Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Glutathione Medicines as Geroprotectors: Molecular Effects in Inflammaging Model
*Corresponding author: Ekaterina Mironova, PharmaVam CJSC, 190121 Saint-Petersburg, Russia.
Received: July 23, 2024; Published: July 26, 2024
DOI: 10.34297/AJBSR.2024.23.003075
Abstract
The prevention of accelerated aging, optimization of diagnostic and treatment of age-associated diseases remain a vital task for modern molecular medicine and gerontology. The aging of cells, organs and tissues is a complex process in which a key role belongs to neuroimmune-endocrine regulation mechanisms. The investigation of the effects of glutathione drugs as potential geroprotectors on the expression of signaling molecules that regulate the involution in cells helps to find the signaling molecules, which are potential targets for geroprotection, and thereby extend indications for glutathione-based drugs. The purpose of this study is to investigate the effect of two tripeptide glutathione drugs (V-007 and G-004) on the expression of key signaling molecules participating in the mechanisms of cellular senescence. The study of two signaling molecules involved in cellular senescence has demonstrated that the target of V-007 geroprotective action is prohibitin, and the target of G-004 geroprotective action is telomerase reverse transcriptase (hTERT) and prohibitin. G-004 has a wider and more expressed geroprotective effect, affecting both mitochondrial and nuclear mechanisms of cellular senescence. A further investigation into the effects of V-007 and G-004 will allow to specify the mechanism of their impact on the expression of key signaling gerotropic molecules and thereby map paths for the optimization of their use as medicines for the prevention of premature aging and for treatment of age-associated diseases.
Keywords: Senescence, Aging, Geroprotectors, Glutathione, Signaling molecules, Target diagnostic
Introduction
Aging is a complex biological process the main aspect of which is the accumulation of involutional somatic changes in the body throughout the ontogenesis. “Cellular aging” is a state in which cells undergo an irreversible cell-cycle arrest in response to various cellular stresses. Such irreversible cell-cycle arrest is considered to be the main characteristic of aging cells, but recent studies have revealed yet other properties that characterize this type of cells. Senescent cells express pro-inflammatory cytokines, growth factors, and matrix metalloproteinases, which jointly form the so-called Senescence-Associated Secretory Phenotype (SASP) [1]. Such cells are viable in vitro, in contrast to apoptotic cells which are prone to programmed cell death. Some SASP factors play an important role in inducing a persistent cell-cycle arrest in senescent cells and, presumably, contribute to tumor suppression during cellular aging. Nevertheless, multiple SASP factors may cause a chronic inflammation and/or oncogenesis depending on biological context [2]. A significant contribution into the implementation of inflammaging mechanisms is made by the disruption of redox regulation of the transmission of intra- and intercellular signals and gene expression [3]. A key role in the stable maintenance of the redox status of cells and ensuring redox regulation belongs to the glutathione/oxidized glutathione (GSH/GSSG) system. Tripeptide Glutathione GSH (ϒ-L-glutamyl-L-cysteinyl-L-glycine) is one of the key intracellular antioxidants [4], and being an endogenous antioxidant that affects many cellular functions plays a crucial role in the utilization and regulation of reactive oxygen and nitrogen species (ROS and RNS, respectively) in organisms. GSSG has receptor-mediated effects on intracellular processes [5]. Based on GSSG, pharmacological preparations have been synthesized which have a stabilizing effect on mitochondria, regulating the expression of membrane proteins [6].
These include the preparation G-004 (disodium salt of GSSG with d-metal in nanoconcentration) and the preparation V-007 (complex G-004 with the nucleoside inosine). Both drugs are obtained from the PharmaVam Company (St. Petersburg, Russia).
Since mitochondrial dysfunction plays an important role in the development of cellular aging processes, we investigated the geroprotective properties of these drugs in the inflammaging model.
Various signaling molecules are involved in the inflammaging mechanisms, the key ones are as the follows:
1) Prohibitin-the mitochondrial protein, which ensures the preservation of the mitochondrial membrane and prevents mitochondrial apoptosis;
2) P65-the integral marker of apoptosis (programmed cell death);
3) Ki-67-the integral marker of cell proliferation;
4) SIRT-6-the powerful endogenous geroprotector;
5) hTERT-telomerase reverse transcriptase, the key component of the telomerase complex;
6) Klotho-the protein regulating cellular senescence;
7) Melatonin (receptors)-receptors of the key regulator of biorhythms and geroprotective factor.
