anomaly, fetal heart anomaly, congenital anomalies and malformations, and congenital heart disease. We found only 95 articles published from 2014 to 2024.
Studies that addressed the use of ML and DL algorithms in fetal scanning using MRI and ultrasound written in English were considered for inclusion. Each study was independently reviewed by two evaluators using the full text, abstract, and title as their criteria. To ensure focused analysis, this review incorporated studies meeting the following criteria: 1) written in English, 2) utilizing AI, 3) investigating fetal brain or heart anomalies, and 4) containing complete data. Following the application of the exclusion criteria, we found, as illustrated in Figure 1, 14 articles that were particularly relevant to fetal heart and brain abnormalities.
Research Findings
The research findings are as follows:
Fetal Imaging Techniques
a) Ultrasonography
Ultrasound is a noninvasive, nonradiative, convenient, and inexpensive technique for prenatal imaging diagnosis [30]. Prenatal ultrasound offers a non-invasive method for assessing fetal growth parameters, detecting potential congenital anomalies, and aiding in overall fetal diagnosis through visualization of the fetus and its appendages [31]. With better diagnostic accuracy and high-quality images, it can offer comprehensive information on fetal anatomy [32]. Currently, organ functions, disease diagnosis, and measurement of fetal structures are all accomplished through the widespread use of two-dimensional (2D) imaging and three-dimensional (3D) ultrasound [33]. Pregnant women who receive routine ultrasonography exams can successfully lower the incidence of congenital disabilities. Fetal ultrasonography is currently dealing with some obstacles in the clinical pipeline, though. Numerous factors, including high fetal mobility, pregnant women's thick abdominal wall and inconsistent interpretations between doctors, influence the accuracy of the examination [33].
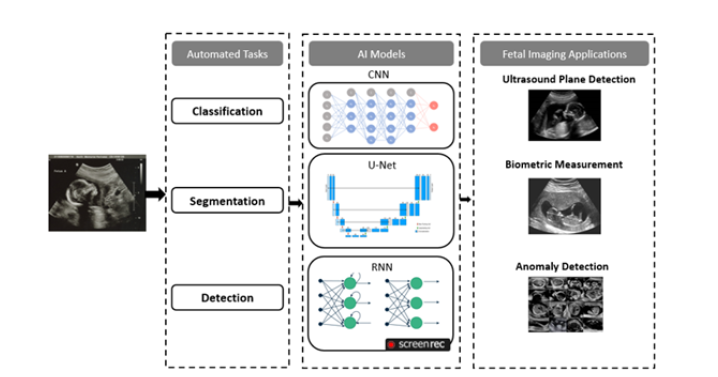
A prenatal ultrasound examination, regardless of gestational age, fulfills two crucial purposes: screening and diagnosis. Over the past ten years, screening and diagnosis based on Artificial Intelligence (AI) have been introduced in an effort to improve diagnostic accuracy for fetal abnormalities [32,33]. These AI systems focus on three key areas: 1) automatically identifying anatomical structures, 2) performing standardized measurements, and 3) classifying the ultrasound images to aid in diagnosis. Because obstetric ultrasound takes a lot of time, using AI could speed up workflow and cut down on examination time [33]. The majority of the related research is still in its early stages [19,34], despite the fact that many machine learning and deep learning methods have been introduced to provide high resolution imaging and accurate measurement for obstetric ultrasound.
b) Magnetic Resonance Imaging
Fetal MRI is a safe alternative to X-rays for examining a developing baby. It uses magnets and radio waves to create detailed pictures, and there are no known risks [35]. The first description of MRI in pregnancy was published in 1983 [36]. Maternal and placental anomalies were the main causes of the early obstetric applications [36,37]. A thorough examination of the placenta, umbilical cord, and amniotic cavity completes fetal Magnetic Resonance Imaging (fMRI) [35]. A precise interpretation of fetal magnetic resonance imaging can yield important data that supports research studies, aids in management decisions, guides therapy, and aids in prenatal counseling. When it comes to diagnosing developmental issues in the fetal brain, MRI has proven to be a reliable method, providing intricate pictures for accurate evaluation. Congenital cardiac lesions have also been studied in more recent times; the findings have greatly improved the counseling of impacted pregnancies [37]. Table 1 provides a brief description of the indications of fetal MRI.
Fetal MRI accurately depicts body anatomy, it has been demonstrated to both complement and outperform fetal ultrasound in various conditions [38]. Expanding its use can hasten the diagnosis and investigation of numerous fetal abnormalities [38]. Additionally, MRI overcomes a number of ultrasound's technical drawbacks, including abnormal fetal position, low amniotic fluid volume, and high maternal body mass index [39]. The quality of ultrasound images can be affected by fetal lying, oligohydramnios, and maternal habitus. With the advent of post-acquisition processing that enables motion correction to be applied to both the brain and the abdominal cavity, MRI can eliminate these problems [35,36,40]. Fetal MRI has been studied using advanced MRI techniques like Diffusion-Weighted Imaging (DWI) and Magnetic Resonance Spectroscopy (MRS) [40]. Applications for both destructive and developmental brain processes could be found with fetal brain diffusion imaging.
