Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Autoimmune diseases of the nervous system: Risk factors and therapeutic challenges
*Corresponding author: Mostafa A Abdel Maksoud, Immunology, Zoology Department, King Saud University, Saudi Arabia.
Received: June 27, 2019; Published: July 10, 2019
DOI: 10.34297/AJBSR.2019.03.000726
Abstract
Autoimmune diseases are a heterogeneous group of pathological conditions that have a rapidly increasing morbidity and mortality rates nowadays. The complex nature and the inscrutable etiology of these diseases have made them a non- negotiable challenge for immunologists. The neurologic autoimmune diseases are considered more dangerous as they affect the system that orchestrate all other body systems. However, the available body of knowledge in this area is so much little than the required and still many questions are needing to be answered. The risk factors that could lead to autoimmune reactivity are still also a matter of debate. This review will focus on the risk factors that could be potentially considered as contributors for the appearance of autoimmune reactivity and their implications in the therapeutic approaches of these diseases.
Keywords: Autoimmune diseases; Nervous system; Etiology; Epigenetics; Immunosenescence; Autoimmune ecology
Prevalence of Autoimmune Diseases
The incidence of autoimmune diseases (ADs) has been tremendously increased during the last years and many research groups around the world have reported clinical complications in autoimmune diseases patients [1-3]. They cumulatively affect 5-10% of the industrial world population and are a significant cause of morbidity and mortality [4]. In fact, the rise in ADs parallels the surge in allergic and cancer conditions while infections are less frequent in the Western societies, supporting the previously described ‘hygiene hypothesis’ [5]. Multiple sclerosis (MS), type 1 diabetes (T1D), inflammatory bowel diseases (IBD) (mainly Crohn’s disease), systemic lupus erythematosus (SLE), primary biliary cirrhosis (PBC), myasthenia gravis (MG), autoimmune thyroiditis (AT), rheumatoid arthritis (RA), bullous pemphigoid, and Celiac disease (CD) are considered as the most familiar representative examples of ADs [6-7]. The prevalence of ADs is distinctively varying worldwide. For example, the prevalence of MS which is the most common inflammatory demyelinating disease involving the central nervous system (CNS) with various clinical types, varies from high rates in North America and Europe (more than 100 cases per 100,000 population) to low levels in Eastern Asia and Sub- Saharan Africa (2 cases per 100,000 population) [7]. Actually, ADs are one of the leading causes of death among young and middleaged women in many countries of the industrialized world [8]. Unfortunately, despite some ADs like MG, RA, CD, Crohn’s disease, ulcerative colitis, T1D, MS, and SLE have gained special attention regarding their prevalence, prevalence data are scares for some other ADs like Guillain Barre syndrome, pemphigoid, phemphigus, dermatomyositis, polymyositis, autoimmune hemolytic anemia, idiopathic thrombocytopenic purpura, or pernicious anemia [9]. Some studies have reported annual % increases per year for Rheumatic, endocrinological, gastrointestinal and neurological autoimmune diseases and the results were 7.1, 6.3, 6.2, and 3.7, respectively [6]. Meanwhile, neurological ADs could be considered as the most aggressive one among the other ADs.
Autoimmune Diseases of the Nervous System
Both of central nervous system (CNS) and peripheral nervous system (PNS) could be targets for ADS either one or together. Autoimmunity in both of the CNS and PNS can manifest as the result of cellular or humoral immune responses to autoantigens. As an example, multiple sclerosis (MS) is a cell-mediated autoimmune disease of the CNS in which both myelin and the cell that produces the myelin are destroyed and consequently, characterized by chronic inflammation, demyelination and gliosis affecting the CNS but usually not the PNS [10]. Limbic encephalitis represents a group of autoimmune conditions characterized by inflammation of the limbic system (Hippocampus and fornix) and other parts of the brain. Despite limbic encephalitis was often associated with an underlying neoplasm (paraneoplastic limbic encephalitis); some cases never have a neoplasm identified (non-paraneoplastic limbic encephalitis) whereas antibodies against proteins associated with voltage-gated potassium channels (LGI1 and CASPR2) were detected [11]. Autoimmune channelopathies have become one of the attractive topics of basic and clinical research in neurologic ADs.
An emerging group of neurological disorders is associated with autoantibodies acting on ligand-gated ion channels (receptors) or on voltage-gated ion channels. The pathogenicity of autoantibodies to ion channels has been demonstrated in most of these conditions, and patients may respond well to immunotherapies that reduce the levels of the pathogenic autoantibodies [12]. On the other hand, chronic inflammatory demyelinating polyneuropathy (CIDP) (also called chronic relapsing polyneuropathy (CRP) or chronic inflammatory demyelinating polyradiculoneuropathy) is considered a typical example of an autoimmune disease restricted to the PNS because it is apparently caused by an autoimmune response against one or several antigen (s) on peripheral nerves [13]. Diseases such as CIDP and myasthenia gravis (MG) are considered antibody-mediated diseases of the PNS and neuromuscular junctions, respectively. MG is caused by antibodies targeting the muscle acetylcholine receptor or other neuromuscular junction proteins like muscle-specific kinase (MUSK). These antibodies cause malfunction of the communication between nerve and muscle leading to muscular weakness and fatigue. MG is the prototypic autoimmune neurologic disease, since the antibody targeting the acetylcholine receptor was identified back in the 1970s. “Other autoimmune neuromuscular diseases include Lambert-Eaton myasthenic syndrome (which is also a disorder of transmission at the neuromuscular junction), neuromyotonia (or Isaacs syndrome, where antibodies target regulatory proteins on the nerves, causing excessive muscle activity), and autoimmune autonomic ganglionopathy (AAG) (antibodies target a receptor on the autonomic nerves, resulting in autonomic failure).
Surprisingly, several single cases with combined demyelination of both CNS and PNS, occurring either sequentially or simultaneously, have been reported [14]. An example of ADs affecting both the CNS and PNS is the overlapping clinical spectrum of Miller–Fisher–/Fisher–Syndrome (FS), Bickerstaff brainstem encephalitis (BBE) and Guillain–Barré-Syndrome (GBS) [15]. It was supposed that all the three diseases may share a common etiology, as supported especially by the presence of common autoantibodies and antecedent infections, and form a continuous spectrum with variable clinical and anatomical involvement of PNS and CNS [16]. Another example is neuropsychiatric lupus (NPSLE) whereas SLE can involve both the CNS and the PNS. In one clinical study there were 19 distinct syndromes described and it was hypothesized that Up to 65% of child SLE patients develop NPSLE at any time during the disease course [17]. Sarcoidosis is a chronic inflammatory disorder that primarily affects the lungs, but can also impact almost every other organ and system in the body. Neurosarcoidosis (NS) is characterized by inflammation and abnormal cell deposits in any part of the nervous system – the brain, spinal cord, or peripheral nerves.