8) In this study we selected (based on the significance of their biological gerotropic properties) two signaling molecules-prohibitin and hTERT-as potential targets for the action of different doses of glutathione-containing drugs V-007 and G-004 (3mg; 1.5mg; 0.3mg).
Prohibitin (PHB) is the multifunctional mitochondrial protein. It is involved in regulation of the respiratory activity of mitochondria and responsible for the stability of organization and the number of copies of mitochondrial DNA. Mitochondrial prohibitin protects cells from the oxidative stress [7,8].
Human Telomerase Reverse Transcriptase (hTERT). The strategy of developments for telomerase activation presumes preventing the age-related shortening of telomeres, which allow to slow down the aging of organism and extend the life-span. Telomerase consists of two components: Telomerase Reverse Transcriptase (hTERT) and RNA molecule (TERC), which is used as a matrix for reverse transcription in the lengthening of telomeres [9]. hTERT plays a key role in the telomerase complex, as it is a protein that performs the main function by catalyzing the formation of a new phosphodiester bond in the synthesis of a telomeric repeat. The expression of the telomerase reverse transcriptase gene correlates with telomerase enzymatic activity in the body [10-12].
Materials and Methods
For the study the cellular senescence (inflammaging) model in culture of human endometrial cells (3rd–5th passages) was used. The endometrial cell line (ECL) (AcceGen Biotech) was cultured with the use of the DMEM/F12 growth medium containing 10% FBS; cultivation conditions: 37°C and 5% CO2. The growth medium was replaced every 2-4 days. Once the cells formed a confluent monolayer, they were dispersed on a 1:3 ratio. The passage was done with 0.25% trypsin solution (Gibco, USA) and 0.02% Versen solution (BioloT, Russia).
The absence of bacterial, fungal, and mycoplasmal pneumonia contamination was confirmed by Microbiological analysis of the ECL. On the 3rd passage, the line was subjected to cryopreservation and stored in liquid nitrogen in Dewar's flask. After decryopreservation, the ECL was characterized by various molecular biological methods. The ECL was mostly a population of mesenchymal stem cells, as evidenced by the following characteristics of the line:
1) The high (above 95%) expression of mesenchymal markers (CD44, CD73, CD90, CD105, CD146) and the low (below 5%) expression of hematopoietic markers (CD34, CD45, HLA-DR of class II);
2) Adhesion to the plastic surface;
3) Fibroblast-like morphology of cells;
4) A multipotent status (ability to differentiate into osteoblasts, chondrocytes, and adipocytes).
5) Karyological analysis demonstrated a normal karyotype of the ECL (46, XX), which remained stable during the cultivation.
The line was scaled to the 3rd passage after the defrosting with the subsequent inoculation of cells onto specially prepared sterile slides in 24-cell trays. Three replications were made for each marker.
Once the culture in wells achieved a monolayer of 70-80%, the culture growth medium was replaced, to which the solution of drugs at different doses were added. V-007 and G-004 were tested at three doses (maximum, intermediate, and minimum). Each dose was contained in 0.1ml of solution. The volume of the drug solution injected into each well was 10% of the volume of the nutrient medium and could not exceed it. Thus, the ratio of the volume of the drug solution to the volume of the nutrient medium was 1:10, while the maximum dose of each tested drug was 3.0mg, the intermediate dose was 1.5mg, and the minimum one was 0.3mg. A separate subgroup was formed for each dose of the drug.
Groups under study:
a) Group 1-human endometrial cell culture (control);
b) Group 2-the inflammaging model (genotoxic stress impact) in human endometrial cell culture;
c) Group 3-V-007 solution at three doses (3mg, 1.5mg, and 0.3mg), without genotoxic stress impact;
d) Group 4-G-004 solution at three doses (3mg, 1.5mg, and 0.3mg), without genotoxic stress impact;
e) Group 5-V-007 solution at three doses (3mg, 1.5mg, and 0.3mg) in the inflammaging model;
f) Group 6-G-004 solution at three doses (3mg, 1.5mg, and 0.3mg) in the inflammaging model.\
g) In 24 hours after the cultivation, the experimental groups were subjected to genotoxic stress for 30 min (exposure to UV irradiation by Philips TUV 8W lamps (Philips, Netherlands), with the wavelength of 253.7 nm (UV light). After UV irradiation, the cells were washed twice with PBS (1×), then placed in the standard volume of the growth medium and incubated under the standard conditions (5%CO2, 37°C).
