Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Electron Microscopy Study of Nerve Cell Death Types in Some Central Nervous System Diseases. A Review
*Corresponding author: Orlando J Castejón, Faculty of Medicine, Zulia University, Maracaibo, Venezuela.
Received: February 09, 2019; Published: May 22, 2019
DOI: 10.34297/AJBSR.2019.03.000637
Abstract
In the present review we describe the nerve cell death types in some nervous central diseases. Cortical biopsies of patients with congenital hydrocephalus, brain trauma, vascular anomalies and brain tumors are examined by means of transmission electron microscopy to study distinct and combined forms of nerve cell death. Most non-pyramidal nerve cells, astrocyte and oligodendrocytes undergo a hybrid oncotic-apoptotic and necrotic continuum process. In a lesser proportion another non-pyramidal nerve cells, astrocytes and oligodendrocytes show apoptosis or oncosis only. Autophagic cell death was rarely seen in non-pyramidal nerve cells in traumatic brain edema. The nerve cell death is discussed in relation with the severity of brain edema, anoxic-ischemic conditions of the tissue, calcium depolarization and Ca ++ influx, resulting in excitotoxic cell death, glutamate excitotoxicity, caspase dependent and independent mechanisms, oxidative stress, and calcium overload.
Introduction
Häcker and Vaux [1] have published an interesting review on a chronology of cell death, listing papers between 1842 and 1995, that illustrates how physiological death has been exhaustively studied. In the last decade, diversity mechanisms of cellular and molecular events have been elucidated in relation with nerve cell death [1], which can be now correlated with nuclear structural alterations in brain edema and associated anoxic-ischemic conditions [3]. studied the ultrastructure of excitotoxic (glutamate) neuronal death in murine cortical culture to explore to which extend these in vitro paradigms resemble in vivo ischemic injury, and described rapid swelling of mitochondria, vacuolation of endoplasmic reticulum and random condensation of chromatin. Two pathways of premortal events of cell death: oncosis and apoptosis, and necrosis, conceptualized as a postmortal stage, have been described [4-7].
For more than 80 years apoptosis and necrosis have been known to be distinct forms of cell death. Apoptosis considered as a normal physiological process during development has been suggested to be involved in the abnormal neuronal death following axonal injury or in neurodegeneration [8-10]. Described delayed, selective neuronal death following cortical injury in rats, and its possible role in memory deficits [11]. reported nerve cell death in rat hydrocephalus [12], found two different morphological types of apoptosis following focal ischemia in the striatal penumbra. [12], described the ultrastructural morphology of neuronal death following reversible focal ischemia in the rat.
Saikumar [13], examined the diversity of death paradigms in hypoxia/reoxigenation injury related with caspase independent as well as caspase dependent processes, and the involvement of several mitochondrial factors [14], described the apoptotic response to trauma of nerve cells using TUNEL histochemistry and agarosa gel electrophoresis and reported acute and delayed patterns of cell death. [15], by means of similar techniques, reported the features of apoptosis in a neonatal rat model of cerebral hypoxia-ischemia, and described DNA fragmentation, chromatin condensation and apoptotic bodies.
Calcium-dependent mechanisms related to excess activation of calcium-dependent enzymes (Calmortin hypothesis) can cause neuronal cell death [16]. Mitochondrial calcium overload is considered a critical event in both apoptotic and necrotic cell death [17,18]. Caspase activation has been postulated to occur in cells undergoing apoptosis [17,19] studied the role of glutamate receptors and voltage-dependent calcium channels in glutamate toxicity in energy-compromised cortical neurons [20], found that autophagy may mediate caspase-independent neuronal death. [21], described programmed cell death as a mechanism of neuronal death in prion diseases.
Walton [22], reviewed the neuronal death and survival in two models of hypoxic-ischemic brain damage. [23], described death of oligodendrocytes and microglial pahgocytosis of myelin preceding immigration of Schwann cells into the spinal cord. [24], suggested that caspase, bcl-2, DAP families and substrates, such as PARP contribute to the mechanism of cell death in ischemic brain injury. [25], described caspase-independent programmed cell death with necrotic morphology. Apoptosis and necrosis after ischemia are associated with differential alterations in metabotropic glutamate receptor signalling pathways [26].
Budd [27] reported mitochondrial and extramitochondrial signaling pathways in NMDA receptor-mediated apoptosis of cerebrocortical cultured neurons. [28], demonstrated the contributing role of glycogen synthase kinase in neuronal apoptosis induced by trophic withdrawal. [29], studied the astroglial trophic support and neuronal cell death. [30], described apoptosis and necrosis after cerebral contusions in humans. [31], showed the involvement of caspase 3 in apoptotic death of cortical neurons evoked by DNA damage. [32], suggested a contributory role of caspase 3 in neuronal and glial cell apoptotic degeneration after traumatic brain injury. [33], found that traumatic brain injury induces temporal and selective alteration of gene expression profiles of TUNNEL-positive neurons. [34], demonstrated that mild cerebral ischemia induces loss of ciclin-dependent kinase inhibitors, and activation of neuron cycle machinery before delayed neuronal cell death. [35], reported that astrocytes contribute to neuronal impairment in beta A toxicity increasing apoptosis in rat hyppocampal neurons. [36], postulated that degeneration and death of neurons is the fundamental process responsible for the clinical manifestations of many neurological disorders of aging, including Alzheimer’s disease, Parkinson’s disease and stroke. [37], reported apoptotic neurodegeneration following trauma in developing rat brain, and suggested the involvement of intrinsic and extrinsic pathways. [38], reported apoptotic cell death in congenitally XTX hydrocephalic rats due to retrograde degeneration from extensive axonal damage. [39], also described thalamic neuronal apoptosis after cortical damage in immature mice. Boldirev et al. (2002) demonstrated oxygen deprivation-induced cell death.
Wang [40] studying hemoglobin-induced cytotoxicity in rat cerebral cortical neurons, suggested that in addition to activation of caspase cascades, parallel pathways of oxidative stress predominate in this model of hemoglobin neurotoxicity. Caspase-dependent and caspase-independent pathways have been implicated mediating neuronal cell death [25, 41-46].
Posttraumatic nerve cell death has been also studied. Apoptosis of neurons and glial cells have been reported after traumatic brain injuries in both humans and animals [47-50] .