It most commonly occurs in the cranial and facial nerves, the hypothalamus and the pituitary gland [18]. Primary angiitis of the central nervous system (PACNS) is a rare inflammatory disorder of the blood vessels of the brain and the spinal cord without any evidence of systemic vasculitis. Antibodies against myelin oligodendrocyte glycoprotein (MOG) have been identified in neuromyelitis optica spectrum disorders (NMOSDs). As in NMOSD, spinal cord and optic nerves are 2 of the most frequently involved CNS sites in combined central and peripheral demyelination (CCPD) syndromes [19] Indeed, the simultaneous occurrence of combined central and peripheral demyelination (CCPD) is rare and data are limited to case reports and small case series [20] without sufficient data referring to etiological aspects of this group of diseases.
Etiology
In general, the etiology of ADs remains to large extent, mysterious [21]. Many research activities have been solely directed to investigate the detailed mechanisms for autoimmune reactivity progression. The situation for neurologic ADs is more sophisticated. However, it seems that autoreactive antibodies are one of the major players [22,23]. The effects of various autoantibodies directed at each specific site of the motor unit cause a specific disease. The question remains standing, why are these autoantibodies formed? Genetic factors may play a relevant role. Like a number of other ADs, myasthenia gravis is associated with a higher-than-expected frequency of certain HLA haplotypes [24]. Probably a number of genetic loci, possibly including B- and T-cell receptor genes, immunoglobulin variable region genes, and class I, II, and Ill MHC molecules, play a cumulative role in determining susceptibility to some ADs, like myasthenia gravis [25]. The associations of myasthenia with these and other markers suggest that there are genes that enhance the ability to produce the autoantibodies of myasthenia. The production of anti-idiotypic (anti-Id) antibodies that may cross-react with autoantigens may also be an important mechanism of auotreactivity in ADs. [26] For example, anti-Id antibodies directed against some primary antimicrobial antibodies have anti-AChR activity [27]. This suggests a mechanism for the induction of the anticarbohydrate antibodies associated with motor neuron diseases and with demyelinating autoimmune neuropathies.
Many viruses use cell-surface carbohydrates as receptors. Viruses may attach to the same carbohydrate structures recognized by the autoantibodies of these neurologic diseases. The proposed mechanism is that the virus recognizes the cell-surface receptor, the viral infection induces the antiviral antibody, the antiviral antibody recognizes the receptor binding site on the virus, the anti-Id antibody is produced against the antiviral antibody, and the anti-Id antibody cross-reacts with the cell-surface receptor and becomes an autoantibody. There are many examples of this mechanism in which immunization with a virus, a hormone, or a drug induces antibodies directed at the cellular receptor for the virus, hormone, or drug. [28]. Cytomegalovirus (CMV) is one of the most common viruses associated with the Guillain-Barre syndrome. Attenuated murine CMV specifically recognizes the sequence of the sugars N-acetylglucosamine-galactose [29]. This is similar to the antigens of autoimmune demyelinating neuropathies. Analysis of a panel of antiviral and antireceptor monoclonal antibodies from mice indicated that some antibodies are produced that are antireceptor autoantibodies and are also anti Id antibodies (react with the antiviral antibodies). Nerve impulse transmission to muscle occurs at the acetylcholine receptor site by the release of acetylcholine, which is counterbalanced appropriately by postsynaptic cholinesterase. In patients with myasthenia gravis, many of these receptor sites are blocked, bound, or degraded by antibodies induced by events arising in the patients’ own thymus glands. As a result of this autoimmune process, patients have a decreased number of normally functioning neuromuscular units and have symptoms of weakness and easy fatigability. Thymectomy is thus a strategy for potentially intervening early in the process of the induction of autoimmunity [25].
Conclusion
Despite the fact that Autoimmune disorders can affect people of all genders, races, and ages, certain risk factors have been proposed by many research groups [30]. In some ADS, some ethnic groups (African American, American Indian, or Latino) are more likely to suffer from the disease than others. Although predisposing genetic risk factors have been identified for various autoimmune diseases, it is understood that they account only for a fraction of the overall disease [31] The gender was introduced as a potential factor when it was noticed that in some diseases, 90% of autoimmune patients are women. In this context, the female hormone could be one of the autoimmune arms that are responsible for developing auotreactivity in some stages [32]. In addition to hormonal factors, environmental factors have been strongly thought to be included in autoimmune reactivity development. Environmental factors make up a significant part of the risk in disease initiation and propagation. The increasing incidence of autoimmune diseases with a high prevalence in Western countries [33] and the rapid evolution of MS in former low prevalence countries like Japan [34] nurture multiple explanatory concepts around environmental triggers.
The so-called hygiene hypothesis aims to explain the increase in autoimmunity in industrialized countries by linking the decrease of infection rates and the increase in autoimmune diseases to a general improvement of hygiene standards [35]. Next to improved hygiene and a gross reduction of infections, changes in dietary habits are one of the most evident Western lifestyle factors potentially associated with the increase in ADs. Additionally, there are many more environmental factors that have been proposed to promote ADs including climate, stress, occupation, cigarette smoking, and diet. The consumption of ‘Westernized food’, including high salt, high fat, high protein, and high sugar intake, has already been associated with increasing prevalence in various diseases. The change of dietary habits has been under intensive investigation, revealing a direct influence on immune homeostasis and on bacterial communities colonizing the gastrointestinal tract (GIT) [36] and the gut microbiota is tightly connected to the immune system and highly involved in immune regulatory processes [37]. IBD has been associated with shifts and variety reduction in the microbiome. This observation has also been made in other autoimmune diseases not directly associated with the GIT [36,38]. However, there is still deficiency in the studies linking the environmental factors to the disease progression in ADs in general and in neurologic ADs in particular.