After 48 hours, the experiment was stopped, the cells were fixed, and an immunocytochemical study was carried out to determine the expression of key signaling molecules involved in the mechanism of cellular senescence. Immunocytochemical study. The immunocytochemical study was performed on slides with a monolayer of cells by the use of primary antibodies to prohibitin (ab75766, 1:500, Abcam, UK) and telomerase reverse transcriptase-hTERT (ab186735, 1:50, Abcam, UK). Secondary antibodies (AlexaFluor 488, 647 (Abcam, UK)) were used for fluorescent staining. All primary antibodies were checked at the standard control staining (positive and negative) to select a protocol and antibody titer.
Morphometry and Computer Analysis of Images. To evaluate the results of immunocytochemical staining, a morphometric study was performed using the microscope image analysis system consisting of an Olympus IX73 microscope, Olympus DP80 digital camera, AMD Ryzen 3 3200G personal computer, and QuPath software. 12-bit (grayscale) images were obtained using a pseudostaining technique. The red and green colors were chosen as pseudocolors for expression of the markers, and the blue for nuclei as the most appropriate for demonstration and subsequent morphometric analysis of images. At least 5 fields of view were analyzed at 600×magnification in each case.
The relative area of marker expression per nucleus was calculated by dividing the total expression area (in pixels) in the field of view by the number of nuclei. Statistical Analysis of Results. The Shapiro-Wilk test was used to test normality of the obtained data. Parametric statistical methods were used in the case where the sample statistic followed the normal distribution, otherwise nonparametric methods (Mann-Whitney, Kruskal-Wallis tests) were applied. However, if the data of at least one sample failed to follow the normal distribution law, then the typical value in the comparison groups was characterized by the median with the upper and lower quartiles Me (Q1÷Q3).
Results and Discussions
The average expression rates obtained in an immunocytochemical investigation of markers in cell culture for the control and inflammaging groups, with the addition of 0.1mL (water for injections) are shown in Table 1.
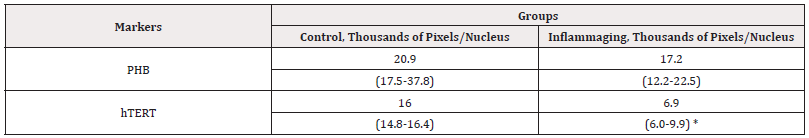
Table 1: The average parameters of markers in cell culture (with the addition of 0.1mL of water for injections).
Note*: Statistic difference in comparison groups at p<0.05.
The logic of the experiments may be summarized as follows:
The expression of PHB and hTERT under physiological condition (the injection be water);
The expression of PHB and hTERT under the high doses of the drugs (3.9mg) and the comparison with the group administered only with water.
The expression of PHB and hTERT under the 10 times less doses of the drugs (3.9mg) and the comparison with the group administered only with water.
The investigation of drugs in the intermediate doses to scope the optimal dose regime for further control of side-effects (Table 1).
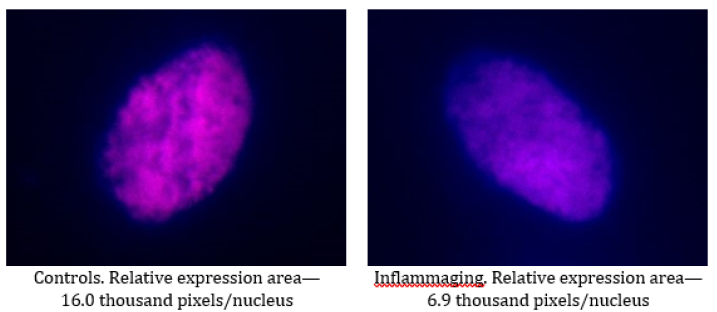
The data presented in Table 1 demonstrate that the expression of hTERT is evidently lower in the group of cells exposed to UV irradiation (6.9 thousand pixels/nucleus), as compared to the control group (16.0 thousand pixels/nucleus), which points to the aging of cells and the efficacy of the selected inflammaging model (Figure 1). The PHB level in the UV-exposed group (17.2 thousand pixels/nucleus) is lower than in the controls (20.9 thousand pixels/nucleus), but this difference is not statistically significant. This could be explained by the fact that PHB being a mitochondrial protein quickly changes its activity in the response to damage, thereby preventing cell death (Figure 1, 2).