Radiologist and clinician understanding of the typical developing fetal anatomy is necessary for accurate interpretation of fetal MRI [35]. When portraying fetal anatomy, fetal age (also known as gestational age, or GA) is a crucial consideration [35]. Current fetal MRI analysis involves examining many sequences and images, often not ideal for precise measurements. Furthermore, a lack of specialists creates delays. AI offers a solution by automating these tasks, leading to a faster, more consistent, and efficient approach to fetal MRI analysis [41]. Fetal MRI analysis is exploring the use of AI models. These models can automatically pinpoint key anatomical structures and segmentation within the scans [42]. A variety of AI models, primarily Convolutional Neural Network and Res-Net, have been employed [43-46], covering all gestational age weeks (17–38 week). The accuracy of certain models reached 95% [47] or higher. Reconstructing images and preprocessing and postprocessing fetal images could be aided by AI [48]. Artificial Intelligence (AI) can also be used for placenta detection, fetal brain segmentation [49], fetal brain extraction, congenital heart disease prediction and gestational age prediction (Table 1).
c) Fetal Echocardiography
Fetal echocardiography is a specialized ultrasound exam that checks the developing baby's heart. To improve the accuracy of diagnosis, fetal echocardiography combines both grayscale and color Doppler ultrasound [50]. Grayscale images show the structure of the heart, while color Doppler allows visualization of blood flow within the heart chambers and major vessels. It uses various ultrasound views, including the upper abdomen, four-chamber view, five-chamber view, short axis view, three-vessel-trachea view, and longitudinal views of the aortic arch, ductal arch, and systemic veins [50,51].
Fetal echocardiography is an extremely specific and sensitive diagnostic procedure [50,52]. These days, fetal echocardiography is regarded as an essential part of the standard fetal anomaly scan. Most countries offer this type of scan in an effort to detect serious malformations, still, the detection rates of antenatal CHD are lower than those of the majority of other major structural anomalies [52]. Table 2 summarizes the use of ML and DL algorithms for analyzing fetal echocardiograms.
Fetal Abnormalities
a) Fetal Brain Anomalies
With a 1% incidence rate, anomalies of the CNS rank second among the most prevalent congenital fetal malformations [58]. The second-trimester anomaly scan uses special ultrasound techniques, like trans-ventricular and trans-cerebellar views, to examine the fetal skull. With the advancement of AI-assisted ultrasound diagnosis, it was possible to identify fetal brain anomalies with 92.93% accuracy [28]; as a result, AI has been predicted to replace other screening techniques for fetal malformations of the central nervous system. Table 3 summarizes the studies done to predict fetal brain anomalies.
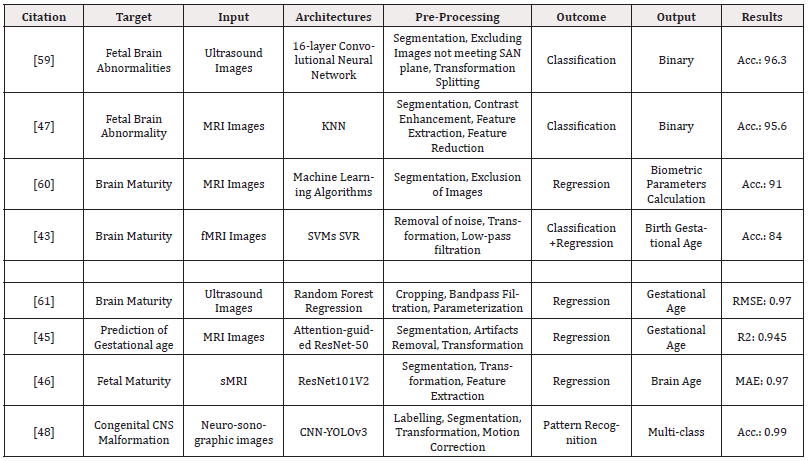
Table 3: Summarized Literature on the applications of AI algorithms for the prediction of brain abnormalities in fetuses using ultrasonography and MRI.
b) Fetal Heart Anomalies
Due to its constant motion and small size, the fetal heart is an intricate organ to study and track. The most prevalent fetal heart malformations are congenital heart diseases [59]. Sonographers use an ultrasound anomaly scan to diagnose fetal malformations during the 1st or 2nd trimester of pregnancy. Nevertheless, congenital cardiac disease detection rates are still low [60]. Owing to these difficulties, AI algorithms have emerged to automate ultrasound evaluations of fetus in order to increase prediction rates and fetal heart evaluation accuracy [61]. Table 4 summarizes the most recent studies which have developed ML and DL algorithms to detect or predict fetal heart diseases.
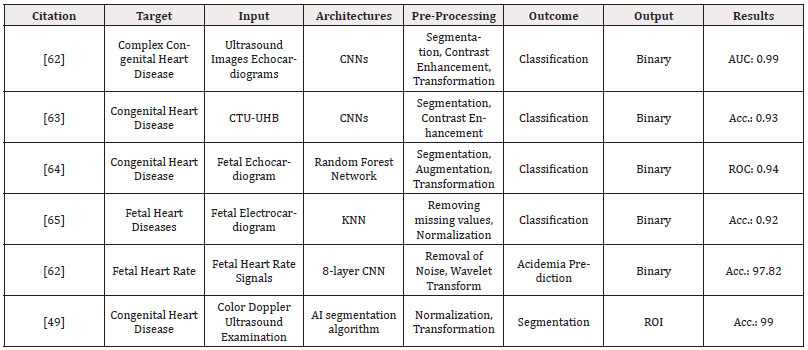
Table 4: Summarized Literature on the applications of AI algorithms for the prediction of heart abnormalities in fetus using ultrasonography and MRI.