Immunosenescence & Autoimmunity
The decline in immunocompetence with age is accompanied by the increase in the incidence of ADs. Aging of the immune system, or immunosenescence, is characterized by a decline of both T and B cell function, and paradoxically the presence of low-grade chronic inflammation [39]. It is now globally accepted that aging is related to increased reactivity to self-antigens and loss of tolerance. These was supported by the observations that elderly people experience general systemic inflammation and, at the same time, they aggravate degenerative diseases, which, in turn, increase the risk of developing ADs [40-41]. For example, rheumatoid factors (RFs) are present in up to 5% of young, healthy individuals, and increases up to 5 times in elderly persons. Similarly, the prevalence of antinuclear antibodies (ANAs) is higher in healthy individuals over 70 years of age compared to healthy younger adults [42]. It was hypothesized that the aging-associated increase in inflammatory cytokines and chemokines such as TNF-α, C-reactive protein (CRP), IL-8, Monocyte chemoattractant protein 1 (MCP1), and regulated on activation, normal T cell expressed and secreted (RANTES) could be an important contributor for the development of auotreactivity [43]. Clinical studies have shown that there is a change from Th1 to Th4 (mainly IL-4 and IL-6) in the cytokine profile as age advances [44]. IL-6 is a potent proinflammatory cytokine closely related to disability in patients with RA; therefore, it represents a therapeutic target for this disease [45].
In addition, there are reports of an imbalance between Th17 and Treg cells. A considerable number of IL-17-secreting naïve CD4+ T helper cells have been detected in the elderly in contrast to reduced IL-17-secreting memory CD4+ T helper cells [46]. Autoantibody production such as rheumatoid factor as well as antinuclear, antiphospholipid, and antithyroglobulin antibodies are present during aging [47,48]. Autoantibody production has been attributed to altered T and B cell function [49], especially to the decrease in antibody affinity maturation. This evidence supports the idea that autoantibody levels may be closely related to the clinical characteristics of elderly and to patients with ADs. A striking feature of the aging process is the involution of the thymus. Reduced thymic output has been postulated to induce compensatory auto proliferation of T cells, which can then lead to premature T cell senescence and contribute to immune system abnormalities associated with autoimmunity and aging [50]. Additionally, alterations in apoptosis in T cells may be an important mechanism of autoimmune disease and immunosenescence.
Expansion of CD4+ and CD8- senescent T cells that have lost the expression of CD28 emerge in normal aging and in several autoimmune diseases including T1D, RA, and MS [51] Reports of telomere length (in peripheral blood mononuclear cells (PBMCs)) alteration in patients with ADs such as RA [52] scleroderma (SSc) [53], SLE [54], Wegener’s disease [55], psoriasis, and atopic dermatitis [56], suggest an excessive cell replication with its corresponding telomere erosion. These findings have been interpreted as evidence of T cell accelerated proliferation in the autoimmune process. In aged animals, the antibodies produced are generally of lower affinity and are less protective than those produced by young animals [57]. Dissection of splenic B cell subsets has revealed significant alterations in sub-population distribution as mice age [58]. Specifically, the percentage of naïve follicular B cells declines dramatically, whereas subsets of antigen-experienced cells increase, including poly/self-reactive subtypes such as marginal zone (MZ) and CD5+ B1-like cells, and memory B cells.
Additionally, an increase in lifespan of mature B cells in the periphery of aged mice has been reported [59]. It is therefore possible that autoimmunity may increase with aging by accumulation of longlived B cells with specificity against “neoantigens” that form during one’s life. Others have also postulated that self-reactive memory B cells may become reactivated later in life (recall memory) due to ageassociated reduction in immune tolerance, loss of tissue integrity leading to the exposure of neo-self-antigens, or re-exposure to similar environmental agent(s) that result in aberrant autoimmune response through molecular mimicry [57]. Briefly, there are three main processes that could explain the age- associated autoimmune reactivity phenomenon: 1. thymic involution, 2. intrinsic damage, and 3. chronic antigenic stimulation. Thus, the microenvironments, soluble factors, surface and signal transduction molecules as well as processes such as telomere erosion and infection diseases can result from a decreased immunity through aging. Understanding these mechanisms will make it possible to establish appropriate treatment strategies, optimize responses to pathogens, and improve the quality of life for the elderly
Genetics of Autoimmune Diseases
Despite many research papers have focused on the genetics of ADs, few have discussed the epigenetics of these group of diseases. Epigenetics is defined as the study of all inheritable and potentially reversible changes in genome function that do not alter the nucleotide sequence within the DNA. Epigenetic mechanisms such as DNA methylation, histone modification, nucleosome positioning, and microRNAs (miRNAs) are essential to carry out key functions in the regulation of gene expression. Therefore, the epigenetic mechanisms are a window to understanding the possible mechanisms involved in the pathogenesis of ADs [60]. It was found (Table 1) that the epigenetic changes occurring in elderly people may affect important genes involved in ADs [60- 61]. Growing evidence supports epigenetic dysregulation as a potential explanation for the higher incidence of autoimmune and neoplastic diseases associated with increasing age [62]. Epigenetic mechanisms linking aging to cancer include hypermethylation of the promoter of tumor suppressor genes such as RB1, p16, and Wnt associated factors, aberrant Dnmt activity, loss of genomic imprinting, and chromosomal translocations in hypomethylated DNA sequences [63,64].
Additionally, several groups have reported that microRNAs (miRNAs), a new class of small non-protein-coding RNAs that negatively affect gene expression at the post-transcriptional level, are regulated by epigenetic mechanisms, exhibit changes in expression during aging, and have an important role in cancer [65], aging and growth control [66]. Interestingly, two groups reported that the expression pattern of miR-155 and miR-146 miRNAs is altered in synovial tissue in RA, suggesting a potential role of miRNA in the pathogenesis of other disorders acquired with age [67]. Epigenetic modifications may also provide a mechanistic link between immunosenescence and autoimmunity. In this context, the role of lymphocyte function-associated antigen-1 (LFA-1; CD11a/CD18) in the development of autoimmunity and aging was investigated. LFA-1 is an integrin adhesion molecule involved in T cell activation, whose expression increases progressively throughout life [68]. Interestingly, LFA-1 is overexpressed in T cells from patients with SLE, with the extent of the overexpression directly relating to disease activity and responsible for T cell auto reactivity in vitro, and a lupus-like disease in vivo [69]. Additionally, inhibiting T cell DNA methylation with the DNA methyltransferase inhibitor 5-azacytidine increases LFA-1 overexpression.