The data presented in Table 2 demonstrate that the differences in the expression of hTERT are insignificant in the UV-exposed group (13.7 thousand pixels/nucleus) as compared to the control group (11.5 thousand pixels/nucleus) (Figure 3) as well as to the inflammaging group with the injection by water (6.9 thousand pixels/nucleus) (Table 1).
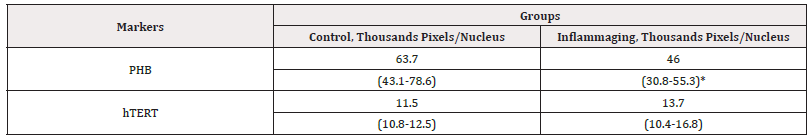
Table 2: The average parameters of markers in cell culture under the action of V-007 (3mg).
Note*: Statistic difference in comparison groups at p<0.05.
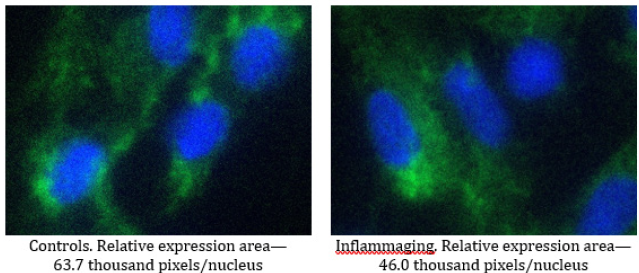
The level of prohibitin in the UV-exposed group (46.0 thousand pixels/nucleus) was significantly lower than in the control group (63.7 thousand pixels/nucleus) (Figure 4) and exhibited significant differences in the inflammaging group with the injection by water (17.2 thousand pixels/nucleus) (Table 1). These results demonstrate that V-007 has no impact on cell division despite an increase in enzyme production under the drug administration. In the case of V-007 administration, prohibitin showed a considerable increase of expression both in normal cells with the drug administration and in UV-exposed cells, but failed to achieve the target values of control levels. These outcomes prove that the target for V-007 geroprotective action is prohibitin which promotes mitochondrial metabolism to fight oxidative stress and alteration (Figure 3,4).
The results presented in Table 3 illustrate that the differences in hTERT expression are insignificant in the UV-exposed group (15.7 thousand pixels/nucleus) in comparison with the control group (13.2 thousand pixels/nucleus) Figure 5 (Table 3).
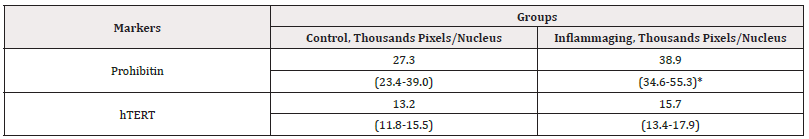
Table 3: The average parameters of markers in cell culture under the action of G-004 (3mg).
Note*: Statistic difference in comparison groups at p<0.05.
However, the comparison with the inflammaging group with the injection by water (6.9 thousand pixels/nucleus) (Table 1) revealed significant difference characterized by the increasing of hTERT expression, which demonstrates the G-004 promoting effect on telomerase activity (reparation) in the case of damage. The level of prohibitin in the UV-exposed group (38.9 thousand pixels/nucleus) was significantly higher than in the control group (27.3 thousand pixels/nucleus) (Figure 6) and in the inflammaging group with the injection by water (17.2 thousand pixels/nucleus) (Table 1). The comparison with the values of V-007 administration showed no significant differences (46.0 thousand pixels/nucleus). Thus, the obtained data points to the effect of G-004 on the up-regulation of mitochondrial chaperone proteins along with an increased resistance of cells to oxidative stress. Our study revealed the same effect of G-004 and V-007 at the dose of 3 mg on mitochondrial metabolism and resistance to the oxidative stress (Figure 5,6).