Bezvenyuk [51] demonstrated that chromatin condensation preceded nuclear DNA fragmentation, as uncoupled event during neuronal cell death. [52], found nucleolar disintegration after rat perinatal asphyxia. [53], demonstrated that sustained calpain activation induced disruption of lysosome membrane after ischemia and executes necrosis of CA1 neurons in primates. [54], found that steroid-triggered programmed cell death of motoneuron is autophagic and involves structural changes in mitochondria. [55], described apoptosis induced by oxidative stress and the role played by caspases and intracellular calcium ions. [56], demonstrated that a concurrent increase in caspase-3 and cathepsin-B suggest that necrotic and apoptotic cell death are concomitantly activated in ischemic neurons. [57], reported type I TUNEL positive cells in the ischemic cortex leading to necrotic changes, and type II TUNEL positive cells showing hybrid forms of cell death, featured by concurrent apoptotic and necrotic alterations. [58], demonstrated that ischemia leads to apoptosis and necrosis-like neuron death in the ischemic rat hippocampus.
According to Rhagupathi [59], apoptotic and necrotic neurons have been identified after traumatic brain injuries. [60], correlated regional cerebral blood flow and the presence of apoptotic cells, 3 hours after hypoxic-ischemic injury in preterm lambs. [61], related brain edema and elevated intracranial pressure in animal models of traumatic brain injuries and found that apoptotic cell count has a positive correlation with cerebral water content, and elevated intracranial pressure. [62], postulated that cerebral ischemia triggers a complex cascade of cellular events, such as calcium depolarization and Ca ++ influx, resulting in excitotoxic cell death. Haku [63], found persistent neuronal death in immature rats after hypoxia and ischemia. [64], proposed that calpain-induced calcineurin activation in part mediates delayed neuronal death in brain ischemia. [65], demonstrated in vitro activation of caspase 8 pathways and induction of cell death in cultured hippocampal neurons. [66], reported that nerve cell death in vascular demencia occurs as a consequence of ischemic insults such as hemorrhage and hipoperfusion that trigger neurodegeneration. [67], summarize the arguments that implicate calpain in neuronal apoptosis and neurodegenerative diseases. [68], described ultrastructural changes of apoptosis and secondary cell death after compression in cortical neurons. [50], postulated a continuum processes of oncosis, apoptosis, necrosis, or oncosis or apoptosis only, and autophagic cell death in the human edematous cerebral cortex associated to congenital hydrocephalus, brain trauma, vascular anomaly, and brain tumors. According to aging is neuroprotective during global ischemia, but leads to increased caspase-3 apoptotic activity in hippocampal neurons.
Brief cerebral ischemia leads to selective neuronal necrosis (SNN), which is characterized by neuronal death with sparing of glial and vascular elements of the central nervous system [69]. N-methyl-D-aspartate receptors (NMDARs), which are critical for excitotoxicity in neurons, triggers microglia activation in vitro and secretion of factors that induce cell death of cortical neurons [70,71], described neuronal cell death and degeneration through increased nitroxidative stress and tau phosphorylation in HIV-1 transgenic rats.
Li [72], recently described the ultrastructural characteristics of neuronal death and white matter injury in mouse brain tissues after intracerebral hemorrhage, and the coexistence of ferroptosis, autophagy, and necrosis.
According to Formella [73], KillerRed-activated neurons in the spinal cord undergo stress and cell death after induction of reactive oxygen species (ROS). [74], reported recent studies suggesting a toxic role for non-phosphorylated and non-aggregated tau when it is located in the brain extracellular space, and that phosphorylated and/or aggregated intracellular tau protein was causative of neuronal death.
In the present review we analyze by means of conventional transmission electron microscopy, and using strict morphological criteria, the distinct and combined pathways of neuronal and glial cell death in the human edematous cerebral cortex associated to four different pathological entities: congenital hydrocephalus, brain trauma, vascular anomaly and brain tumors. Oncosis and apoptosis, as separate nerve cell death pathways, and a continuum oncosisapoptosis leading to necrosis are described.
The main goal of this review is to describe how different insults to nerve cells induce diversity mechanisms of nerve cell death. To examine how brain edema and their associated anoxic-ischemic conditions interact to cause a characteristic nerve cell death type (oncosis or apoptosis) or hybrids forms of nerve cell death leading to necrosis.
Nerve cell death in congenital hydrocephalus
In congenital hydrocephalus and associated lumbar meningomielocele, nonpyramidal neurons undergo coexisting oncotic and apoptotic processes characterized by remarkably swollen mitochondria and endoplasmic reticulum canaliculi and cisterns, and dense nucleoplasm with a condensed chromatin (Figure 1).
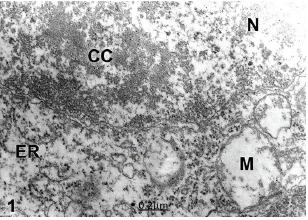
Figure 1:Congenital hydrocephalus. Lumbar meningomielocele. Right parietal cortex. Non-pyramidal neuron (NP) showing overlapped oncotic and apoptotic processes of nerve cell death characterized by nuclear (N) chromatin condensation (CC) and swollen electron lucent cytoplasm. Note the notably swollen mitochondria (M). X 60.000.
In Arnold-Chiari malformation and hypertensive communicant hydrocephalus, the non- pyramidal nerve cells and oligodendrocytes exhibit oncotic cell death featured by a decondensed nuclear chromatin, enlarged and disrupted perinuclear cistern widely communicated with the endoplasmic reticulum and the dilated extracellular space (Figure 2), suggesting that the oncotic cell death is due to the high pressure exerted by the interstitial hydrocephalic edema [75].
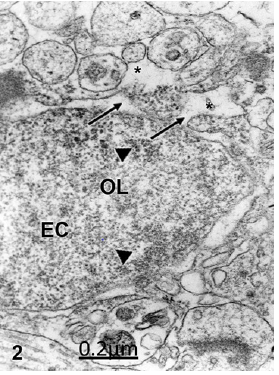
Figure 2: Arnold-Chiari malformation. Communicant hydrocephalus. Frontal cortex. Oncotic oligodendroglia (OL) cell death featured by wide communications between the perinuclear cistern and rough endoplasmic reticulum (arrows) and the extracellular space (asterisks). Note the decondensed state of nuclear euchromatin (EC) featured by granular and fibrillar organization (arrowheads). X 60.000.
Oligodendroglial cell death have been described in Arnold Chiari malformation and related with axonal demyelination process [76], The astrocytes mostly show a combined oncotic and apoptotic cell death characterized by strong chromatin condensation, disrupted swollen cytoplasm and fragmented limiting plasma membranes (Figure 3).

Figure 3: Right temporal cortex. Right parietal cortex. Arnold-Chiari malformation. Hydrocephalus. Fibrous astrocyte (A) showing a combined oncotic and apoptotic cell death process characterized by a disrupted cytoplasm, and fragmented plasma membrane (arrows), shrunken lobulated nucleus (N), and strong chromatin condensation (CC). X 36.000
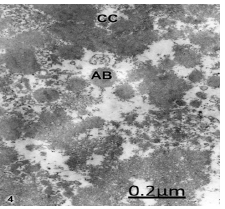
Figure 4: Congenital hydrocephalus. Frontal cortex. Nucleus of a non-pyramidal neuron showing the typical features of apoptosis characterized by strong chromatin condensation (CC) and formation of apoptotic bodies (AB). X 60.000.