Furthermore, [71] reported that in MRL/MpJ-Fas lpr mice which develop a systemic autoimmune disease with similarities to SLE, loss of LFA-1 significantly protected mice from the development of murine lupus, as measured by attenuated autoantibody formation, and inhibited development of glomerulonephritis, and increased survival compared to control MRL/MpJ-Fas Lpr mice [70]. It was demonstrated that regions flanking the promoter of the ITGAL gene, which encodes for the CD11a chain of LFA-1, demethylate during aging, providing evidence that age-dependent decreases in T cell DNA methylation may contribute to the changes in T cell function and gene expression that occur in aging [71]. Since anti-DNA antibodies are induced by LFA-1 overexpression, these changes may contribute to the development of anti-nuclear antibodies with aging [72]. Indeed, the identification of cell-specific targets of epigenetic deregulation could be used as clinical markers for diagnosis, disease progression, and therapy approaches.
Autoimmune ecology
The influence of environmental exposure on the risk of developing ADs is crucial and hence, the term ‘’autoimmune ecology’’ has been recently introduced. In fact, environment, more than genetics, shapes immune system. Autoimmune ecology is closer to exposome, that is all the exposures – internal and external – across the lifespan, interacting with hereditary factors (both genetics and epigenetics) to favor or protect against autoimmunity and its outcomes [73]. In this context, the immune response to environmental agents in general, and microbiota, cigarette smoking, alcohol and coffee consumption, socioeconomic status (SES), gender and sex hormones, vitamin D, organic solvents, and vaccines in particular will be the whole point [74]. The effect of different environmental factors on the development of ADs has attracted many research groups around the world. Panel findings on studies of the role of environmental factors and development of ADs are shown in (Table 2) [75]. The environmental risk factors associated with ADs are varied as are the underlying immune mechanisms that lead to these disorders. However, many risk factors have been suggested to be potential contributors that have effects on innate immunity, such as toll-like receptor (TLR) activation by xenobiotics, adjuvant effects, and inflammatory responses; B-cell activation; direct effect impairing the immune function, such as T helper 17 (Th17) and T regulatory cells (Treg); post-translational modifications (PTMs) of self-antigens, and epigenetic modifications, mainly DNA methylation (Table 3) [76].
Note: EPW, expert panel workshop; ADs, autoimmune diseases; RA, rheumatoid arthritis; SSc, systemic sclerosis; SLE, systemic lupus erythematosus; ANCA, anti-neutrophil cytoplasmic antibody; ACPA, anticitrullinated protein antibody; RF, rheumatoid factor; MS, multiple sclerosis; HT, hashimoto’s thyroiditis; GD, graves’ disease; CrD, crohn’s disease; UC, ulcerative colitis; T1DM, type 1 diabetes mellitus; GSE, gluten-sensitive enteropathy; DEPAP, 1,2-di-oleyl ester; EBV, Epstein–Barr virus; UV, ultraviolet.
Note: TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; TLRs, toll-like receptors; Th, T helper cells; UV, ultraviolet.
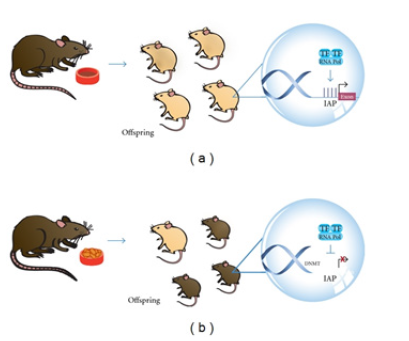
Figure 1: Epigenetic-environmental interaction [77]. Offspring of pregnant Agouti rodents fed with food rich in methyl donors had a different color of coat (brown, (b)) due to an increased DNA methylation status in the viable yellow allele (A vy allele), in comparison to offspring of pregnant rodents fed a normal diet (yellow or mottle, (a)). Intracisternal A Particle (IAP), Transcription Factor (TF), RNA Polymerase (RNA Pol), Methylated Cytosine (M).
Epigenetics interaction with environment was elucidated through a fantastic study on the pregnant Agouti rodents. In this study, researchers fed pregnant Agouti rodents with food rich in methyl donors such as folate, methionine, and choline. They found that, in comparison to offspring of pregnant rodents fed a normal diet (yellow or mottle, (Figure 1) (a)), the offspring of these rodents had a different color of coat (brown, Figure 1 (b)) due to an increased DNA methylation status in the viable yellow allele (A vy allele). These authors demonstrated that the percentage of phenotypes with a darker brown coat rises as increasing levels of methyl supplement are added to the diet. The lack of a methyl supplement has important implications because it indicates a pattern of future obesity and insulin resistance. In other words, mice with yellow or mottle coats have altered metabolism and obesity. It also results in increased cancer susceptibility, adult diabetes, and twice the mortality seen in normal mice [77].
Microbial Infections & Autoimmunity
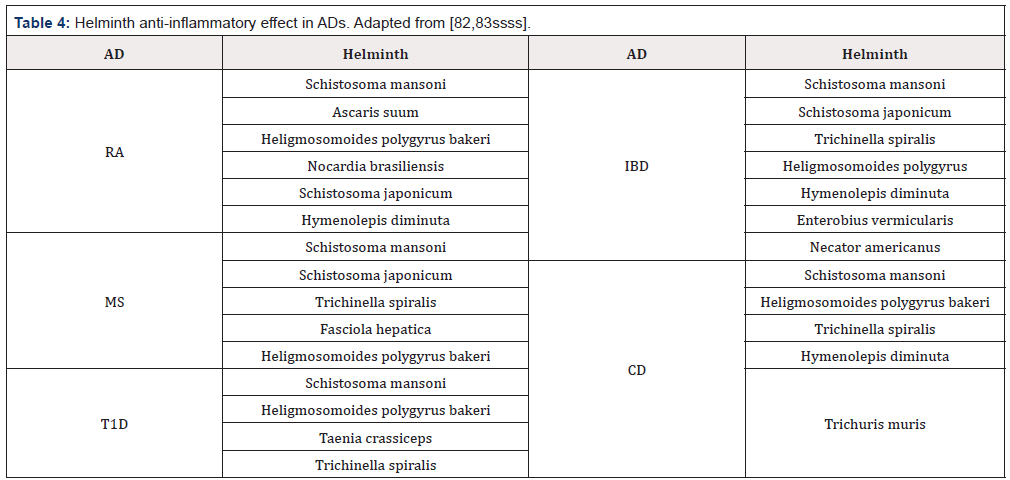
Microbial infections are one of the major players in the environmental factors that modulate the development of ADs and there is an ambiguous relation between both of them [22]. Infections may play both a causative role and a protective role in the pathogenesis of ADs. Infections can be triggers of ADs as has been shown in animal models [78]. There are different mechanisms by which infection may trigger ADs. Molecular mimicry, epitope spreading, bystander activation and clearance deficiency are the most studied ones. On the other hand, infections may prevent the development of autoimmunity or even withdrawal of an autoimmune process. This happens as a result of the interaction between microorganisms and the host [79]. Parasitic helminths modulate the immune response towards an anti-inflammatory profile which favors their survival in the host. Thus, helminths are suppressors of the immunological pro-inflammatory process [80]. According to the hygiene hypothesis, the increase in ADs is the result of the reduction in exposure to microorganisms and parasites during childhood [81]. This was supported by the geographic relationship observed worldwide. Many inversed associations have been reported between parasitic infections and protection from ADs (Table 4). Many parasites are able to change the cytokine profile from a pro-inflammatory to an anti-inflammatory profile. This change creates the perfect environmental conditions for them to survive and extend their lives within the host.