AI Algorithms
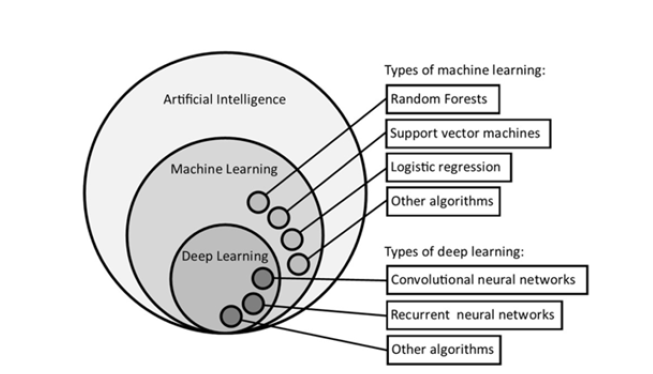
A lot of machine learning and deep learning algorithms are being utilized for the prediction of fetal brain and heart anomalies. This part of the review concludes the most commonly used algorithms for fetal analysis. Machine Learning (ML) lets computers teach themselves to improve at specific tasks over time, without needing explicit programming for every step. Neural networks organized into multiple layers (usually more than four, up to hundreds) are the basis of a particular kind of machine learning called Deep Learning (DL). In computer vision, image classification works by automatically finding hidden patterns in images, layer by layer. This lets the computer "understand" the image and make predictions. The relationship between AI, ML, and DL is illustrated in Figure 2, which also provides examples of ML techniques like logistic regression, random forests, and support vector machines, as well as DL techniques like convolutional and recurrent neural networks. Numerous additional machine learning techniques have been created, each with advantages and disadvantages depending on the particular issue. Although a thorough explanation of these techniques is outside the purview of this review, we do point the reader toward some very good online learning resources.
"Supervised" and "unsupervised" learning techniques comprise the broad categories of machine learning techniques. When there is labeled training data available, supervised learning works best. The algorithm learns how to make particular predictions from the data and applies that knowledge to new, unlabeled data. Unsupervised learning is another technique. Unlike supervised learning where data is labeled, unsupervised learning gives the algorithm unlabeled data and asks it to identify patterns or groupings within that data on its own. Since supervised learning is the focus of most of the medical AI literature, unsupervised learning can be an extremely useful technique for spotting patterns in patient data that have never been noticed before.
In medical AI, neural networks are the state-of-the-art. The creation of these models has led to superhuman performance in some medical tasks. Numerous neural network types have been created to work best for particular tasks (e.g., recurrent networks for language processing, and convolutional networks for computer vision). Convolutional Neural Networks (CNNs) have been implemented to analyze images obtained through fetal echocardiography and second-trimester screening scans. This analysis focuses on fetuses between 18 and 24 weeks of gestation. They discovered that it was possible to differentiate between the presence of inborn cardiac anomalies and normal heart development [60,62]. Neural networks are composed of multiple layers of interconnected processing units known as perceptron’s. Data, such as an image from a fetal echocardiogram, is fed into the first layer. Each subsequent layer builds upon the previous one, progressively extracting more complex features from the data as it travels through the network. This layered processing architecture allows the network to learn and make predictions based on the information it has analyzed.
Image Pre-Processing and Post-Processing
Obtaining high-quality images of a constantly moving target is one of the main challenges in fetal imaging, and motion-correction and pre-processing tools offer promising solutions. Traditionally, this required a skilled technician to re-acquire sequences and adjust acquisition planes on a regular basis. This requires a lot of time and is subject to operator variability. Pregnant patients lying motionless in an enclosed magnetic resonance imaging scanner may find it difficult to endure prolonged scan times. Fetal motion correction that is accurate and automated during initialization may result in better-quality images and possibly shorter scan times.
S Oldham, et al. [63] described a deep learning algorithm that used 15 key points to automatically detect fetal landmarks and estimate fetal poses. This allowed an automated parameter readjustment and potentially save technician time and MRI acquisitions. The researchers achieved a high degree of accuracy in predicting fetal pose using their model. The mean error between the predicted pose and the actual pose acquired was less than 4.5 millimeters. This rapid prediction (under 1 second) was achieved by comparing the model's output with real fetal pose data.
Time-efficient and repeatable tissue segmentation can be achieved through the use of artificial intelligence algorithms for image post-processing. Recent works have focused a lot of attention on the fetal brain. Historically, 2D images have been manually delineated to create 3D reconstructions of the fetal brain. N Khalili, et al. [64] reported the successful segmentation of a varied set of fetal brain, as well as fetal brain images deteriorated by artifact, using the U-net algorithm. Biparietal (BPD) and trans-cerebellum diameter, which are typically measured manually, can also be accurately performed by a CNN.
Performance Metrics
AUC was used by a few articles in this review, while the accuracy metric was used by mostly. Just 61.3% of the studies assessed sensitivity. Many studies (38%) evaluated performance with limited methods and lacked details on accuracy. While focused on creating good prediction systems for fetal complications, none explored practical use in clinical settings.
Traditional AI Applications in Fetal Imaging
This part of the review provides a brief overview of the conventional techniques which are being utilized in fetal analysis through various imaging modalities. Finding the correct fetal position during an ultrasound (fetal standard plane) heavily relies on the expertise of the technician. However, challenges arise because ultrasound images can vary significantly within the same exam (high intra-class variability) and appear quite different between different pregnancies (low inter-class similarity). This makes it difficult to develop automated systems that rely on pre-programmed image recognition [65]. AI is applicable in this situation. Using their feature representation capabilities, DCNNs can distinguish between similar ultrasonic views without the need for any manually created features.
AI algorithms are also playing a role in the intelligent measurement of head circumference. For the purpose of determining fetal abnormalities, assessing fetal growth parameters, developmental progress, establishing gestational age and weight, HC is an important biometric indicator [66,67]. Interobserver variation and partial boundary missing in cranial ultrasonography images can affect the accuracy of fetal HC measurement. Low contrast and artifacts are also problems with ultrasound images. Because of this, even sonographers with extensive experience find that measuring fetal HC by hand is difficult and time-consuming. In fetal ultrasound, the precise and effective quantification of HC is essential.