The summarized hTERT and PHB expression levels in normal cell culture and in UV-exposed culture (inflammaging), depending from the effect of drugs (V-007, G-004, the injection by water), are presented in (Table 4).
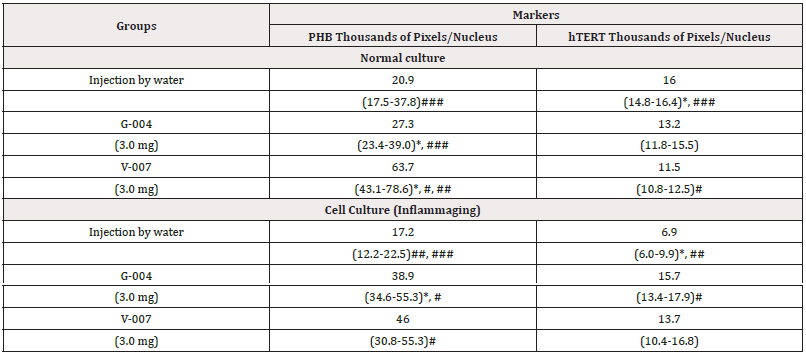
Table 4: The average parameters of markers in cell cultures.
Note*: Statistic difference between normal culture and UV-exposed culture (inflammaging), at p<0.05; #, ##, ###-statistic difference between markers within one culture (#-with injection water, ##-G-004, and ###-V-007), at p<0.05.
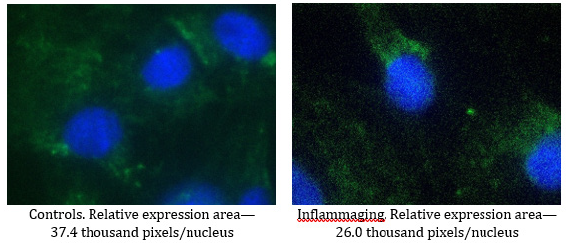
When studying the drugs at the dose of 0.3 mg (Table 5), the differences in the expression of hTERT are significant in the UV-exposed group (14.6 thousand pixels/nucleus) as compared to the control group (12.1 thousand pixels/nucleus) (Figure 7) and the inflammaging group with the injection by water (6.9 thousand pixels/nucleus) (Table 4). The prohibitin level in the UV-exposed group (26.0 thousand pixels/nucleus) was different from the control group (37.4 thousand pixels/nucleus) (Figure 8) and exhibited significant differences from the inflammaging group with the injection by water (17.2 thousand pixels/nucleus) (Table 4). Significant differences (p<0.05) were found between prohibitin expression at V-007 concentrations of 3.0 mg (63.7 thousand of pixels/nucleus) and 0.3mg (37.4 thousand of pixels/nucleus) only in the groups which were not exposed to UV irradiation. The obtained data confirm the conclusion that the target for V-007 geroprotective action (for both doses) is prohibitin which promotes mitochondrial metabolism to fight oxidative stress and alteration (Figure 7,8).
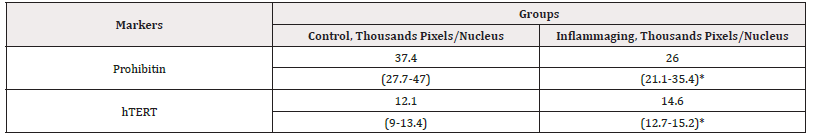
Table 5: Average parameters of markers in cell culture with the administration of V-007 (0.3 mg).
Note*: Statistic difference in comparison groups at p<0.05.
The average parameters of markers in cell culture with G-004 administration (at the dose of 0.3mg) are presented in (Table 6).
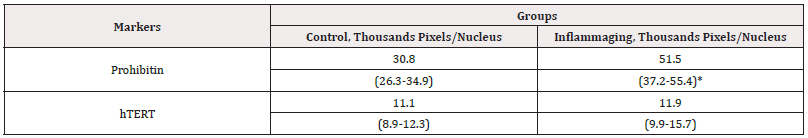
Table 6: The average parameters of markers in cell culture with the administration of G-004 (0.3 mg).
Note*: Statistic difference in comparison groups at p<0.05.