AD: Autoimmune disease, RA: rheumatoid arthritis, MS: Multiple sclerosis, T1D: diabetes type 1, IBD: inflammatory bowel disease, CD: Crohn’s disease.
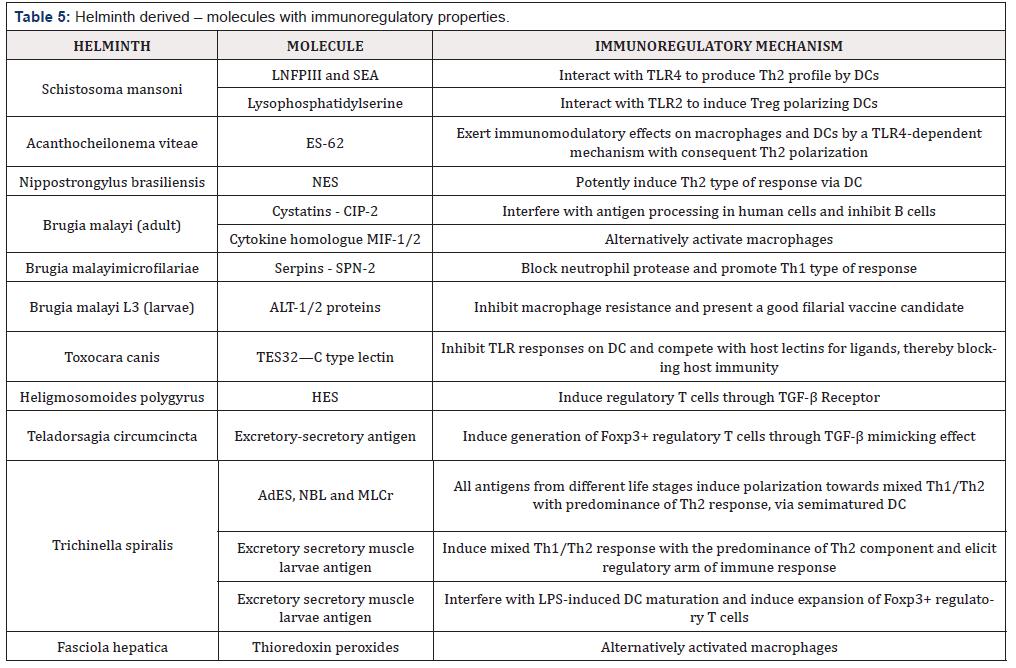
Note: Adapted from [85] LNFPIII: Lacto-N-fucopentaose III, SEA: soluble egg antigen, TLR: Toll like receptor, DC: dendritic cells, NES: Excretory-secretory antigen, Cystatins: cysteine protease inhibitors, MIF: migration inhibitory factor, Serpins: serine protease inhibitors, ALT: Abundant larval transcript, HES: Excretory-secretory antigen, AdES: Adult excretory-secretory antigen, NBL: newborn larvae antigen, MLCr: crude muscle larvae antigen, LPS: Lipopolysaccharide.
They may promote the inhibition of IFNα, IL-1β, and IL-17 to suppress the Th1 and Th17 response. They also promote the production of IL-4, IL-10, TGF-β, and the activation of regulatory cells including Treg, Breg, regulatory dendritic cells, and macrophages [82-83]. There is production of different molecules during helminth infections. Some of them are probably having a regulatory effect on the host immune system (Table 5) [84]. It is noteworthy that microbiota is the first barrier against pathogenic microorganisms. They may produce molecules against the pathogens during infection because they occupy the same niche, thus competing for the same place. Fluctuations in microbiota population have been described in patients with ADs [85]. Germ free (GF) mice show deficiencies in T lymphocyte differentiation within the lamina propria in the presence of IgA in mucosal layers and alterations in the homeostasis of Th populations (Th1, Th17, and Treg). In addition, most of the studies have established a relationship between the microbiota inhabiting the gut and its influence on health. Usually, microorganisms that live in the gut are not pathogenic under healthy conditions, and they have a positive effect on the host [86].
Nevertheless, some commensal bacteria may drive the preferential development of Treg while others promote Th17 response and inflammation. These bacteria favor the production of regulatory molecules and cytokines, e.g., Foxp3 and IL-10, which characterize the regulatory cells, Treg in particular. Specifically, species such as Bacteroides fragilis and the genus Lactobacillus and Bififobacterium greatly promote the presence of Treg in the gut. In contrast, a pathogenic phenotype characterized by Th17 response and pro-inflammatory cytokines is promoted by segmented filamentous bacteria such as Firmicutes. Moreover, it has been demonstrated that this kind of bacteria is able to induce the production of IgA in the small intestine. Th17 response certainly has its own positive role in the case of infection control, but it is also critical in the development of inflammatory and autoimmune diseases [87]. Consequently, it has been suggested that differences in the modern western diet could be causing the rapid increase in diseases such as asthma [88]. For example, one study shows how a switching from a low fat, vegetable rich diet to a high fat, high sugar diet could alter the microbiota within one day [89]. Away from the microbiota and the helminths, we have found that plasmodium chabaudi infection in BWF1 mice (the animal model for SLE) have several positive impacts on the disease progression when compared with the non-infected lupic mice [90-93]. Interestingly, when animals were injected with gamma-irradiated parasites, despite being unable to abrogate the lupus-associated pathology, they were in some parameters not as worse as the lupic mice. Indeed, the protective effects that the parasites may exert in autoimmune disease either in animal models or in human subjects needs more and more efforts especially for the aggressive category of ADS, the neurologic ADs.
