Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Methods of Chitin Production a Short Review
*Corresponding author: Luciano Pighinelli, Research director of Biomatter Lab, Porto Alegre, Brazil.
Received: May 29, 2019; Published: June 14, 2019
DOI: 10.34297/AJBSR.2019.03.000682
Introduction
In addition to be the second most abundant natural polymer in nature, a renown has a great potential as a biomaterial in the area of biotechnology, because it is biocompatible, bio reactive and biodegradable [1,2]. Such characteristics, diverse applications in areas such as agriculture, food, environmental, and as two areas with greater focus: pharmaceutical and health [3,4]. Its structure consists of N-acetyl-d-glucosamine units with β- (1,4) bonds, having as main characteristic the insolubility in water and some organic acids [5]. Chitin belongs to the group of structural polysaccharides, together with cellulose, the second polymer being more abundant in the biosphere [6-9]. Due to its structural nature, a product release system was not found in any of the arthropod exoskeleton, in the structures of molluscs [10], in the cell wall of fungi [11,12], protozoa and bacteria, egg shells of nematodes [13,14], the shrimp fishery residue being the most widely used source [15].Throughout the decades of research and handling of this polymer, many methods of extraction have been developed, being the chemical method most found in the literature, being also used in the means of production of industrial chitin.
The USA, Japan, India, Canada, China, South Korea, Russia and Norway generally use the reject of crustacean fishing for production. The use of strong acids and bases in the chitin extraction process generates critical points to the process, such as: high cost of the materials involved, generation of chemical effluent and final product with low levels of purity [16,17]. Biological processes become more attractive because they have an affordable cost of production, do not generate high risk effluent (such as the chemical process) and high-quality final product [18,19]. All the processes found in the literature are an objective: to obtain chitin by separating the proteins and minerals from the raw material used [20]. Chitin, besides having great biotechnological value, generates by-products (such as chitosan) that also have added value and even more relevant properties. In this paper we discuss the already known processes of obtaining chitin known and registered in the literature of 2010 up to the present moment: Chemical, enzymatic and biological processes relating the different methods of obtaining and with the objective to identify the particularities of each process regarding the industrial viability and economically balancing them so that the reader concludes the best process for their research, also the possibility of executing quality improvements in these processes.
We will also discuss the polymorphic structures of α- and β-chitin and the different methods of obtaining each, since different processes are required in each of them due to their structures, properties and reactivity. The main objective of this review is to be able to relate the different processes of obtaining chitin with the most suitable applications for the method, based on such relation in aspects such as degree of purity and economic applicability.
Chitin
Economic Aspect
The main industrial source used for the extraction of the biopolymer is the reject of the fishing industry, reusing mainly the shell of prawns, crabs and lobsters [21,22]. According to Ioelovich, the production of chitin and its derivatives is estimated at 100 billion tons per year [23]. Gortari & Hours states that the bark discarded by the fishing trade can reach 70% of the total weight of the material [24]. According to Ameh, these carapaces have about 20-30% chitin, varying according to species and season, since chitin is naturally produced on a scale of 1010 to 1011 tons each year in a study conducted by Ifuku et al. [25,26]. Jaganathan estimates that chitin costs $ 220 per kilogram on the world market [27].
Chemical Aspect
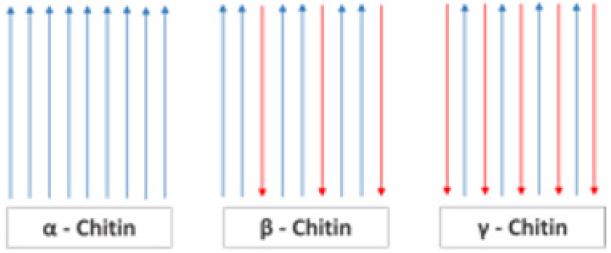
Chitin is a natural polymer composed of units of β-Dglucosamine molecules and N-acetyl groups, forming monomers that will be bound by beta (1→ 4) bonds [4]. Its degree of acetylation (DA) must be above 50% to be characterized as chitin [1]. Thus, it is called β (1→4) -N-acetyl-D-glucosamine (Figure 1) [28,29]. The polymer should have acetylation degree greater than 50% to be called chitin Due to the beta bonds between carbons 1 and 4, chitin becomes an extremely stable polymer [30]. Its polymeric extension renders it insoluble in water and practically all organic acids due to the intense hydrogen bonds [31-35]. Chitin contains, on average, 6.5% of nitrogen and its main derivative, chitosan, can reach up to 9.5% of hydrogen content. In obtaining the chitosan, the polymer undergoes a deacetylation process, eliminating about 80% of the acetyl groups to obtain the amino group [36]. Chitin has statistically ordered allomorphic configurations. These arrangements, or microfibers, can be characterized in: α-chitin, β-chitin and γ-chitin (Figure 2) [37].
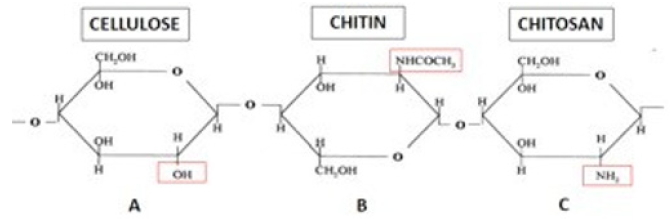
Note: Molecular representation of (a) cellulose, (b) chitin and (c) chitosan. Figure 1: Difference between cellulose, chitin and chitosan.