In congenital hydrocephalus nerve cells suffering an evident apoptotic process are observed, the formation of typical apoptotic bodies (Figure 4).
In congenital hydrocephalus and Arnold-Chiari malformations we are dealing with immature brain, where oncosis, apoptosis and necrosis also occurred as a continuum [50,75]. In these cases, the nerve cell populations exhibit high vulnerability, and support the hypothesis that excitotoxic neuronal death in the immature brain is not a uniform event [77], but rather overlapped morphological processes with a distinct phenotype of neurodegeneration.
Del Biggio and Zhan [11] reported nerve cell death in immature rat brain following the induction of hydrocephalus. [15], reported the features of apoptosis in a neonatal rat model of cerebral hypoxia-ischemia and described DNA fragmentation, chromatin condensation and apoptotic bodies. [38], reported apoptotic cell death in congenitally XTX hydrocephalic rats due to retrograde degeneration from extensive axonal damage. [39], also described thalamic neuronal apoptosis after cortical damage in immature mice. [37], reported apoptotic neurodegeneration following trauma in developing rat brain and suggested the involvement of intrinsic and extrinsic pathways. [63], found persistent neuronal death in immature rats after hypoxia and ischemia. [26,[77-79]], postulated a continuum of apoptotic, necrotic, and overlapping morphologies in excitotoxic neuronal death in newborn rat brains. According to [80], hypoxic-ischemic neuronal degeneration in the developing CNS triggers two separates waves of neurodegeneration, the first being excitotoxic and the second being apoptotic.
Nerve cell death in complicated human traumatic brain injuries
In severe brain trauma complicated with subdural or epidural hematoma, some non-pyramidal nerve cells exhibit oncotic cell death type featured by electron lucent cytoplasm and nucleoplasm, lobulated nucleus, presence of intranuclear inclusions, intact and disrupted nuclear pore complexes, enlarged perinuclear cistern, rough endoplasmic reticulum canaliculi and cisterns and swollen clear an dense. Degenerated mitochondria were considered as marker of lethal injury [81], (Figure 5).

Figure 5: Brain trauma. Right epidural hematoma. Right temporal cortex. Oncotic nerve cell death of a non-pyramidal neuron characterized by flocculent precipitate of cytoplasmic matrix, degranulated and vacuolated endoplasmic reticulum (ER), irregularly enlarged perinuclear cistern (PC), intranuclear inclusion (II), intact (short arrow) and disrupted (long arrow) nuclear pores, The decondensed nuclear chromatin exhibits granular and fibrillar organization. X 60.000.
Another non-pyramidal nerve cells exhibit a combined pathway of apoptotic and autophagic cell death leading to necrosis, characterized by chromatin condensation, clear nucleoplasm, dark and isolated profiles of endoplasmic reticulum, degenerated mitochondria, disrupted multivesicular bodies, numerous clear vacuoles, apparently of lysosomal origin, and numerous small dense like-lysosomal bodies [50], (Figure 6).
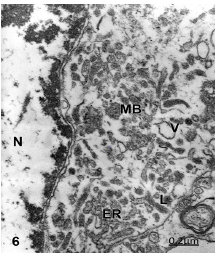
Figure 6:Brain trauma. Right epidural hematoma. Right temporal cortex. Non-pyramidal neuron showing an overlapped process of oncotic, apoptotic and autophagic cell death characterized by disintegrated and dark endoplasmic reticulum profiles (ER), nuclear (N) fragmented peripheral heterochromatin (arrow), chromatin condensation (CC), lysosomal bodies (L), disrupted vesicular bodies (MB), large vacuole (V), and electron lucent cytoplasm and nucleoplasm. X 24.000.
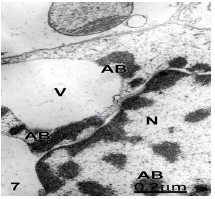
Figure 7: Brain trauma. Right epidural hematoma. Glycogen-depleted astrocyte showing apoptotic cell death and exhibiting apoptotic bodies (AB) in the nucleoplasm (N) and in the cytoplasm. This latter shows a huge vacuole (V). X 60.000.
Xue [20], found that autophagy may mediate caspaseindependent neuronal death. Autophagic cell death may be mediated by lysosomal cysteine proteases (cathepsyns) to caspase [82-84]. Autophagic cell death also is seen in neurodegenerative diseases [85]. Some non-pyramidal neurons and astrocytes show typical apoptotic cell death characterized by chromatin condensation of nuclear chromatin, disrupted perinuclear cistern, and presence of apoptotic bodies in the nucleus and cytoplasm (Figure 7).

Figure 8:Brain trauma complicated with right epidural hematoma. Right temporal cortex. Astrocyte cell (A) showing oncotic cell death and necrosis characterized by electron translucent and swollen nucleoplasm (N). Small masses of condensed peripheral heterochromatin (CC) are observed resembling a picknotic nucleus. The cytoplasm appears devoid of cytomembranes and most cell organelles. A degenerated mitochondrion (M) with a granular matrix and few compacted cristae is seen. The neighbouring collapsed neuropile exhibits a degenerated synaptic ending (SE). X 24.000.
Apoptosis after traumatic brain injuries has been widely reported [30-33,47-49,61,86-89]. Apoptosis has been also found in axonal injury and neurodegenerative diseases, such as amyotrophic lateral sclerosis and Alzheimer’s [8,90], consider that in complete absence of oxygen, cells undergo cell death through apoptosis. Oxidative stress exerts a pivotal role in the generation of apoptosis [91,92]. In addition, [86], demonstrated apoptosis after cortical impact, and expression of proteins associated with DNA damage and cyclin D1. [15], found apoptosis in nerve and glial cells in areas with milder forms of ischemic damage. Some astrocytes displayed a coexisting oncotic cell death and necrosis showing chromatin condensation, empty, electron lucent and disrupted cytoplasm, and swollen degenerated mitochondria [50] (Figure 8).
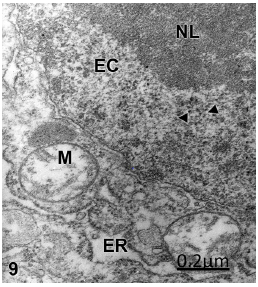
Figure 9: Brain trauma. Severe frontal contusion. Left frontal cortex. Non-pyramidal neuron showing oncotic cell death, and displaying a prominent nucleolus (NL), dense euchromatin (EC), numerous perichromatinic granules (arrowheads), swollen mitochondria (M), and enlarged endoplasmic reticulum (ER). X 36.000.