The data presented in Table 6 and on Figure 9 illustrate that the differences in hTERT expression are insignificant in the UV-exposed group (11.9 thousand pixels/nucleus) in comparison with the control group (11.1 thousand pixels/nucleus). The prohibitin level in the UV-exposed group (51.5 thousand pixels/nucleus) was significantly lower than that in the control group (30.8 thousand pixels/nucleus) (Figure 10) and in the inflammaging group with the injection by water (17.2 thousand pixels/nucleus) (Table 4). Significant differences were found between hTERT expression at G-004 concentrations of 3.0 mg (13.2 thousand of pixels/nucleus) and 0.3mg (11.1 thousand of pixels/nucleus) in the groups that were not exposed to UV irradiation. Our study has shown that G-004 as well as V-007 at the dose of 0.3 mg possess a stimulating effect on prohibitin expression in normal and damaged tissues (Figures 9,10).
The summarized hTERT and PHB expression levels in normal cell culture and in UV-exposed culture (inflammaging), depending from the effect of medications (V-007, G-004, the injection by water), are presented in (Table 7).
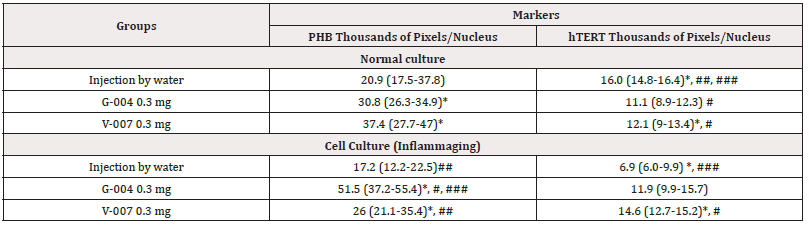
Table 7: The average parameters of markers in cell cultures.
Note*: Statistic difference between normal culture and UV-exposed culture (inflammaging), at p 0.05; #, ##, ###-statistic difference between markers within one culture (#-with injection water, ##-G-004, and ###-V-007), at p<0.05’.
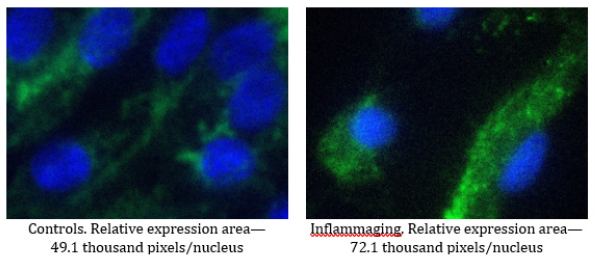
Our study confirms that both molecules, hTERT and PHB, are targets for the geroprotective effect of G-004 (at both doses). We also investigate intermediate doses of the drugs (1.5mg). As evident from data presented in Table 8, the differences in the expression of hTERT are significant in the UV-exposed group (12.4 thousand pixels/nucleus) as compared to the control group (8.3 thousand pixels/nucleus) (Figure 11) and the inflammaging group with the injection by water (6.9 thousand pixels/nucleus) (Table 7). The level of prohibitin in the UV-exposed group (72.1 thousand pixels/nucleus) was different from that in the control group (49.1 thousand pixels/nucleus) (Figure 12) and exhibited significant differences from the inflammaging group with the injection by water (17.2 thousand pixels/nucleus) (Table 7). Significant differences (p<0.05) were found between prohibitin expression at V-007 concentrations of 1.5 mg and 0.3 mg (26.0 thousand of pixels/nucleus) in the UV-exposed groups (Table 8).
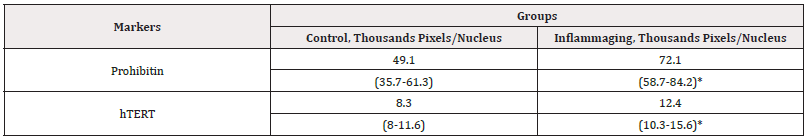
Table 8: The average parameters of markers in cell culture with the injection of V-007 (1.5 mg).
Note*: Statistic difference in comparison groups at p<0.05.
The obtained data confirm that the target for V-007 geroprotective action is prohibitin which promotes mitochondrial metabolism to fight the oxidative stress and alteration. The highest expression of prohibitin (72.1 thousand pixels/nucleus) was observed at V-007 concentration of 1.5 mg in the UV-exposed group (Figure 11,12).