The primary factor used to determine fetal weight is abdominal circumference [68], which has significant clinical significance when assessing fetal development and performing early screening for oversized fetuses or intrauterine growth restriction [69]. Reducing fetal morbidity and mortality from these diseases can be achieved by increasing the measurement's accuracy. Sonographers in clinical practice are required to manually locate the standard plane of the abdomen. The accuracy of measuring AC can be impacted by variations in fetal posture, oligohydramnios, and the thickness of the abdominal wall in pregnant women [68,69].
To lessen the workload on sonographers, a quick and precise way to measure AC is needed. Segmentation of the fetal abdomen in ultrasound images is crucial for clinical applications. CNNs have demonstrated exceptional performance in this task compared to other methods. Additionally, measuring the Nuchal Translucency (NT), the fluid buildup at the back of the fetal neck [70], is important. Increased NT thickness can be associated with higher risks of birth defects like Down syndrome and poor pregnancy outcomes [70], [71,72]. To accurately measure NT thickness and enable the early detection of fetal structural abnormalities and genetic defects, the fetus should be placed in the standard sagittal plane. Nevertheless, it is challenging to obtain intelligent NT thickness measurement and standard plane acquisition. These difficulties include the fetus's mobility in the early stages of gestation, the short fetal parietal rump length, and the low signal-to-noise ratio of ultrasound images. Experts spend 25.56% less time on critical biometric tasks than unskilled sonographers [72].
Gestation age estimation is a significant additional use of AI in conjunction with fetal brain ultrasonography. Early pregnancy uses ultrasound measurements of fetal landmarks as a proven method for estimating gestational age. But as time passes and fetal growth and development variability is ignored, the error in ultrasound-estimated Gestational Age (GA) increases, and in certain studies, the error exceeds two weeks [72,73]. Thus, it is worthwhile to investigate the creation of a precise and trustworthy model for mid- and late-stage GA assessment (Figure 3).
Discussion
Several papers on the application of ML and DL to fetal ultrasound and MRI assessment are included in this review. DL algorithms obtain high-performance predictions by extracting important features from a small number of training samples using a CNN with multiple hidden layers. By automating the identification of fetal heart and brain, the neural networks are intended to improve fetal imaging assessment procedures. This will maximize the technique's accuracy and reduce examination time. The reviewed studies described a wide range of techniques, all of which were successful in meeting their goals by obtaining accuracy rates higher than 90% when it came to identifying the fetal brain and heart anomalies or their biometric measurements [74,75]. These results represent an improving accuracy and automation of fetal parameter estimations. The most prevalent birth defects in the heart are congenital cardiac conditions [59]. The goal of integrating ML and DL into ultrasonography evaluations is to improve detection rates of congenital cardiac conditions with greater accuracy. Research shows AI algorithms can effectively identify fetal structures as early as the first trimester, regardless of gestational age. This paves the way for developing a reliable protocol using Deep Learning (DL) architectures to create an automated and intelligent clinical decision support system specifically for early-stage fetal echocardiography.
The emergence of such algorithms highlights the adaptability of AI in fetal imaging. AI has the potential to improve antenatal care by offering more precise and effective ways to recognize and diagnose fetal abnormalities. These developments highlight how AI is revolutionizing the fetal analysis and present a bright future for advancements in fetal healthcare. With a 1% incidence rate, anomalies of the central nervous system rank second among congenital fetal malformations [58]. With high accuracy rates of up to 99% in identifying fetal brain standard planes, AI-assisted ultrasound diagnosis presents a viable substitute screening technique for fetal malformations of the central nervous system. A remarkable accuracy of over 96% has been achieved in the detection of fetal involving congenital brain anomalies [74].
This method highlights the effectiveness of AI algorithms as useful resources for novice medical practitioners that can greatly aid in enhancing diagnostic proficiency. Sonographers can measure the nuchal translucency in cases with Down syndrome and automatically identify the neck region in ultrasound images with the help of artificial intelligence. Our thorough analysis covers a wide range of ML and DL algorithms, current research, the benefits and drawbacks of each, possible roadblocks, and the expected uses of these algorithms in gynecology. This extensive study makes it clear that AI has a great deal of promise for antenatal diagnosis especially in fetal abnormalities. In fetal medicine, it may overcome diagnostic obstacles, expand available treatment options, and ultimately lead to better patient outcomes.
Challenges and Limitations
This review found a gap in research exploring the full potential of Machine Learning (ML) and Deep Learning (DL) for analyzing fetal ultrasounds and MRIs. While many studies focused on fetal brain image analysis and data processing (segmentation), there's a lack of research on applying these techniques for diagnosis. Numerous use cases are demonstrated, including those for automated fetal biometric measurements [75], prognostication [9], and disease classification [48].
Training and testing AI algorithms for fetal heart and brain anomaly prediction are hampered by small datasets, typically under 200-400 cases, often originating from a single source. To bridge this gap and ensure clinical relevance for diverse populations, fostering international collaboration is crucial. This would involve data sharing, establishing multicenter databases, and strengthening scientist-clinician partnerships.