“Take-home” message
The ADs affecting nervous system have not yet gained enough attention despite being vigorously aggressive. The search for the interaction between the different risk factors and focusing on the environmental (especially microbial) ones may lead to a promising resolution for the therapeutic limitation in this category of diseases.
Figure1: Epigenetic-environmental interaction [77]. Offspring of pregnant Agouti rodents fed with food rich in methyl donors had a different color of coat (brown, (b)) due to an increased DNA methylation status in the viable yellow allele (A vy allele), in comparison to offspring of pregnant rodents fed a normal diet (yellow or mottle, (a)). Intracisternal A Particle (IAP), Transcription Factor (TF), RNA Polymerase (RNA Pol), Methylated Cytosine (M).
References
- Díaz-Ramírez GS, Marín-Zuluaga JI, Donado-Gómez JH, Muñoz-Maya O, Santos-Sánchez Ó, et al. (2018) Characterization of patients with autoimmune hepatitis at a university hospital in Medellín-Colombia: cohort study. Gastroenterol Hepatol 41(2): 87-96.
- Hor JY, Lim TT, Chia YK, Ching YM, Cheah CF, et al. (2018) Prevalence of neuromyelitis optica spectrum disorder in the multi-ethnic Penang Island, Malaysia, and a review of worldwide prevalence. Mult Scler Relat Disord 19: 20-24.
- Alyafei F, Soliman A, Alkhalaf F, Sabt A, De Sanctis V, et al. (2018) Prevalence of β-cell antibodies and associated autoimmune diseases in children and adolescents with type 1 diabetes (T1DM) versus type 2 diabetes (T2DM) in Qatar. Acta Biomed 89(S5): 32-39.
- Shapira Y, Agmon-Levin N, Shoenfeld Y (2010) Defining and analyzing geoepidemiology and human autoimmunity. J Autoimmun 34(3): J168-J77.
- Bach JF (2002) The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 347(12): 911-920.
- Lerner A, Jeremias P, Matthias T (2015) The World Incidence and Prevalence of Autoimmune Diseases is Increasing. International Journal of Celiac Disease 3(4): 151-155.
- Cooper GS, Stroehla BC (2003) The epidemiology of autoimmune diseases. Autoimmun Rev 2(3): 119-125.
- E Leray, T Moreau, A Fromont, Edan G (2016) Epidemiology of multiple sclerosis. Rev Neurol 172(1): 3-13.
- Coopera GS, Bynumb ML, Somers EC (2009) Recent Insights in the Epidemiology of Autoimmune Diseases: Improved Prevalence Estimates and Understanding of Clustering of Diseases. J Autoimmun 33(3-4): 197- 207.
- Ruth Ann Marrie, Nadia Reider, Jeffrey Cohen, Olaf Stuve, Per S Sorensen, et al. (2015) A systematic review of the incidence and prevalence of autoimmune disease in multiple sclerosis. Mult Scler 21(3): 282-293
- Irani SR, Alexander S, Waters P (2010) Antibodies to Kv potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain 133(9): 2734-2748.
- Joubert B, Honnorat J (2015) Autoimmune channelopathies in paraneoplastic neurological syndromes. Biochim Biophys Acta 1848(10 Pt B): 2665-2676.
- Feasby TE, Hahn AF, Koopman WJ, Lee DH (1990) Central lesions in chronic inflammatory demyelinating polyneuropathy: an MRI study. Neurology 40(3 Pt 1): 476-478.
- J Katchanov, JD Lu¨nemann, F Masuhr, D Becker, M Ahmadi, et al. (2004) Acute combined central and peripheral inflammatory demyelination. J Neurol Neurosurg Psychiatry 2004; 75(12):1784-1786.
- Yuki N (2009) Fisher syndrome and Bickerstaff brainstem encephalitis (Fisher-Bickerstaff syndrome). J Neuroimmunol 215(1-2): 1-9.
- Christoph Kamm, Uwe K Zettl (2012) Autoimmune disorders affecting both the central and peripheral nervous system. Autoimmun Rev 11(3): 196-202.
- Levy DM, Kamphuis S (2012) Systemic lupus erythematosus in children and adolescents. Pediatr Clin North Am 59(2): 345-364.
- Ungprasert P, Matteson EL (2017) Neurosarcoidosis. Rheum Dis Clin North Am 43(4): 593-606.
- Vazquez Do Campo R, Stephens A, Marin Collazo IV, Rubin DI (2018) MOG antibodies in combined central and peripheral demyelination syndromes. Neurol Neuroimmunol Neuroinflamm 5(6): e503.
- H Zéphir, T Stojkovic, P Latour, A Lacour, J de Seze, et al. (2008) Relapsing demyelinating disease affecting both the central and peripheral nervous systems. J Neurol Neurosurg Psychiatry 79(9): 1032-1039.
- T Adamovic, EM Riou, G Bernard, M Vanasse, JC Décarie, et al. (2008) Acute combined central and Peripheral nervous system demyelination in children, Pediatr Neurol 39(5): 307-316.
- Abdel Maksoud ma, Al quraishy SA (2018) Autoimmune Diseases and Infections: A Controversial Relationship. Current Immunology Reviews 14(1): 60-65.
- Siloşi I, Siloşi CA, Boldeanu MV, Cojocaru M, Biciuşcă V, et al. (2016) The role of autoantibodies in health and disease. Rom J Morphol Embryol 57(2): 633-638.
- Suurmond J, Diamond B (2015) Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity. J Clin Invest 125(6): 2194-2202.
- Nacu A, Bunpan JA, Lisnic V, Furlund Owe J, Gilhus N E (2015) Complicating autoimmune diseases in myasthenia gravis: a review. Autoimmunity. 48(6): 362-368.
- Newsom Davis J, Willcox N, Schluep M, Harcourt G, Vincent A, et al. (1987) Immunological heterogeneity and cellular mechanisms in myasthenia gravis. Ann NY Acad Sci 505:12-16.