The average parameters of markers in cell culture with G-004 administration (1.5mg) are presented in (Table 9).
As evident from data presented in Table 9, the differences in the expression of hTERT are insignificant in the UV-exposed group (16.8 thousand pixels/nucleus) as compared to the control group (14.0 thousand pixels/nucleus) (Figure 13) and the inflammaging group with the injection by water (6.9 thousand pixels/nucleus) (Table 10). The data presented in Table 9 and on Figure 14 illustrate that the differences in prohibitin expression are significantly higher (p<0.05) in the UV-exposed group (40.7 thousand pixels/nucleus) in comparison with the control group (29.0 thousand pixels/nucleus). The prohibitin level in the UV-exposed group (40.7 thousand pixels/nucleus) was significantly lower than that in the control group (29.0 thousand pixels/nucleus) (Figure 14) and in the inflammaging group with the injection by water (17.2 thousand pixels/nucleus) (Table 10) (Figure s13, 14).
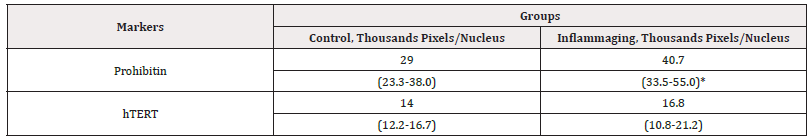
Table 9: The average parameters of markers in cell culture with the injection by G-004 (1.5 mg).
Note*: Statistic difference in comparison groups at p<0.05.
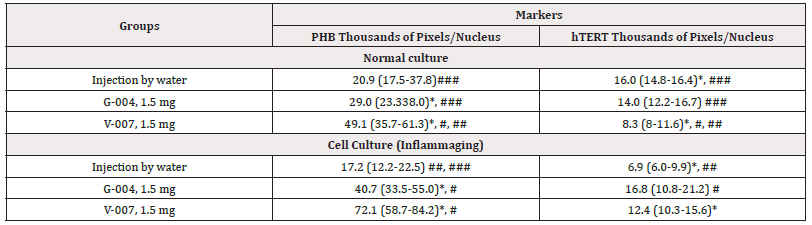
Table 10: The average parameters of markers in cell cultures.
Note*: Statistic difference between normal culture and UV-exposed culture (inflammaging), at p<0.05; #, ##, ###-statistic difference between markers within one culture (#-with the injection by water, ##-G-004, and ###-V-007), at p<0.05.
Significant differences were found between hTERT expression at G-004 concentrations of 1.5 mg (14.0 thousand of pixels/nucleus) and 0.3mg (11.1 thousand of pixels/nucleus) in the groups which were not exposed to UV irradiation. Our study has shown that G-004 at the dose of 1.5mg exerts an effect on hTERT expression in normal undamaged tissues. The aggregated indicators of hTERT and PHB expression in normal cell culture and in UV-exposed culture (inflammaging), depending on the effect of medications (V-007, G-004, injection water), are presented in Table 10.
The obtained data allows us to establish the geroprotective effect of V-007 and G004 drugs in the inflammaging model in endometrial cell culture. It has been shown that the targets for G-004 at all doses are hTERT and prohibitin, a key mitochondrial protein/chaperone, and for V-007 at the same doses it is prohibitin only. Thus, the geroprotective activity of G-004 is manifest both in the promotion of the functional activity of telomerase (promoting reparation) as well as in the promotion of mitochondrial metabolism (increasing of the resistance to the oxidative stress), while the geroprotective activity of V-007 is manifest in an increased resistance of cells to the oxidative stress. A further investigation of the effects of V-007 and G-004 will allow to specify the mechanism of their impact on the expression of key signaling gerotropic molecules and thereby map paths for the optimization of their use as medicines for the prevention of premature aging and treatment of age-associated pathologies. It appears promising to examine, on a larger scale, other signaling molecules as possible pharmacological targets, which will open prospects for the improvement of geroprotective and general regulatory effects of glutathione-containing drugs.