Research on applying AI for disease classification and prognosis in fetal MRI has been limited. The inherent complexity of the task and the requirement for large datasets of rare fetal conditions pose significant challenges. For instance, [76-81] tested CNN’s ability to detect brain abnormalities using 225 fetal MRIs of varying gestational ages that are publicly available. This resulted in a 95% accuracy rate in identifying pathologies such as polymicrogyria, agenesis of the corpus callosum, colpocephaly, and Dandy-Walker spectrum malformations among other abnormal fetal brains. While radiologists experienced in interpreting fetal MRI studies would be able to identify these particular pathologies with ease, using this algorithm. However, it could be helpful in prioritizing which MRI studies need to be reviewed immediately over those that are not.
Based on our scoping review and our own thoughts, we think there are a few gaps that could open up interesting directions for future fetal imaging techniques and artificial intelligence research. Overall, what we found lacking was research on the potential applications of Natural Language Processing (NLP) in clinical governance and communication of important findings to clinicians. Additionally, very few image classification studies had been published to support the diagnosis and prognostication of fetal ultrasound and MRI, and even fewer publications contributed to the real-world evidence of enhanced clinical workflow and efficiency in practice. Given the serious consequences and high stakes associated with inaccurate fetal imaging results, it will be crucial to demonstrate the reliability of any new technology before implementing it routinely and to pursue patient acceptability.
Conclusion
Deep learning (DL), a subset of artificial intelligence, is so good at identifying patterns in images, it is especially useful for practitioners who use image-based data to diagnose and make decisions in healthcare settings. AI is positioned as a potential adjunct or alternative screening method for fetal anomaly identification due to its significant advancements in recent years and its improved capacity to detect prenatal fetal brain and heart malformations. Research demonstrates AI's potential for precise cardiac and brain structure detection. Studies have shown that CNNs in particular, perform as well as experts in terms of prediction and similarity when it comes to differentiating between cardiac or brain anomalies and normal development. Artificial Intelligence (AI) tools have proven remarkably accurate at automatically measuring fetal head biometry and identifying brain structures and planes. Additionally, there was a decrease in false-negative results in the diagnosis of fetal brain abnormalities and anomaly detection performance that was comparable to that of the experienced sonographers. It is clear that Artificial Intelligence (AI) has the potential to improve prenatal care by offering more precise and effective ways to recognize and diagnose fetal abnormalities. These developments highlight how AI is revolutionizing the field and present a bright future for advancements in fetal healthcare.
Acknowledgements
The authors acknowledge the support from Buch International Hospital, Multan, Pakistan.
Conflict of Interest
None.
References
- A C Rossi and F Prefumo (2013) Accuracy of ultrasonography at 11-14 weeks of gestation for detection of fetal structural anomalies: A systematic review. Obstet Gynecol 122 (6): 1160-1167.
- D Selvathi and R Chandralekha (2022) Fetal biometric based abnormality detection during prenatal development using deep learning techniques. Multidimens Syst Signal Process 33(1): 1-15.
- L Davidson and M R Boland (2021) Towards deep phenotyping pregnancy: a systematic review on artificial intelligence and machine learning methods to improve pregnancy outcomes. Brief Bioinform 22(5): 1-29.
- A L Depla, L De Wit, T J Steenhuis, M G Slieker, D N Voormolen, et al. (2021) Effect of maternal diabetes on fetal heart function on echocardiography: systematic review and meta-analysis. Ultrasound Obstet Gynecol 57(4): 539-550.
- J Courtney, Weston Troja, Kathryn J Owens, Heather M Brockway, Andrea C Hinton, et al. (2020) Abnormalities of placental development and function are associated with the different fetal growth patterns of hypoplastic left heart syndrome and transposition of the great arteries. Placenta 101: 57-65.
- TG Day, B Kainz, J Hajnal, R Razavi and JM Simpson (2021) Artificial intelligence, fetal echocardiography, and congenital heart disease. Prenat Diagn 41(6): 733-742.
- K Kaur, C Singh and Y Kumar (2023) Diagnosis and Detection of Congenital Diseases in New-Borns or Fetuses Using Artificial Intelligence Techniques: A Systematic Review. Archives of Computational Methods in Engineering 30(5): 3031-3058.
- W Shi, Guohui Yan, Yamin Li, Haotian Li, Tingting Liu, et al. (2020) Fetal brain age estimation and anomaly detection using attention-based deep ensembles with uncertainty. Neuroimage 223: 117316.
- L Shen, Jimmy Zheng, Edward H Lee, Katie Shpanskaya, Emily S McKenna, et al. (2022) Attention-guided deep learning for gestational age prediction using fetal brain MRI. Sci Rep 12(1): 1408.
- N J Wald and A Kennard (1998) Routine Ultrasound Scanning for Congenital Abnormalities. Ann N Y Acad Sci 847(1): 173-180.
- A Sarma and Spruthi (2023) Congenital Brain Malformations- Update on Newer Classification and Genetic Basis. Semin Roentgenol 58(1): 6-27.
- A Ahmad, A Tariq, HK Hussain and AY Gill (2023) Equity and Artificial Intelligence in Surgical Care: A Comprehensive Review of Current Challenges and Promising Solutions. BULLET: Jurnal Multidisiplin Ilmu 2(2): 443-445.
- A Ahmad, HK Hussain, H Tanveer, T Kiruthiga and K Gupta (2023) The Intelligent Heart Rate Monitoring Model for Survivability Prediction of Cardiac Arrest Patients Using Deep Cardiac Learning Model. Proceedings of the International Conference on Intelligent Systems for Communication, IoT and Security 376-381.
- HK Hussain, A Ahmad, MA Adam, T Kiruthiga and K Gupta (2023) Prediction of Blood Lactate Levels in Children after Cardiac Surgery using Machine Learning Algorithms, Proceedings of the 3rd International Conference on Artificial Intelligence and Smart Energy ICAIS 2023 1163-1169.
