Editorial 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Is Cannabidiol the Cure-all Remedy we Have Been Looking for?
*Corresponding author: Olga Ostrovsky, Surgical Research, Department of Surgery, Cooper Research Institute, 401 Haddon Ave, Camden, NJ 08103, USA.
Received: May 22, 2019; Published: May 28, 2019
DOI: 10.34297/AJBSR.2019.03.000645
Abstract
Cannabidiol (CBD), the second major component of the plant Cannabis sativa, has become increasingly popular for its potential anxiolytic, antiinflammatory, antiemetic, and antipsychotic effects. It is non-psychotropic and has no abusive potential thus far, however scientific evidence on the pharmacological effects of CBD are limited to preclinical studies. More large and well-controlled clinical trials are needed to establish the clinical applications of CBD.
Cannabis sativa is one of three primary species from the Cannabaceae family, known for its cannabinoid extract, delta-9-tetrahydrocannabinol (Δ9-THC,). Δ9-THC is the most abundant component of Cannabis sativa and is popular for its psychoactive or euphoric effects. On the contrary, the second major component of the plant Cannabis sativa-cannabidiol (CBD)-does not have psychoactive effects; it has received the latest media buzz and marketing claims as a miracle cure for anxiety, depression, pain, inflammation, insomnia, and even cancer, without the high.
CBD can be derived from varieties of Cannabis, commonly referred to as hemp or marijuana. Hemp and marijuana differ by the amount of Δ9-THC in the plant. The Agriculture Improvement Act of 2018 (or 2018 Farm Bill) defines “hemp” as any part or derivative of the plant Cannabis sativa, with a Δ9-THC concentration of no more than 0.3% by dry weight, in contrast to up to 30% of Δ9-THC which is in marijuana [1]. The 100-fold difference in the psychoactive component of Cannabis sativa is likely the growing public’s interest in the potential therapeutic effects of CBD.
Products advertised to contain CBD have been increasingly found in oils, creams, cosmetics, gummies, and even dog treats. The increasing availability of cannabis-derived products is due to the passage of the 2018 Farm Bill which legalized hemp cultivation and declassified it as a Schedule I controlled substance. Now hemp-derived CBD is widely available in almost all 50 states. With the overwhelming ease in purchasing CBD products, what is the scientific evidence on CBD as a cure all drug and is it safe to use? There are a variety of pharmacologic effects of CBD reported in preclinical studies such as anxiolytic, anti-inflammatory, antiemetic, and antipsychotic properties (Figure 1).

Figure 1: Pharmacologic effects of CBD [2].
In vitro and In vivo research efforts have been growing to explore its effects in a wide range of diseases: Alzheimer’s, Parkinson’s, multiple sclerosis, epilepsy, Huntington’s, hypoxic-ischemic injury, pain, anxiety, depression, cancer, nausea, inflammatory, auto-immune, cardiovascular, and diabetes [2]. For example, in an in vitro study of Alzheimer’s disease with A-stimulated PC12 neuronal cells, CBD inhibited hyperphosphorylation of tau protein and the formation of neurofibrillary tangles through the Wnt/-catenin pathway [3]. In a study of 21 patients with Parkinson’s disease treated with either placebo, 75mg or 300mg/day CBD in a double-blind trial for 6 weeks, significant improvement of quality of life was seen with CBD 300mg/day compared to placebo [4]. CBD was also shown to inhibit breast cancer growth and metastasis through modulation of EGF/EGFR signaling pathway [5]. The only successful Phase III clinical trial thus far, has led to the U.S. Food and Drug Administration approval of Epidiolex (CBD oral solution) in 2018, for the treatment of two rare forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome. Epidiolex has made headway as the first FDA-approved drug containing a purified drug substance derived from Cannabis sativa.
In addition to attempts on identifying therapeutic effects of CBD, its biosafety profile has been explored as early as in the 1970s. The World Health Organization states that CBD “exhibits no effects indicative of any abuse or dependence potential…to date, there is no evidence of recreational use of CBD or any public health related problems associate with use of pure CBD” [6]. In vivo studies have shown CBD in doses ranging 3-30 mg/kg did not affect physiological parameters such as heart rate, blood pressure, body temperature; no effect was observed in motor function, gastrointestinal transit, or memory either [2,7]. Acute or chronic uses of CBD across different doses, whether oral (65-660 mg), inhalation (0.15 mg/ kg), or intravenous injections (5-30 mg), did not induce side effects in human studies [7]. However, there are still other potential side effects of CBD observed in preclinical studies, such as drug interactions, immune modifications, and decreased receptor activity [7].
CBD is metabolized in the liver by the CYP3A4 enzyme, and in mice studies, has been shown to inactivate cytochrome P450 isozymes in the short term, while inducing them after repeated administration [8]. Many drugs undergo this hepatic metabolism, and thus raises the question whether CBD inhibits the metabolism of other drugs affecting changes in drug and CBD concentrations. This is especially important as many of the diseases which CBD claims to have an affect are managed by multiple pharmacologic agents. Though there are increasing preclinical studies identifying CBD’s safety profile, many of the studies involve animals with different metabolic profiles between species, and the studies involve various doses of CBD across varying short-term periods. Due to the lack of concrete scientific evidence, the availability of CBD oil, granules, crystals, etc., is growing and becoming readily available on the market in doses ranging from 1 mg to 1500 mg. These CBD products often contain unknown purity, variable and much lower doses than used in clinical trials with very limited instructions on usage. For example, a 10 ml bottle of 3% CBD oil contains 1mg per drop (Table 1).