- Williams WV, Guy HR, Cohen JA, Weiner DB, Greene MI (1988) Molecular and immunologic analyses of a functional internal image formed by an antireceptor antibody. Ann Inst Pasteur Immunol 139(6):659-675.
- Dwyer DS, Vakil M, Bradley RJ, Oh SJ, Kearney JF (1987) A possible cause of myasthenia gravis: Idiotypic networks involving bacterial antigens. Ann NY Acad Sci 505: 461-471.
- Krah DL, Choppin PW (1988) Mice immunized with measles virus develop antibodies to a cell surface receptor for binding virus. J Virol 62(5): 1565-1572.
- Ravindranath R, Graves MC (1990) Attenuated murine cytomegalovirus binds to Nacetylglucosamine and shift to virulence may involve recognition of sialic acids. J Virol 64(11): 5430-5440.
- Lerner A, Jeremias P, Matthias T (2015) The World Incidence and Prevalence of Autoimmune Diseases is Increasing. International Journal of Celiac Disease 3(4): 151-155.
- Ceccarelli F, Agmon Levin N, Perricone C (2016) Genetic Factors of Autoimmune Diseases. J Immunol Res 2016: 3476023.
- Moulton VR (2018) Sex Hormones in Acquired Immunity and Autoimmune Disease. Front Immunol 9: 2279.
- Bach JF (2002) The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 347(12): 911-920.
- Houzen H, Niino M, Hata D, Nakano F, Kikuchi S, et al. (2008) Increasing prevalence and incidence of multiple sclerosis in northern Japan. Mult Scler 14(7): 887-892.
- Fleming J, Fabry Z (2007) The hygiene hypothesis and multiple sclerosis. Ann Neurol 61(2): 85-89.
- Conlon MA, Bird AR (2015) The impact of diet and lifestyle on gut microbiota and human health. Nutrients 7(1): 17-44.
- Kuhn KA, Stappenbeck TS (2013) Peripheral education of the immune system by the colonic microbiota. Semin Immunol 25(5): 364-369.
- Salonen A, de Vos WM (2014) Impact of diet on human intestinal microbiota and health. Annu Rev Food Sci Technol 5: 239-262.
- Grolleau Julius A, Yung R L (2008) The Role of Epigenetics in Aging and Autoimmunity. Autoimmunity 41(4): 329-335.
- Mohan SV, Liao YJ, Kim JW, Goronzy JJ, Weyand C (2011) Giant cell arteritis: immune and vascular aging as disease risk factors. Arthritis Res Ther 13(4): 231-239.
- Goronzy JJ, Shao L, Weyand CM (2010) Immune aging and rheumatoid arthritis. Rheum Dis Clin North Am 36(2): 297-310.
- Hasler P, Zouali M (2005) Immune receptor signaling, aging, and autoimmunity. Cell Immunol 233(2):102-108.
- Agrawal A, Sridharan A, Prakash S, Agrawal H (2012) Dendritic cells and aging: consequences for autoimmunity. Expert Rev Clin Immunol 8(1): 73-80.
- Desai A, Grolleau Julius A, Yung R (2010) Leukocyte function in the aging immune system. J Leukoc Biol 87(6): 1001-1009.
- Murakami M, Nishimoto N (2011) The value of blocking IL-6 outside of rheumatoid arthritis: current perspective. Curr Opin Rheu-matol 23(3): 273-277.
- Lee JS, Lee WW, Kim SH, Kang Y, Lee N, et al. (2011) Age-associated alteration in naive and memory Th17 cell response in humans. Clin Immunol 140(1): 84-91.
- Hasler P, Zouali M (2005) Immune receptor signaling, aging, and autoimmunity. Cell Immunol 233(2): 102-108.
- Johnson TE (2006) Recent results: biomarkers of aging. Exp Gerontol 41(12): 1243-1246.
- Agarwal S, Busse PJ (2010) Innate and adaptive immunosenescence. Ann Allergy Asthma Immunol 104(3): 183-190.
- Duggal NA, Pollock RD, Lazarus NR, Harridge S, Lord JM (2018) Major features of immunesenescence, including reduced thymic output, are ameliorated by high levels of physical activity in adulthood. Aging Cell 17(2): e12750.
- Goronzy JJ, Weyand CM (2003) Aging, autoimmunity and arthritis: T-cell senescence and contraction of T-cell repertoire diversity - catalysts of autoimmunity and chronic inflammation. Arthritis Res Ther 5(5): 225- 234.
- Koetz K, Bryl E, Spickschen K, O’Fallon WM, Goronzy JJ, et al. (2000) T cell homeostasis in patients with rheumatoid arthritis. Proc Natl Acad Sci U S A 97(16): 9203-9208.
- Artlett CM, Black CM, Briggs DC, Stevens CO, Welsh KI (1996) Telomere reduction in scleroderma patients: a possible cause for chromosomal instability. Br J Rheumatol 35(8): 732-737.
- Honda M, Mengesha E, Albano S, Nichols WS, Wallace DJ, et al. (2001) Telomere shortening and decreased replicative potential, contrasted by continued proliferation of telomerase-positive CD8+CD28(lo) T cells in patients with systemic lupus erythematosus. Clin Immunol 99(2): 211- 221.
- Vogt S, Iking Konert C, Hug F, Andrassy K, Hänsch GM (2003) Shortening of telomeres: Evidence for replicative senescence of T cells derived from patients with Wegener’s granulomatosis. Kidney Int 63(6): 2144-2151.
- Wu K, Higashi N, Hansen ER, Lund M, Bang K, et al. (2000) Telomerase activity is increased and telomere length shortened in T cells from blood of patients with atopic dermatitis and psoriasis. J Immunol 165(8): 4742-4747.
- Stacy S, Krolick KA, Infante AJ, Kraig E (2002) Immunological memory and late onset autoimmunity. Mech Ageing Dev 123(8): 975-985
- Johnson SA, Cambier JC (2004) Ageing, autoimmunity and arthritis: senescence of the B cell compartment - implications for humoral immunity. Arthritis Res Ther 6(4):131-139.
- Kline GH, Hayden TA, Klinman NR (1999) B cell maintenance in aged mice reflects both increased B cell longevity and decreased B cell generation. J Immunol 162(6): 3342-3349.
- Paula Quintero Ronderos, Gladis Montoya Ortiz (2012) Epigenetics and Autoimmune Diseases. Autoimmune Dis 2012: 593720.