Some non-pyramidal neurons suffer only oncotic cell death exhibiting a prominent nucleolus, numerous perichromatinic granules embedded in a dense euchromatin (Figure 9).
Apoptotic and oncotic cell death as separate entities are observed in a lesser proportion in non-pyramidal nerve cells, astrocytes and oligodendrocytes [50].
Nerve cell death types in brain tumors
In some brain tumors, oncotic or ischemic cell death of nonpyramidal neurons is observed characterized by an electron dense nucleoplasm, presence of perichromatinic granules, absence of chromatin condensation, and notably swollen mitochondria, endoplasmic reticulum cisterns and vacuoles (Figure 1).
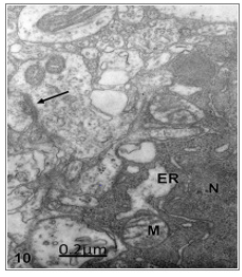
Figure 10: Cystic craniopharyngioma. Right temporal cortex. Dark ischemic nerve cell showing oncotic and necrotic cell death featured by dark nucleoplasm (N) and cytoplasm, lacunar enlargement of endoplasmic reticulum (ER), and swollen mitochondria (M). The arrow labels a degenerated axodendritic contact in the neighbouring neuropile. X 36.000.
Macrophages are able to induce apoptosis in a number of tumor cells, including glioma. It is known that apoptosis of cells is executed on either a death receptor-dependent or independent pathway [39].
According to Mora [93], appropriately activated microglial cells induce secreted proteic factors that decreased proliferation and migration of glioma cells and efficiently killed them by a process of autophagy-dependent death. Cell death was characterized by the early accumulation of acidic vesicles, phosphatidylserine exposure, appearance of double-membrane cytoplasmic vesicles, extensive zeiosis and a very late loss of DNA in cells that had lost membrane integrity.
Inhibition of paraquat-induced autophagy accelerates the apoptotic cell death in neuroblastoma SH-SY5Y cells. The cells succumbed to cell death with hallmarks of apoptosis such as phosphatidylserine exposure, caspase activation, and chromatin condensation [94]. These Authors related a relationship between autophagy and apoptotic cell death in human neuroblastoma cells treated with paraquat.
Nerve growth factor (NGF) is known to regulate both cancer cell survival and death signaling, depending on the cellular circumstances, in various cell types. NGF strongly upregulated the protein level of tropomyosin-related kinase A (TrkA) in TrkAinducible SK-N-MC cancer cells [95].
Nerve cell death in vascular anomaly
In vascular anomalies a combined apoptotic and oncotic cell death is observed in neurons and oligodendrocyte cells featured by chromatin condensation, severely swollen perinuclear cistern and necrotic endoplasmic reticulum (Figure 11).
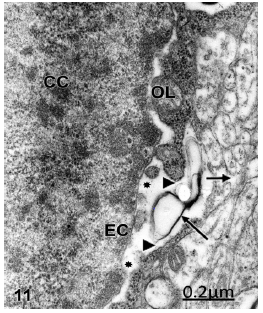
Figure 11:Right temporal cortex. Anomaly of anterior cerebral artery. Oligodendroglial cell undergoing apoptotic cell death showing chromatin condensation (CC), granular and fibrillar organization of euchromatin (EC), enlarged perinuclear cistern (asterisks), communication of perinuclear cistern with the thin band of cytoplasm (arrowheads), necrosis of endoplasmic reticulum membranes (long arrow), and communication of cytoplasm with the extracellular space of neighbouring neuropile (short arrow). X36.000.
In the periphery surrounding the ischemic core (the so-called penumbra) neurons, astrocytes, microglia, oligodendrocytes, pericytes, and endothelial cells react to detrimental factors such as excitotoxicity, oxidative stress, and inflammation in different ways [96].
Brain ischemia may lead to stroke and is characterized by key molecular events that initiate apoptosis in many cells, such as overproduction of free radicals, Ca2+ overload and excitotoxicity. These changes may trigger either apoptosis or necrosis. Apoptosis results in DNA fragmentation, degradation of cytoskeletal and nuclear proteins, cross-linking of proteins, formation of apoptotic bodies, expression of ligands for phagocytic cell receptors and finally uptake by phagocytic cells [97,98].
Overlapped cell death types
The different overlapped cell death types observed in the human edematous cerebral cortex are considered leading to necrosis conceptualized as the changes that occur after nerve cell death [5, 6,99]. In most nerve cells an oncotic-apoptotic cell death continuum is observed where distinctions between this cell death types are becoming blurred. A continuum apoptosis-necrosis was earlier reported by [77-79]. Although apoptosis and necrosis are mediated through distinct pathways, different pathological conditions lead to the continuum oncosis, apoptosis and necrosis, depending of the severity of brain parenchymatous edema, the age of the patients, and the moderate or severe anoxic-ischemic conditions involved.
The continuum oncosis-apoptosis-necrosis observed in severe traumatic head injuries complicated with hematomas or hygroma, is mainly related with the degree of existing vasogenic and cytotoxic brain edema, mainly mitochondrial edema.
Molecular mechanisms underlying nerve cell death
Diverse molecular mechanisms have been proposed to explain nerve cell death, such as caspase independent as well as caspase dependent processes Tanabe et al., 1999; [7,15,17,25,31,41- 46,64,65,67,100,101]. which are proposed to trigger the activation of calpains, the cytosolic Ca2+ activated cysteine proteases, which may in turn degrade cytoplasmic proteins. Activated calpain may in turn activate lysosomal cathepsyn and induce necrosis. Calciumdependent mechanisms related to excess activation of calciumdependent enzymes (Calmortin hypothesis) can cause neuronal cell death [16]. Mitochondrial calcium overload is considered a critical event in both apoptotic and necrotic cell death [18,102]. The ability of high levels of Ca 2+ to induce swelling and disruption of mitochondria [102] cause opening of permeability transition pore and loss of energy metabolism, a key event leading to necrosis [45]. The pivotal role of mitochondrial calcium uptake in neuronal cell apoptosis and necrosis has been emphasized by [17]. The damage of the blood brain barrier and the perivascular hemorhages in human traumatic brain injuries [103] could induce hemoglobin citotoxicity, caspase activation and parallel pathways of oxidative stress [40].
Concluding Remarks
In the present review we describe the nerve cell death types in some nervous central diseases. Cortical biopsies from patients with clinical diagnosis of congenital hydrocephalus, brain trauma, vascular anomalies and brain tumors were examined by means of transmission electron microscopy to study the distinct and combined forms of nerve cell death [104-127]. Most non-pyramidal nerve cells, astrocytes and oligodendrocytes undergo an oncoticapoptotic- necrotic continuum. In a lesser proportion another non-pyramidal nerve cells, astrocytes and oligodendrocytes show apoptosis or oncosis only. Autophagic cell death was rarely seen in non-pyramidal nerve cells in traumatic brain edema. The nerve cell death is discussed in relation with the severity of brain edema, anoxic-ischemic conditions of the tissue, caspase dependent and independent mechanisms, oxidative stress, and calcium overload.