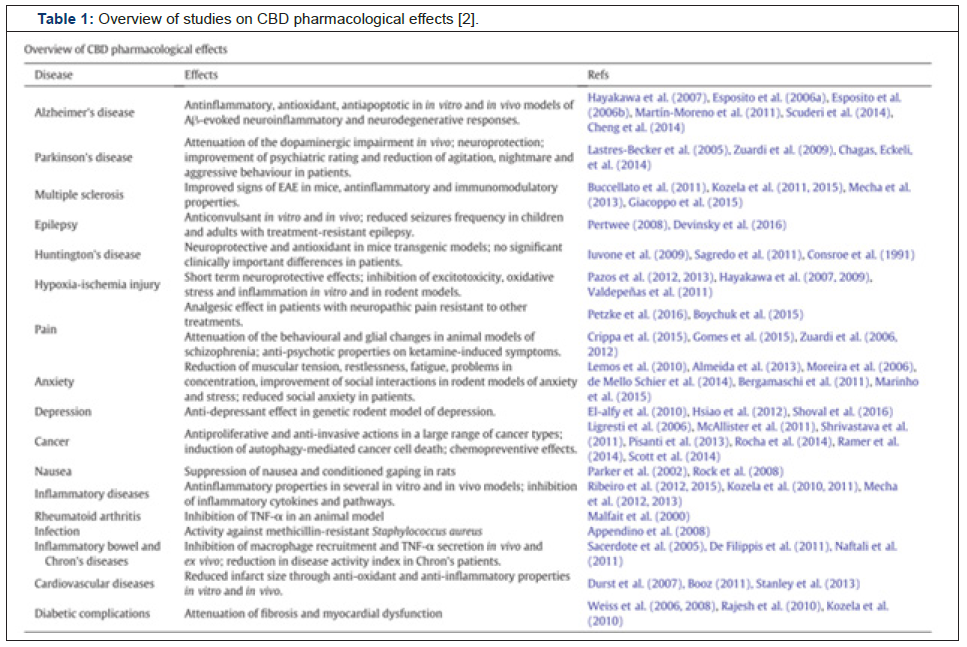
Table 1: Overview of studies on CBD pharmacological effects [2].
The previously mentioned Parkinson’s study found 300 mg/day to be an effective dose, which means a person would have to consume the entire bottle of CBD oil to achieve a therapeutic effect seen in the study. Since hemp-derived CBD is now legal but not well regulated, the development of CBD-based products for medical purposes has potential health dangers to users regarding the quality and purity of the product, effective doses, or route of administration of CBD for treatment of various diseases. Moreover, current commercially available CBD products are not regulated on its purity of CBD content. They may contain other cannabinoids (as there are hundreds of other chemical entities from the plant) that we do not yet know the full effects.
In order to maintain public safety within this booming industry, there is a strong need for adequate, long term, well-controlled clinical trials to better understand the biosafety profile and interactions of CBD. Evidence based therapeutic strategies could then be recommended for CBD dosage and administration for individual disease types. Preclinical studies have shown pharmacological potential and promise in CBD, but more large and robust clinical trials are needed to establish the clinical applications of CBD.
References
- Farm Bill (2018) Agriculture Improvement Act of 2018.
- Pisanti S, Malfitano AM, Ciaglia E, Lamberti A, Ranieri R, et al. (2017) Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol Ther 175: 133-150.
- Esposito G, De Filippis D, Carnuccio R, Izzo A, Iuvone T (2006) The marijuana component cannabidiol inhibits β-amyloid-induced tau protein hyperphosphorylation through Wnt/β-catenin pathway rescue in PC12 cells. J Mol Med 84(3): 253-258.
- Chagas MHN, Zuardi AQ, Tumas V, Pena-Pereira MA, Sobreira ET, et al. (2014) Effects of cannabidiol in the treatment of patients with Parkinson’s disease: an exploratory double-blind trial. J Psychopharmacol 28(11): 1088-1098.
- Elbaz M, Nasser MW, Ravi J, Wani NA, Ahirwar DK, et al. (2015) Modulation of the tumor microenvironment and inhibition of EGF/EGFR pathway: Novel anti-tumor mechanisms of Cannabidiol in breast cancer. Mol Oncol 9(4): 906-919.
- Cannabidiol Pre-Review Report (2017) Expert Committee on Drug Dependence 39th Meeting. Geneva, p. 6-10.
- Bergamaschi MM, Queiroz RH, Zuardi AW, Crippa JA (2011) Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Saf 6(4): 237-249.
- Iffland K, Grotenhermen F (2017) An Update on Safety and Side Effects of Cannabidiol: A review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res 2(1): 139-154.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.