- A Ahmad, A Tariq, HK Hussain, Ahmad and Y Gill (2023) Revolutionizing Healthcare: How Deep Learning is poised to Change the Landscape of Medical Diagnosis and Treatment, Journal of Computer Networks, Architecture and High Performance Computing 5(2): 458-471.
- H Khawar Hussain, A Tariq, AY Gill and A Ahmad (2022) Transforming Healthcare: The Rapid Rise of Artificial Intelligence Revolutionizing Healthcare Applications. BULLET: Jurnal Multidisplin Ilmu 1(2).
- A Shiwlani, M Khan, A Mannan, K Sherani and MU Qayyum (2024) Synergies of AI and Smart Technology: Revolutionizing Cancer Medicine, Vaccine Development, and Patient Care. International Journal of Social Humanities and Life Sciences 1 (1): 10-18.
- M Yaqub, B Kelly, AT Papageorghiou and JA Noble (2017) A Deep Learning Solution for Automatic Fetal Neurosonographic Diagnostic Plane Verification Using Clinical Standard Constraints. Ultrasound Med Biol 43(12): 2925-2933.
- H Chen, Lingyun Wu, Qi Dou, Jing Qin, Shengli Li, Jie Zhi Cheng, et al. (2017) Ultrasound Standard Plane Detection Using a Composite Neural Network Framework. IEEE Trans Cybern 47 (6): 1576-1583.
- CF Baumgartner, Konstantinos Kamnitsas, Jacqueline Matthew, Tara P Fletcher, Sandra Smith, et al. (2017) SonoNet: Real-Time Detection and Localisation of Fetal Standard Scan Planes in Freehand Ultrasound, IEEE Trans Med Imaging 36(11): 2204-2215.
- J Spilka, G Georgoulas, P Karvelis, V Chudáček, CD Stylios, et al. (2014) Discriminating Normal from ‘Abnormal’ Pregnancy Cases Using an Automated FHR Evaluation Method. Lecture Notes in Computer Science including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics 8445: 521-531.
- Q Lin, Siyuan Shi, Yujuan Zhang, Shaoli Yin, Xuye Liu, et al. (2022) How much can AI see in early pregnancy: A multi‐center study of fetus head characterization in week 10-14 in ultrasound using deep learning. Comput Methods Programs Biomed 226: 107170.
- I U Khan, Nida Aslam, Fatima M Anis, Samiha Mirza, Alanoud AlOwayed, et al. (2022) Amniotic Fluid Classification and Artificial Intelligence: Challenges and Opportunities. Sensors 22(12): 4570.
- AM Ponsiglione, C Cosentino, G Cesarelli, F Amato, and M Romano (2021) A Comprehensive Review of Techniques for Processing and Analyzing Fetal Heart Rate Signals. Sensors 21(18): 6136.
- G Someshwaran and V Sarada (2022) A Research Review on Fetal Heart Disease Detection Techniques. Proceedings - 2022 6th International Conference on Intelligent Computing and Control Systems ICICCS 1674-1681.
- M Ribeiro, João Monteiro Santos, Luísa Castro, Luís Antunes, Cristina Costa Santos, et al. (2021) Non-linear Methods Predominant in Fetal Heart Rate Analysis: A Systematic Review. Front Med Lausanne 8: 661226.
- T Ciceri, Luca Casartelli, Florian Montano, Stefania Conte, Letizia Squarcina, et al. (2024) Fetal brain MRI atlases and datasets: A review. Neuroimage 292: 120603.
- S Xiao, Junmin Zhang, Ye Zhu, Zisang Zhang, Haiyan Cao, et al. (2023) Application and Progress of Artificial Intelligence in Fetal Ultrasound. J Clin Med (9): 3298.
- Z Leibovitz, T Lerman Sagie and L Haddad (2022) Fetal Brain Development: Regulating Processes and Related Malformations. Life 12(6): 809.
- L S Chitty, GH Hunt, J Moore, and MO Lobb (1991) Effectiveness of routine ultrasonography in detecting fetal structural abnormalities in a low-risk population. BMJ 303(6811): 1165-1169.
- J Sonek (2007) First trimester ultrasonography in screening and detection of fetal anomalies. Am J Med Genet C Semin Med Genet 145C (1): 45-61.
- Chitty LS, Hunt GH, Moore J, Lobb MO, (1991) Effectiveness of routine ultrasonography in detecting fetal structural abnormalities in a low-risk population Br Med J 303(6811): 1165-1169.
- S Xiao, Junmin Zhang, Ye Zhu, Zisang Zhang, Haiyan Cao, et al. (2023) Application and Progress of Artificial Intelligence in Fetal Ultrasound J Clin Med 12(9): 3298.
- Xie HN, Wang N, He M, Zhang LH, Cai HM, et al. (2020) Using deep-learning algorithms to classify fetal brain ultrasound images as normal or abnormal. Ultrasound in Obst Gynecol 56(4): 579-587.
- Saleem SN (2014) Fetal MRI: An approach to practice: A review. J Adv Res 5(5): 507-523.
- Coakley FV, Glenn OA, Qayyum A, Barkovich AJ, Goldstein R, et al. (2004) Fetal MRI: A Developing Technique for the Developing Patient. AJR Am J Roentgenol 182(1): 243-252.
- Lloyd DFA, Kuberan Pushparajah, John Simpson M, Joshua van Amerom FP, Milou van Poppel PM, et al. (2019) Three-dimensional visualisation of the fetal heart using prenatal MRI with motion-corrected slice-volume registration: a prospective, single-centre cohort study. The Lancet 393(10181): 1619-1627.