References
- Häcker G, Vaux DL (1997) A chronology of cell death. Apoptosis 2(3): 247-256.
- Vaux DL (1993) Toward and understanding of the molecular mechanisms of physiological cell death. Proc Natl Acad Sci 90(3): 786-789.
- Regan RF, Panter SS, Witz A, Tilly JL, Giffard RG (1995) Ultrastructure of excitotoxic neuronal death in murine cortical culture. Brain Res 705(1- 2): 188-198.
- Agid Y, Blin J (1987) Nerve cell death in degenerative diseases of the central nervous system: clinical aspects. Ciba Found Symp 126: 3-29.
- Majno G, Joris I (1995) Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol 146(1): 3-15.
- Trump BF, Berezesky IK, Chang SH, Phelps PC (1997) The pathways of cell death: oncosis, apoptosis, and necrosis. Toxicol Pathol 25(1): 82-88.
- Yuan J, Lipinski M (2003) Diversity in the mechanisms of neuronal cell death. Neuron 40(2): 401-413.
- Lo AC, Houenou LJ, Oppenheim RW (1995) Apoptosis in the nervous system: morphological features, methods, pathology, and prevention. Arch Histol Cytol 58(2): 139-149.
- Hutchin JB, Barger SW (1998) Why neurons die cell death in the nervous system. Anat Rec 253(3): 79-90.
- Colicos MA, Dixon CE, Dash PK (1996) Delayed, selective neuronal death following experimental cortical impact injury in rats: possible role in memory deficits. Brain Res 739(1-2): 111-119.
- Del Bigio MR, Zhang YW (1998) Cell death, axonal damage, and cell birth in the immature rat brain following induction of hydrocephalus. Exp. Neurol. 154(1): 157-169.
- Ben Ari Y, Charriaut Marlangue C, Aggoun Zouaoui D, Margalli I, Borrega F, et al. (1998) Ultrastructural morphology of neuronal death following reversible focal ischemia in the rat. Apoptosis 3(2): 133-141.
- Saikumar P, Dong Z, Wainberg JM, Venkatachalam MA (1998) Mechanisms of cell death in hypoxia/reoxygenation injury. Oncogene 17(25): 3341-3349.
- Conti AC, Raghupathi R, Trojanowski JQ, McIntosh TK (1998) Experimental brain injury induces regionally distinct apoptosis during the acute and delayed post-traumatic period. J Neurosci 18(15): 5663- 5672.
- Pulera MR, Adans LM, Liu H, Santos DG, Nishimura RN, et al. (1998) Apoptosis in a neonatal rat model of cerebral hypoxia-ischemia. Stroke 29(12): 2622-2630.
- Bennet MR, Huzlin KR (1996) Neuronal cell death in the mammalian nervous system: the calmortin hypothesis. Gen. Pharmacol 27(3): 407- 419.
- Krutman II, Mattson MP (1999) Pivotal role of mitochondrial calcium uptake in neuronal cell apoptosis and necrosis. J Neurochem 72(2): 529- 540.
- Abe K (1999) Neurons, necrotic vs. apoptotic changes. In Cerebral Ischemia. Walz W (Ed). Humana Press, New Jersey, USA, pp. 217-232.
- Kimura M, Katayama K, Nishizawa Y (1999) Role of glutamate receptors and voltage-dependent calcium channels in glutamate toxicity in energycompromised cortical neurons. Japan J. Pharmacol 80(4): 351-358.
- Xue L, Fletcher GC, Tolkovsky AM (1999) Autophagy is activated by apoptotic signalling in sympathetic neurons: an alternative mechanism of death execution. Mol Cell Neurosci 14(3): 180-198.
- Chretien F, Dorandeu A, Adle Biassette H, Ereau T, Wingertsmann L, et al. (1999) A process of programmed cell death as a mechanism of neuronal death in prion diseases. Clin Exp Pathol 47(3-4): 181-191.
- Walton M, Connor B, Lawlor P, Young D, Sirimanne E, et al. (1999) Neuronal death and survival in tow models of hypoxic-ischemic brain damage. Brain Res Rev 29(2-3): 137-168.
- Li Y, Field PM, Raisman G (1999) Death of oligodendrocytes and microglial phagocytosis of myelin precede immigration of Schwann cells into the spinal cord. J Neurocytol 28(4-5): 417-427.
- Hara H (1999) Involvement of caspase on apoptosis in ischemia-induced neuronal cell death: usefulness of caspase inhibitors for stroke therapy. Nippon Yakurigaku Zasshi 113(2): 97-111.
- Kitanaka C, Kuchino Y (1999) Caspase-independent programmed cell death with necrotic morphology. Cell Death Differ 6(6): 508-515.
- Martin LJ, Siebr FE, Traystman RJ (2000) Apoptosis and necrosis occur in separate neuronal populations in hippocampus and cerebellum after ischemia and are associated with differential alterations in metabotropic glutamate receptor signaling pathways. J Cereb Blood Flow Metab 20(1): 153-167.
- Budd SL, Tenneti L, Lishnak T, Lipton SA (2000) Mitochondrial and extramitochondrial apoptotic signalling pathways in cerebrocortical neurons. Proc Natl Acad Sci 97(11): 6161-6166.
- Hetman M, Cavanaugh JE, Kimelman D Xia, M Zhengui (2000) Role of glycogen Synthase Kinase-3β in neuronal apoptosis induced by trophic withdrawal. J Neurosci 20(7): 2567-2574.
- Ohgoh M, Shimizu H, Ogura H, Nishizawa Y (2000) Astroglial trophic support and neuronal cell death: influence of cellular energy level on type of cell death induced by mitochondrial toxin in cultured rat cortical neurons. J Neurochem 75(3): 925-933.
- Ng I, Yo TT, Tang WY, Soong R, Ng PY, Smith DR (2000) Apoptosis occurs after cerebral contusions in humans. Neurosurgery 46(4): 949-956.
- Keramaris E, Stefanis L, Maclaurin J, Harada N, Takaku K, et al. (2000) Involvement of caspase 3 in apoptotic death of cortical neurons evoked by DNA damage. Mol Cell Neurosci 15(4): 368-379.
- Beer R, Franz G, Srinivasan A, Hayes RL, Pike BR, et al. (2000) Temporal profile and cell subtype distribution of activated caspase-3 following experimental traumatic brain injury. J Neurochem 75(3): 1264-1273.