- Zhang Z, Deng C, Lu Q, Richardson B (2002) Age-dependent DNA methylation changes in the ITGAL (CD11a) promoter. Mech Ageing Dev 123(9): 1257-1268.
- Annabelle Grolleau Julius, Raymond L Yung (2008) The Role of Epigenetics in Aging and Autoimmunity. Autoimmunity 41(4): 329-335.
- Liu L, Wylie RC, Andrews LG, Tollefsbol TO (2003) Aging, cancer and nutrition: the DNA methylation connection. Mech Ageing Dev 124(10- 12): 989-998.
- Robert MF, Morin S, Beaulieu N, Gauthier F, Chute IC, et al. (2003) DNMT1 is required to maintain CpG methylation and aberrant gene silencing in human cancer cells. Nat Genet 33(1): 61-65.
- Lujambio A, Esteller M (2009) How epigenetics can explain human metastasis: a new role for microRNAs. Cell Cycle 8(3): 377-382
- Kato M, Slack FJ (2008) microRNAs: small molecules with big roles -C. elegans to human cancer. Biology of the Cell 100:71-81.
- Stanczyk J, Pedrioli DM, Brentano F, Sanchez Pernaute O, Kolling C, et al. (2008) Altered expression of MicroRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum 58(4): 1001- 1009.
- Pallis M, Robins A, Powell R (1993) Quantitative analysis of lymphocyte CD11a using standardized flow cytometry. Scand J Immunol 38(6): 559- 564.
- Lu Q, Kaplan M, Ray D, Ray D, Zacharek S, et al. (2002) Demethylation of ITGAL (CD11a) regulatory sequences in systemic lupus erythematosus. Arthritis Rheum 46(5):1282- 1291.
- Kevil CG, Hicks MJ, He X, Zhang J, Ballantyne CM, et al. (2004) Loss of LFA- 1, but not Mac-1, protects MRL/MpJ-Fas(lpr) mice from autoimmune disease. Am J Pathol 165(2): 609-616.
- Zhang Z, Deng C, Lu Q, Richardson B (2002) Age-dependent DNA methylation changes in the ITGAL (CD11a) promoter. Mech Ageing Dev 123(9): 1257-1268.
- Juan Manuel Anaya, Carolina Ramirez Santana, Maria A Alzate, Nicolas Molano Gonzalez, Adriana Rojas Villarraga (2016) The Autoimmune Ecology. Front Immunol 7: 139.
- Anaya JM, Restrepo Jiménez P, Ramírez-Santana C (2018) The autoimmune ecology: an update. Curr Opin Rheumatol 30(4): 350-360.
- Parks C, Miller F, Pollard K, Selmi C, Germolec D, et al. (2014) Expert panel workshop consensus statement on the role of the environment in the development of autoimmune disease. Int J Mol Sci 15(8): 14269- 14297.
- Hurst J, von Landenberg P (2008) Toll-like receptors and autoimmunity. Autoimmun Rev 7(3): 204-208.
- Cooney CA, Dave AA, Wolff GL (2002) Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr 132(8): 2393S-2400S.
- Pollard KM, Hultman P, Kono DH (2010) Toxicology of autoimmune diseases. Chem Res Toxicol 23(3): 455-466.
- Zandman Goddard G, Shoenfeld Y (2009) Parasitic infection and autoimmunity. Lupus 18(13): 1144-1148.
- Zaccone P, Fehérvári Z, Jones FM, Sidobre S, Kronenberg M, et al. (2003) Schistosoma mansoni antigens modulate the activity of the innate immune re-sponse and prevent onset of type 1 diabetes. Eur J Immunol 33(5): 1439-1449.
- Strachan DP (2000) Family size, infection and atopy: the first decade of the “hygiene hypothesis”. Thorax 55(1): S2-10.
- Elliott DE, Weinstock J V (2012) Helminth-host immunological interactions: prevention and control of immune-mediated diseases. Ann N Y Acad Sci 1247: 83-96.
- Kuijk LM, Van Die I (2010) Worms to the rescue: can worm glycans protect from autoimmune diseases?. IUBMB life 62(4): 303-312.
- Aranzamendi C, Sofronic Milosavljevic L, Pinelli E (2013) Helminths: Immunoregulation and Inflammatory Diseases-Which Side Are Trichinella spp. and Toxocara spp. on? J Parasitol Res p. 11.
- Kosiewicz MM, Zirnheld AL, Alard P (2011) Gut microbiota, immunity, and disease: a complex relationship. Front Microbiol 2: 180.
- Ivanov II, Honda K (2012) Intestinal commensal microbes as immune modulators. Cell Host Microbe 12(4): 496-508.
- Cho I, Blaser MJ (2012) The human microbiome: at the interface of health and disease. Nat Rev Genet 13(4): 260-270.
- Devereux G (2006) The increase in the prevalence of asthma and allergy: food for thought. Nat Rev Immunol 6(11): 869-874.
- Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, et al. (2009) The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med 1(6): 6-14.
- Gamal Badr, Ayat Sayed, Mostafa A Abdel Maksoud, Amany O Mohamed, Azza El-Amir, et al. (2015) Infection of Female BWF1 Lupus Mice with Malaria Parasite Attenuates B Cell Autoreactivity by Modulating the CXCL12/CXCR4 Axis and Its Downstream Signals PI3K/AKT, NFκB and ERK. PLoS One 10(4): e0125340.
- Saleh Al Quraishy, Mostafa A Abdel Maksoud, Azza El Amir, Fathy A Abdel Ghaffar, Gamal Badr (2013) Malarial Infection of Female BWF1 Lupus Mice Alters the Redox State in Kidney and Liver Tissues and Confers Protection against Lupus Nephritis. Oxid Med Cell Longev 2013: 156562.
- Mostafa A Abdel Maksoud, Fathy A Abdel Ghaffar, Azza El Amir, Saleh Al Quraishy, Gamal Badr (2016) Infection with Plasmodium chabaudi diminishes plasma immune complexes and ameliorate the histopathological alterations in different organs of female BWF1 lupus mice. Eur Rev Med Pharmacol Sci 20(4): 733-744.
- Mostafa A Abdel Maksoud, Fathy A Abdel Ghaffar, Azza El Amir, Saleh Al Quraishy, Gamal Badr (2018) Altered renal immune complexes deposition in female BWF1 lupus mice following Plasmodium chabaudi infection. Saudi J Biol Sci. 25(8): 1609-1616.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.