- Kim AG, Gabriella Norwitz, Monita Karmakar, Maria Ladino Torres, Deborah Berman R, et al. (2020) Discordant prenatal ultrasound and fetal MRI in CDH: wherein lies the truth? J Pediatr Surg 55(9): 1879-1884.
- Prayer D, Malinger G, De Catte L, De Keersmaecker B, Gonçalves LF, et al. (2023) ISUOG Practice Guidelines (updated): performance of fetal magnetic resonance imaging. Ultrasound Obstet Gynecol 61(2): 278-287.
- Raybaud C, Levrier O, Brunel H, Girard N, Farnarier P (2003) Child’s nervous system: ChNS: official journal of the International Society for Pediatric Neurosurgery. Childs Nerv Syst 19(7-8): 455-470.
- Manganaro L, Silvia Capuani, Marco Gennarini, Valentina Miceli, Roberta Ninkova, et al. (2023) Fetal MRI: what’s new? A short review. Eur Radiol Exp 7(1): 41.
- Meshaka R, Gaunt T, Shelmerdine SC (2023) Artificial intelligence applied to fetal MRI: A scoping review of current research, Br J Radiol 96(1147): 20211205.
- Christopher Smyser D, Nico Dosenbach UF, Tara Smyser A, Abraham Snyder Z, Cynthia Rogers E, et al. (2016) Prediction of brain maturity in infants using machine-learning algorithms Neuroimage 136: 1-9.
- Seyed Sadegh Mohseni Salehi, Seyed Raein Hashemi, Clemente Velasco Annis, Abdelhakim Ouaalam, Judy A Estroff, et al. (2018) Real-time automatic fetal brain extraction in fetal MRI by deep learning. Proceedings - International Symposium on Biomedical Imaging 720-724.
- Liyue Shen, Jimmy Zheng, Edward H Lee, Katie Shpanskaya, Emily S McKenna, et al. (2022) Attention-guided deep learning for gestational age prediction using fetal brain MRI. Scientific Reports 12(1): 1408.
- Jinwoo Hong, Hyuk Jin Yun, Gilsoon Park, Seonggyu Kim, Yangming Ou, et al. (2021) Optimal Method for Fetal Brain Age Prediction Using Multiplanar Slices from Structural Magnetic Resonance Imaging. Front Neurosci 15: 714252.
- Attallah O, Sharkas MA, Gadelkarim H (2019) Fetal Brain Abnormality Classification from MRI Images of Different Gestational Age. Brain Sci 9(9): 231.
- Lin M, He X, Guo H, He M, Zhang L, et al. (2022) Use of real-time artificial intelligence in detection of abnormal image patterns in standard sonographic reference planes in screening for fetal intracranial malformations. Ultrasound in Obst Gynecol 59(3): 304-316.
- Syed Naveed Mohsin, Abubakar Gapizov, Chukwuyem Ekhator, Noor Ain U, Saeed Ahmad, et al. (2023) The Role of Artificial Intelligence in Prediction, Risk Stratification, and Personalized Treatment Planning for Congenital Heart Diseases Cureus 15(8): e44374.
- Chaoui R, Abuhamad A, Martins J, Heling KS (2020) Recent Development in Three and Four Dimension Fetal Echocardiography. Fetal Diagn Ther 47(5): 345-353.
- W Lee, L Allan, J S Carvalho, R Chaoui, J Copel, et al. ISUOG consensus statement: what constitutes a fetal echocardiogram? Ultrasound Obstet Gynecol 32(2): 239-242.
- TG Day, B Kainz, J Hajnal, R Razavi, JM Simpson (2021) Artificial intelligence, fetal echocardiography, and congenital heart disease. Prenat Diagn 41(6): 733-742.
- J Dong, S Liu, Y Liao, H Wen, B Lei, et al. (2020) A Generic Quality Control Framework for Fetal Ultrasound Cardiac Four-Chamber Planes. IEEE J Biomed Health Inform 24(4): 931-942.
- EJ Topol (2019) High-performance medicine: the convergence of human and artificial intelligence. Nature Medicine 25(1): 44-56.
- H Chen, D Ni, J Qin, S Li, X Yang, et al. (2015) Standard Plane Localization in Fetal Ultrasound via Domain Transferred Deep Neural Networks. IEEE J Biomed Health Inform 19(5): 1627-1636.
- CF Baumgartner, K Kamnitsas, J Matthew, TP Fletcher, S Smith, et al. (2017) SonoNet: Real-Time Detection and Localisation of Fetal Standard Scan Planes in Freehand Ultrasound. IEEE Trans Med Imaging 36(11):2204-2215.
- TK Le, VT Truong, BP Nguyen, TH Nguyen, W Mazur, et al. (2020) APPLICATION OF MACHINE LEARNING IN SCREENING OF CONGENITAL HEART DISEASES USING FETAL ECHOCARDIOGRAPHY. J Am Coll Cardiol 75(11): 648.
- D Paladini, G Malinger, R Birnbaum, A Monteagudo, G Pilu, et al. (2021) ISUOG Practice Guidelines (updated): sonographic examination of the fetal central nervous system. Part 2: performance of targeted neurosonography. Ultrasound in Obstetrics & Gynecology 57(4): 661-671.
- H N Xie, N Wang, M He, L H Zhang, H M Ca, et al. (2020) Using deep-learning algorithms to classify fetal brain ultrasound images as normal or abnormal. Ultrasound Obstet Gynecol 56(4): 579-587.