- O’Dell DM, Raghuapathi R, Crino PB, Eberwine JH, McIntosh TK (2000) Traumatic brain injury alters the molecular fingerprint of TUNNEL positive cortical neurons In vivo: A single-cell analysis. J Neurosci 20(13): 4821-4828.
- Katchanov J, Herms Ch, Gertz K, Hauck L, Waeber Ch, et al. (2001) Mild cerebral ischemia induces loss of cyclin-dependent kinase inhibitors and activation of cell cycle machinery before delayed neuronal cell death. J Neurosci 21(14): 5045-5053.
- Malchiodi Albedi F, Domenici MR, Paradisi S, Bernardo A, Ajmone Cat MA, et al. (2001) Astrocytes contribute to neuronal impairment in beta A toxicity increasing apoptosis in rat hippocampal neurons. Glia 34(1): 68-72.
- Mattson MP, Duan W, Pedersen WA, Culmsee C (2001) Neurodegenerative disorders and ischemic brain diseases. Apoptosis 6(1-2): 69-81.
- Felderhoff Mueser U, Sifringer M, Pesditschek S, Kuckuck H, Moysich A, et al. (2002) Pathways leading to apoptotic neurodegeneration following trauma to the developing rat brain. Neurobiol Dis 11(2): 231-245.
- Mori F, Tanji K, Yoshida Y, Wakabayashi K (2002) Thalamic retrograde degeneration in the congenitally hydrocephalic rat Iattributable to apoptotic cell death Neuropathology, 22(3): 186-193.
- Natale JE, Cheng Y, Martin LJ (2002) Thalamic neuron apoptosis emerge rapidly after cortical damage in immature mice. Neuroscience, 112(3): 665-676.
- Wang X, Mori T, Sumii T, Lo EH (2002) Hemoglobin-induced cytotoxicity in rat cerebral cortical neurons: caspase activation and oxidative stress. Stroke 33(7): 1882-1888.
- Johnson MD, Kinoshita Y, Xiang H, Ghatan S, Morrison RS (1999) Contribution of p53-dependent caspase activation to neuronal cell death declines with neuronal maturation. J Neurosci 19(8): 2996-3006.
- Hamabe W, Fukushima N, Yoshida A, Ueda H (2000) Serum-free induced neuronal apoptosis-like cell death independent of caspase activity. Brain Res Mol Brain Res 78(1-2): 186-191.
- Nicotera P, Leist M, Fava E, Berliocchi L, Volbracht C (2000) Energy requirement for caspase activation and neuronal cell death. Brain Pathol 10(2): 276-282.
- Cregan SP, Fortin A, Maclaurin JG, Calleghan SM, Cecconi F, et al. (2002) Apoptosis-inducing factor is involved in the regulation of caspaseindependent neuronal cell death. J Cell Biol 158(3): 507-517.
- Lang Rollin IC, Rideout HJ, Noticewala M, Stefanis L (2003) Mechanisms of caspase-independent neuronal death: energy depletion and free radical generation. J Neurosci 23(35): 11015-11025.
- Chang LK, Schmidt RE, Johnson EM JR (2003) Alternating metabolic pathways in NGF-deprived sympathetic neurons affect caspaseindependent death. J Cell Biol 162(2): 245-256.
- Raghupathi R, Graham DI, McIntosh TK (2000) Apoptosis after traumatic brain injury. J Neurotrauma 17(10): 927-938.
- Raghupathi R, Muir JK, Fulp CT, Pittman RN, McIntosh TL (2003) Acute activation of mitogen-activated protein kinases following traumatic brain injury in the rat: implications for posttraumatic cell death. Exp Neurol 183(2): 438-448.
- Casha S, Yu WR, Fehlings MG (2001) Oligodendroglial apoptosis occurs along degenerating axons and is associated with FAS and p75 expression following spinal cord injury in the rat. Neuroscience 103(1): 203-218.
- Castejón OJ, Arismendi GJ (2006) Nerve cell death types in the edematous human cerebral cortex. J Submicrosc Cytol Pathol 38(1): 21-36.
- Bezvenyuk Z, Miettinen R, Solovyan V (2003) Chromatin condensation during glutamate-induced excitotoxicity of cerebellar granule neurons precedes disintegration of nuclear DNA into high molecular weight DNA fragments. Brain Res Mol Brain Res 110(3): 140-146.
- Kastner P, Mosgoeller W, Fang Kircher S, Kitzmueller E, Kirchner L, et al. (2003) Deficient brain RNA polymerase and altered nucleolar structure persists until day 8 after perinatal asphyxia of the rat. Pediatr Res 53(1): 62-71.
- Yamashima T, Tonchev AB, Tsukada T, Saido TC, Imajoh Ohmi S, et al. (2003) Sustained calpain activation associated with lysosomal rupture executes necrosis of the postischemic CA1 neurons in primates. Hippocampus 13(7): 791-800.
- Kinck G, Hoff man KL, Rodriguez EM, Zee MC, Weeks JC (2003) Steroidtriggered programmed cell death of a motoneurons is autophagic and involves structural changes in mitochondria. J Comp Neurol 457(4): 384-403.
- Annunziato L, Amoroso S, Pannacione A, Cataldi M, Pignataro G, et al. (2003) Apoptosis induced in neuronal cells by oxidative stress: role played by caspases and intracellular calcium ions. Toxicol Lett 139(2-3): 125-133.
- Unal Cevik I, Kilinç M, Can A, Gürsoy Ozdemir Y, Dalkara T (2004) Apoptotic and necrotic death mechanisms are concomitantly activated in the same cell after cerebral ischemia. Stroke 35(9): 2189-2194.
- Wei L, Ying DJ, Cui L, Langsford J, Ping Yu S (2004) Necrosis, apoptosis and hybrid death in the cortex and thalamus after barrel cortex ischemia in rats. Brain Res 1022(1-2): 54-61.
- Muller GJ, Stadelmann C, Bastholm L, Elling F, Lassmann H, et al. (2004) Ischemia leads to apoptosis-and necrosis-like neuron death in the ischemic rat hippocampus. Brain Pathol 14(4): 415-424.
- Raghupathi R (2004) Cell death mechanisms following traumatic brain injury. Brain Pathol 14(2): 215-222.
- Hilario E, Rey Santano MC, Goni De Cerio F, Alvarez FJ, Gastiosoro E, et al. (2005) Cerebral blood flow and morphological changes alter hypoxicischaemic injury in preterm lambs. Acta Paediatr 94(7): 903-911.
- Yang XF, Liu WG, Shen H, Gong JB, Yu J, et al. (2005) Correlation of cell apoptosis with brain edema and elevated intracranial pressure in traumatic brain injury. Chin J Traumatol 8(2): 96-100.