Conclusions
The expression of prohibitin and telomerase reverse transcriptase (hTERT) was statistically lower in the UV-exposed group (the inflammaging model) in comparison with the control group, which points to the dynamic aging of cells in culture and the efficacy of the selected inflammaging model. The investigation of geroprotective properties of V-007 and G-004 have demonstrated their effect at all applied doses (0.3mg, 3.0mg, 1.5mg) on the expression of signaling molecules in endometrial cell culture both in normal passaging and under conditions of senescence modeling (inflammaging). V-007 does not exert an effect on telomerase activity of cells but significantly promotes the expression of prohibitin, which stimulates mitochondrial metabolism in inflammaging conditions. The effect of V-007 varies at different concentrations: at doses of 1.5 and 3.0mg it affects prohibitin with a significant increase of expression.
The highest expression of prohibitin (72.1 thousand pixels/nucleus) was observed at V-007 concentration of 1.5mg in the UV-exposed group. At the dose of 0.3mg in the case of damaged cells, no effect was observed, and the expression increased only in undamaged cells. When evaluating the effect on hTERT expression, an increase in enzyme activity was only found for the concentration of 0.3mg. G-004 significantly increases telomerase activity and prohibitin expression in inflammaging conditions, that enhances the reparation and cell resistance to oxidative stress, and at the dose of 1.5mg G-004 exerts an effect on hTERT expression predominantly in undamaged tissues. The study of two signaling molecules involved in cellular senescence has demonstrated that the target of V-007 geroprotective action is prohibitin, and the target of G-004 geroprotective action is hTERT and prohibitin. G-004 has a wider and more expressed geroprotective effect, affecting both mitochondrial and nuclear mechanisms of cellular senescence.
Author Contributions
Conceptualization IK, SM and TK; methodology, EM, TZ and AP; software, PS, YK; validation, MD; formal analysis, TZ; investigation, EM; resources, TK; data curation, IK; writing-original draft preparation, EM and SM; writing-review and editing, IK; visualization, TZ; supervision, EM; project administration, TZ; funding acquisition, IK. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the PharmaVam Company (St.Petersburg, Russia), 01/10-2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Coppé J, Patil C, Rodier F, Sun, Y, Muñoz D, et al. (2008) Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biology 6(12): 2853-2868.
- Childs G, Baker D, Kirkland J, Campisi J, van Deursen J (2014) Senescence and apoptosis: dueling or complementary cell fates? EMBO Reports 15(11): 1139-1153.
- Wei Y, Jia S, Ding Y, Xia S, Giunta S (2023) Balanced basal-levels of ROS (redox-biology), and very-low-levels of pro-inflammatory cytokines (cold-inflammaging), as signaling molecules can prevent or slow-down overt-inflammaging, and the aging-associated decline of adaptive-homeostasis. Exp Gerontol 172: 112067.
- Xu D, Hu MJ, Wang YQ, Cui YL (2019) Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 24(6): 1123.
- Chen TH, Wang HC, Chang CJ, Lee SY (2024) Mitochondrial Glutathione in Cellular Redox Homeostasis and Disease Manifestation. Int J Mol Sci 25(2): 1314.
- Kvetnoy I, Mironova E, Krylova Y, Zubareva T, Leontyeva D (2022) Mitochondrial proteins as molecular targets for glutathione-based drugs. Am J Biomed Res 15(4): 452-459.
- Chowdhury D, Kumar D, Sarma P, Tangutur A, Pal Bhadra M (2017) PHB in Cardiovascular and Other Diseases: Present Knowledge and Implications. Curr Drug Targets 18(16): 1836-1851.
- Da Costa CA, Duplan E, Rouland L, Checler F (2018) The transcription factor function of Parkin: breaking the dogma. Front Neurosci 12: 965.
- Chen JH, Hales CN, Ozanne, SE (2007) DNA damage, cellular senescence and organismal ageing: causal or correlative? Nucleic Acids Res 35(22): 7417-7428.
- Yeh JK, Wang CY (2016) Telomeres and telomerase in cardiovascular diseases. Genes (Basel) 7(9): 58.
- Richardson GD, Breault D, Horrocks G, Cormack S, Hole N, et al. (2012) Telomerase expression in the mammalian heart. FASEB J 26(12): 4832-4840.
- Lü M, Liao Z, Zhao X, Fan Y, Lin X, et al. (2012) hTERT-based therapy: A universal anticancer approach (Review). Oncol Rep 28: 1945-1952.













 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.