- J She, Haiying Huang, Zhijun Ye, Wei Huang, Yan Sun, et al. (2023) Automatic biometry of fetal brain MRIs using deep and machine learning techniques. Sci Rep 13(1): 1-11.
- A I L Namburete, RV Stebbing, B Kemp, M Yaqub, AT Papageorghiou, et al. (2015) Learning-based prediction of gestational age from ultrasound images of the fetal brain. Med Image Anal 21(1): 72-86.
- R Arnaout, L Curran, Y Zhao, JC Levine, E Chinn, et al. (2021) an ensemble of neural networks provides expert-level prenatal detection of complex congenital heart disease. Nature Medicine 27(5): 882-891.
- JH Miao and KH Miao (2018) Cardiotocographic Diagnosis of Fetal Health based on Multiclass Morphologic Pattern Predictions using Deep Learning Classification. International Journal of Advanced Computer Science and Applications 9(5): 1-11.
- VT Truong, Binh P Nguyen, Thanh Hoang Nguyen, Wojciech Mazur, Eugene S Chung, et al. (2022) Application of machine learning in screening for congenital heart diseases using fetal echocardiography. Int J Cardiovasc Imaging 38(5): 1007-1015.
- L Pullagura, M Rao Dontha and S Kakumanu (2021) Recognition of Fetal heart diseases through machine learning techniques. Ann Rom Soc Cell Biol 25(6): 2601-2615.
- Anda, AS Marcu, CL Patru , D Ruican, R Nagy, et al. (2023) Learning deep architectures for the interpretation of first-trimester fetal echocardiography (LIFE) - a study protocol for developing an automated intelligent decision support system for early fetal echocardiography. BMC Pregnancy Childbirth 23(1): 1-10.
- R Arnaout, L Curran, Y Zhao, JC Levine, E Chinn, et al. (2021) An ensemble of neural networks provides expert-level prenatal detection of complex congenital heart disease. Nature Medicine 27(5): 882-891.
- ME Philip, A Sowmya, H Avnet, A Ferreira, G Stevenson, et al. (2019) Convolutional neural networks for automated fetal cardiac assessment using 4d b-mode ultrasound. Proceedings - International Symposium on Biomedical Imaging 2019: 824-828.
- Z Zhao, Y Deng, Y Zhang, Y Zhang, X Zhang et al. (2019) DeepFHR: Intelligent prediction of fetal Acidemia using fetal heart rate signals based on convolutional neural network. BMC Med Inform Decis Mak 19(1): 286.
- S Oldham, A Arnatkevic̆iūtė, RE Smith, J Tiego, MA Bellgrove, et al. The efficacy of different preprocessing steps in reducing motion-related confounds in diffusion MRI connectomics. Neuroimage 222: 117252.
- N Khalili, E Turk, MJNL Benders, P Moeskops, NHP Claessens, et al. (2019) Automatic extraction of the intracranial volume in fetal and neonatal MR scans using convolutional neural networks. Neuroimage Clin 24: 102061.
- H Chen, Lingyun Wu, Qi Dou, Jing Qin, S Li, et al. (2017) Ultrasound Standard Plane Detection Using a Composite Neural Network Framework. IEEE Trans Cybern 47(6): 1576-1583.
- Jing Li, Yi Wang, Baiying Lei, Jie-Zhi Cheng, Jing Qin, et al. (2018) Automatic Fetal Head Circumference Measurement in Ultrasound Using Random Forest and Fast Ellipse Fitting. IEEE J Biomed Health Inform 22(1): 215-223.
- Zahra Sobhaninia, Shima Rafiei, Ali Emami, Nader Karimi, Kayvan Najarian, et al. (2019) Fetal Ultrasound Image Segmentation for Measuring Biometric Parameters Using Multi-Task Deep Learning. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS 31(8): 6545-6548.
- J Jang, Y Park, B Kim, SM Lee, JY Kwonet al. (2018) Automatic estimation of fetal abdominal circumference from ultrasound images. IEEE J Biomed Health Inform 22(5): 1512-1520.
- F He, Y Wang, Y Xiu, Y Zhang, L Chen (2021) Artificial Intelligence in Prenatal Ultrasound Diagnosis, Front Med (Lausanne). 8: 729978.
- Robail Yasrab, Zeyu Fu, He Zhao, Lok Hin Lee, Harshita Sharma, et al. (2023) A Machine Learning Method for Automated Description and Workflow Analysis of First Trimester Ultrasound Scans. IEEE Trans Med Imaging 42(5): 1301-1313.
- Yasrab et al., A Machine Learning Method for Automated Description and Workflow Analysis of First Trimester Ultrasound Scans, IEEE Trans Med Imaging, vol. 42, no. 5, pp. 1301-1313, May 2023,
- K O Kagan, D Wright, A Baker, D Sahota and KH Nicolaides (2008) Screening for trisomy 21 by maternal age, fetal nuchal translucency thickness, free beta-human chorionic gonadotropin and pregnancy-associated plasma protein-A. Ultrasound Obstet Gynecol 31(6): 618-624.
- L Zhang, Di Dong, Yongqing Sun, Chaoen Hu, Congxin Sun, et al. (2022) Development and Validation of a Deep Learning Model to Screen for Trisomy 21 During the First Trimester from Nuchal Ultrasonographic Images. JAMA Netw Open 5(6): e2217854-e2217854.
- O Attallah, H Gadelkarim and MA Sharkas (2018) Detecting and Classifying Fetal Brain Abnormalities Using Machine Learning Techniques. Proceedings - 17th IEEE International Conference on Machine Learning and Applications, ICMLA 2018 1371-1376.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.