- Harukuni I, Bhardwaj C (2006) Mechanisms of brain injury after global cerebral ischemia. Neurol Clin 24(1): 1-21.
- Haku T, Miyasaka N, Kuroiwa T, Kubota T, Aso T (2006) Transient ADC change precedes persistent neuronal death in hypoxic-ischemic model in immature rats. Brain Res 1100(1): 136-141.
- Shioda N, Moriguchi S, Shirasaki Y, Fukunaga K (2006) Generation of constitutively active calcineurin by calpain contributes to delayed neuronal death following mouse brain ischemia. J Neurochem 98(1): 310-320.
- Meller R, Clayton C, Torrey DJ, Schindler CK, Lan JQ, et al. (2006) Activation of the caspase 8 pathway mediates seizure-induced cell death in cultured hippocampal neurons. Epilepsy Res 70(1): 3-14.
- Francis P (2006) Targeting cell death in dementia. Alzheimer Dis Assoc Disord 20(2 Suppl 1): S3-7.
- Raynaud F, Marcilhac A (2006) Implicaton of calpain in neuronal apoptosis. A possible regulation of Alzheimer’s disease. Febs J 273(15): 3437-3443.
- Andersson B, Bjelke B, Sykova E (2005) Temporal profile of ultrastructural changes in cortical neurons after a compression lesion. Physiol Res 55(3): 339-348.
- Arsava EM, Gurer G, Gursoy-Ozdemir Y, Karatas H, Dalkara T (2009) A new model of transient focal cerebral ischemia for inducing selective neuronal necrosis. Brain Res Bull 78(4-5): 226-231.
- Kaindl AM, Degos V, Peineau S, Gouadon E, Chhor V, et al. (2012) Activation of microglial N-methyl-D-aspartate receptors triggers inflammation and neuronal cell death in the developing and mature brain. Ann Neurol 72(4): 536-549.
- Cho YE, Lee MH, Song BJ (2017) Neuronal cell death and degeneration through increased nitroxidative stress and tau phosphorylation in hiv-1 transgenic rats. PLoSOne 12(1): e0169945.
- Li Q, Weiland A, Chen X, Lan X, Han X, et al. (2018) Ultrastructural characteristics of neuronal death and white matter injury in mouse brain tissues after intracerebral hemorrhage: coexistence of ferroptosis, autophagy, and necrosis. Front Neurol 9: 581.
- Formella I, Svahn AJ, Radford RAW, Don EK, Cole NJ, et al. (2018) Realtime visualization of oxidative stress-mediated neurodegeneration of individual spinal motor neurons in vivo. Redox Biol 19: 226-234.
- Sebastián Serrano Á, de Diego García L, Díaz Hernández M (2018) The neurotoxic role of extracellular tau protein. Int J Mol Sci 19(4): pii E998.
- Castejón OJ (1994) Transmission electron microscope study of human hydrocephalic cerebral cortex. J Submicrosc Cytol Pathol 26(1): 29-39.
- Castejón OJ, Castejón HV, Castellano A (2001) Oligodendroglial cell damage and demyelination in infant hydrocephalus. An electron microscopy study. J Submicrosc Cytol Pathol 33(1-2): 33-40.
- Portera Cailiau C, Price DL, Martin LJ (1997) Excitotoxic neuronal death in the immature brain is an apoptosis-necrosis morphological continuum. J Comp Neurol 378(1): 70-87.
- Martin LJ, Al Abdulla NA, Brambrink AM, Kirsch JR, Sieber FE, et al. (1998) Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: A perspective on the contributions of apoptosis and necrosis. Brain Res Bull 46(4): 281-309.
- Martin LJ (2001) Neuronal cell death in nervous system development, disease, and injury (Review). Int J Mol Med 7(5): 455-478.
- Young C, Tenkova T, Dikranian K, Olney JW (2004) Excitotoxic versus apoptotic mechanisms of neuronal cell death in perinatal hypoxia/ ischemia. Curr Mol Med 4(2): 77-85.
- Castejón OJ (2008) The thesis of mitochondria as marker of lethal injury in the traumatic human edematous cerebral cortex. Acta Microscópica 17: 16-26.
- Bursch W (2001) The autophagosomal-lysosomal compartment in programmed cell death. Cell Death Differ 8(6): 569-581.
- Bursch W, Ellinger A (2005) Autophagy-a basic mechanism and a potential role for neurodegeneration. Folia Neuropathol 43(4): 297-310.
- Adhami F, Liao G, Morozov YM, Schloemer A, Schmithorst VJ, et al. (2006) Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy. Am J Pathol 169(2): 566-583.
- Marino G, López Otin C (2004) Autophagy: Molecular mechanisms, physiological functions and relevance in human pathology. Cell Mol Lif Sci 61(12): 1439-1454.
- Kaya SS, Mahmood A, Li Y, Yavuz E, Goksel, M, et al. (1999) Apoptosis and expression of p53 response proteins and cyclin D1 after cortical impact in rat brain. Brain Res 818(1): 23-33.
- Barth M, Schilling L, Schmiedek P (2000) Time course of apoptotic cell death after experimental neurotrauma. Acta Neurochir (Suppl.) 76: 121- 124.
- Yakovlev AG, Di X, Movsesyan V, Mullins PG, Wang G, et al. (2001) Presence of DNA fragmentation and lack of neuroprotective effect in DFF45 knockout mice subjected to traumatic brain injury. Mol Med 7(3): 205-216.
- Bittigau P, Sifringer M, Felderhoff Mueser U, Ikonomidou C (2004). Apoptotic neurodegeneration in the context of traumatic injury to the developing brain. Exp Toxicol Pathol 56(1-2): 83-89.
- Brunelle JK, Chandel NS (2002) Oxygen deprivation induced cell death: an update. Apoptosis 7(6): 475-482.
- Valencia A, Moran J (2001) Role of oxidative stress in the apoptotic cell death of cultured cerebellar granule neurons. J Neurosci Res 64(3): 284- 297.
- Chen GG, Chak EC, Chun YS, Lam IK, Sin FL, et al. (2003) Glioma apoptosis induced by macrophages involves both death receptor-dependent and independent pathways. J Lab Clin Med 141(3): 190-199.
- Mora R, Abschuetz A, Kees T, Dokic I, Joschko N, et al. (2009) TNF-alphaand TRAIL-resistant glioma cells undergo autophagy-dependent cell death induced by activated microglia. Glia 57(5): 561-581.
- González Polo RA, Niso Santano M, Ortíz Ortíz MA, Gómez Martín A, Morán JM, et al. (2007) Inhibition of paraquat-induced autophagy accelerates the apoptotic cell death in neuroblastoma SH-SY5Y cells. Toxicol Sci 97(2): 448-458.
- Jung EJ, Chung KH, Bae DW, Kim CW (2016) Proteomic analysis of novel targets associated with the enhancement of TrkA-induced SK-N-MC cancer cell death caused by NGF. Exp Mol Med 48: e235.
- Puig B, Brenna S, Magnus T (2018) Molecular communication of a dying neuron in stroke. Int J Mol Sci 19(9): pii: E2834.
- Radak D, Katsiki N, Resanovic I, Jovanovic A, Sudar-Milovanovic E, et al. (2017) Apoptosis and acute brain ischemia in ischemic stroke. Curr Vasc Pharmacol 15(2): 115-122.
- Sekerdag E, Solaroglu I, Gursoy Ozdemir Y (2018) Cell Death Mechanisms in Stroke and Novel Molecular and Cellular Treatment Options.Curr Neuropharmacol 16(9): 1396-1415.
- Van Cruchten S.,Van Den Broeck, W., (2002). Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat Histol Embryol 31(4): 214-223.
- Yamamoto K (1999) A predominant apoptotic death pathway of neuronal PC12 cells induced by activated microglia is displaced by a non-apoptotic death pathway following blockage of caspase-3- dependent cascade. J Biol Chem 274(22): 15725-15731.
- Zhang Y, Goodyer C, LeBlanc A (2000) Selective and protracted apoptosis in human primary neurons microinjected with active caspase 3, -6, -7, and -8. J Neurosci 20(2): 8384-8389.
- Morley P, Tauskela JS, Hakim AM (1999) Calcium Overload. In Cerebral Ischemia. (Ed) W Walz New Jersey: Humana Press, pp 69-104.
- Castejón OJ, Valero C, Díaz M (1995) Electron microscopy of cerebral cortex in Arnold-Chiari Type II Malformation. Report of two Cases. J Submicrosc Cytol Pathol 27(3): 401-405
- He Z, Meschia JF, Brott TG, Dickson DW, McKinney M (2006) Aging is neuroprotective during global ischemia but leads to increased caspase-3 and apoptotic activity in hippocampal neurons. Curr Neurovasc Res 3(3): 181-186.
- Andersen L (1990) Electron microscopy and morphometry of nucleoli in rat neurosecretory cell with stimulated and suppressed secretion. Acta Anat 138(3): 220-223.
- Barh BA, Bendiske J (2002) The neuropathogenic contribution of lysosomal dysfunction. J Neurochem 83(3): 481-489.
- Benchoua A, Trioulier Y, Zala D, Gaillard MC, Lefort N, et al. (2006) Involvement of mitochondrial complex II defects in neuronal death produced by N-terminus fragment of mutated huntingtin. Mol Biol Cell 17(4): 1652-1663.
- Boldyrev A, Song R, Dyatlov VA, Lawrence DA, Carpenter DO (2000) Neuronal cell death and reactive oxygen species. Cell Mol Neurobiol 20(4): 433-450.
- Castejón OJ, Valero C, Diaz M (1997) Light and electron microscope study of nerve cells in traumatic oedematous human cerebral cortex. Brain Injury 11(5): 363-388.
- Castejón OJ, Arismendi GJ (2004) Nerve cell nuclear and nucleolar abnormalities in the human oedematous cerebral cortex.An electron microscopic study using cortical biopsies. J Submicrosc Cytol Pathol 36(3-4): 273-283.
- Castejón OJ (2004) Structural patterns of injured mitochondria in human oedematous cerebral cortex. Brain Injury 18(11): 1107-1126.
- Chen Q, Chai YC, Mazumder S, Jiang C, Macklis RM, et al. (2003) The late increase in intracellular free radical oxygen species during apoptosis is associated with cytochrome c release, caspase activation, and mitochondrial dysfunction. Cell Death Differ 10(3): 323-334.
- Daxnerova Z, Marsala M, Marsala J (1995) Graded postishemic reoxygenation attenuates ischemia-reperfusion-induced nuclear and nucleolar damage in lumbosacral dorsal root ganglia neurons. A light and electron microscopic study in rabbit. J Hirnforsch 36(3): 379-391.
- Evans PH (1993) Free radicals in brain metabolism and pathology. Brit Med Bull 49(3): 577-587.
- Garcia Segura LM, Berciano MT, Lafarga M (1993) Nuclear compartmentalization in transcriptionally activated hypothalamic neurons. Biol Cell 77(2): 143-154.
- Ginsberg MD, Watson BD, Busto R (1988) Peroxidative damage to cell membranes following cerebral ischemia. A cause of ischemia brain injury. Neurochem Pathol 9: 171-173.
- Lodish HF, Kong N (1990) Perturbation of cellular calcium blocks secretory proteins from rough endoplasmic reticulum. J Biol Chem 265(19): 10893-10899.
- Malatesta M, Bertoni Freddari C, Fattoretti P, Caporaloni C, Fakan S, et al. (2006) Activation of the caspase 8 pathway mediates seizureinduced cell death in cultured hippocampal neurons. Epilepsy Res 70(1): 3-14.
- Mennel HD, Muller I (1994) Morphometric investigation on nuclear and nucleoli arrangement and AgNOR content in the rat hippocampus under normal and ischemic conditions. Exp. Toxicol Pathol 46(6): 491- 501.
- Nicholls DG, Budd SL, Castillo RF, Ward MW (1999) Glutamate excitotoxicity andneuronal energy metabolism. Ann NY Acad Sci 893: 1-12.
- Paschen W (1996) Glutamate excitotoxicity in transient global cerebral ischemia. Acta Neurobiol Exp (Wars) 56(1): 313-322.
- Paschen W, Doutheil J (1999) Disturbance of endoplasmic reticulum functions: A key mechanism underlying cell damage? In: Current Progress in the Understanding of Secondary Brain Damage from Trauma and Ischemia. Baethmann A, et al. (Eds.) Acta Neurochirug (Suppl) p.1-5.
- Paschen W (2000) Role of calcium in neuronal cell injury: which subcellular compartment is involved? Brain Res Bull 53(4): 409-413.
- Paschen W, Mengesdorf T, Althausen S, Hotop S (2001) Peroxidative stress selectively down-regulates the neuronal stress response activated under conditions of endoplasmic reticulum dysfunction. J Neurochem 76(6): 1916-1924.
- Paschen W (2003) Endoplasmic reticulum: a primary target in various acute disorders and degenerative diseases of the brain. Cell Calcium 34(4-5): 365-383.
- Raghupathi R, McIntosh TK, Smith DH (1995) Cellular responses to experimental brain injury. Brain Pathol 5(4): 437-442.
- Traystman RJ, Kirsch JR, Koehler RC (1991) Oxygen radical mechanisms of brain injury following ischemia and reperfusion. J Appl Physiol 71(4): 1185-1195.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.