Α-Chitin has an antiparallel arrangement of its microfibers that favor hydrogen bonds and give rise to a highly compact structure, resulting in high crystallinity and hardness. It is also known as the most abundant form in nature [38]. Β-chitin has parallel chains, being less crystalline and with less packaging, being more flexible and more reactive. Γ-chitin is a mixed composition of forms α and β [7,9]. Α-chitin can be transformed into β-chitin, but not the reverse [39]. The three allomorphs can be observed by X-ray diffraction and Nuclear Magnetic Resonance (NMR) [40]. The degradability of the polymer occurs through the action of the enzyme chitinase, present in nature [41]. Of course, chitin is found in the microfibrillar form, allowing the production of nanofibers by mechanical or chemical processes, according to [42].
Because it is a natural polymer, chitin has characteristics characteristic of polysaccharides, such as: biocompatibility, biodegradability and non-toxicity. It still has bioactivity, antibacterial activities [42] and antimicrobial [43], anticancer activity, anticoagulant [44] and molecular adsorption property [45,46]. Elieh Ali Komi, Sharma and Dela Cruz highlights the immunogenic property of chitin and chitinase against pathogens in both insects and mammals as well as plants [47,48]. It is physically versatile, can be processed in the form of powder, fibers, membranes, sponges, hydrogels [49], scaffolds [50]. According to Anitha, the structure of sponges is very favorable for health, with emphasis on tissue engineering, as it has a hygroscopic potential and complexing capacity of other substances to the structure [50,51].
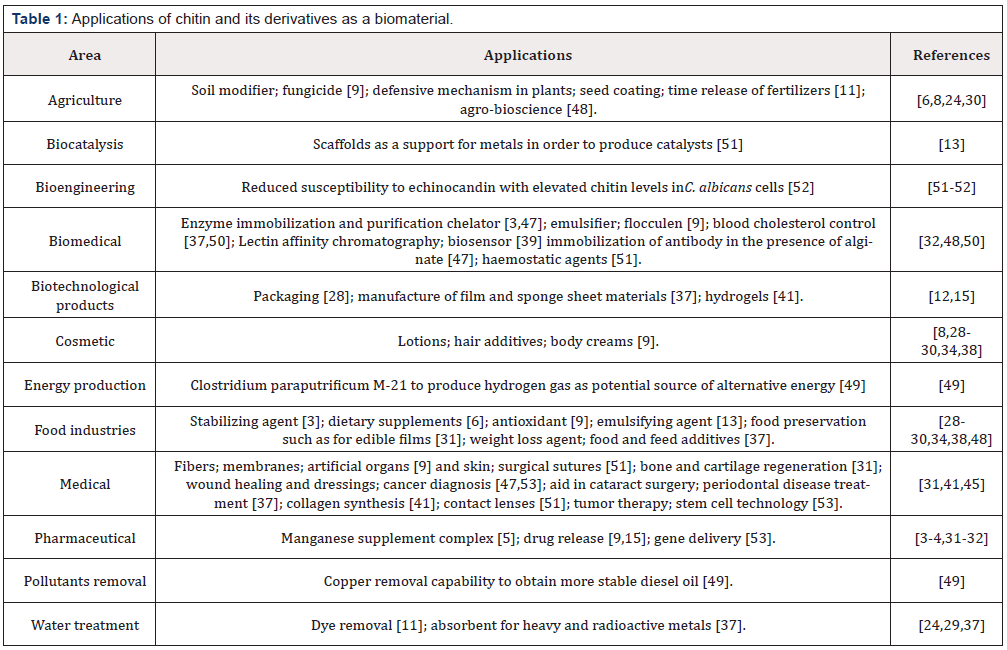
Applications
Amidst all its unique properties, chitin and its derivatives are an excellent biomaterial with unlimited application possibilities. From the chemical industry and agrochemicals to the textile industry and paper industry chitin and derivatives are used as biomaterials potential. Most of the studies found in the literature are directed to the health area, as noted below in Table 1. Kandra, Challa and Kalangi Padma Jyothi presents a vast study on the performance of chitin and chitinase in immunology [49]. Biocomposites of chitin and mainly chitosan is widely studied and developed. Chitosan, because of its greater bioactivity and adhesiveness, becomes more interesting as scaffolds for areas of medicine and pharmacy and for tissue engineering [50].
Extraction and Characterization of Chitin
For decades, chitin has been studied in order to optimize its biomaterial production methods and to better understand its properties and biomaterial potential. Currently, there are several methods of obtaining them in the literature, being the biological processes, with the use of microorganisms and enzymes, chemical and even a combination of both to achieve an even more process efficient. Regardless of the process adopted, the goal is the same: eliminate proteins, minerals, lipids and pigments until only the material of interest is obtained. Today, the most common method in industrial production is the chemical method, due to its productivity and practicality, however, all forms of obtaining chitin are relatively accessible and quite practical [50].
Chemical Process of Obtaining
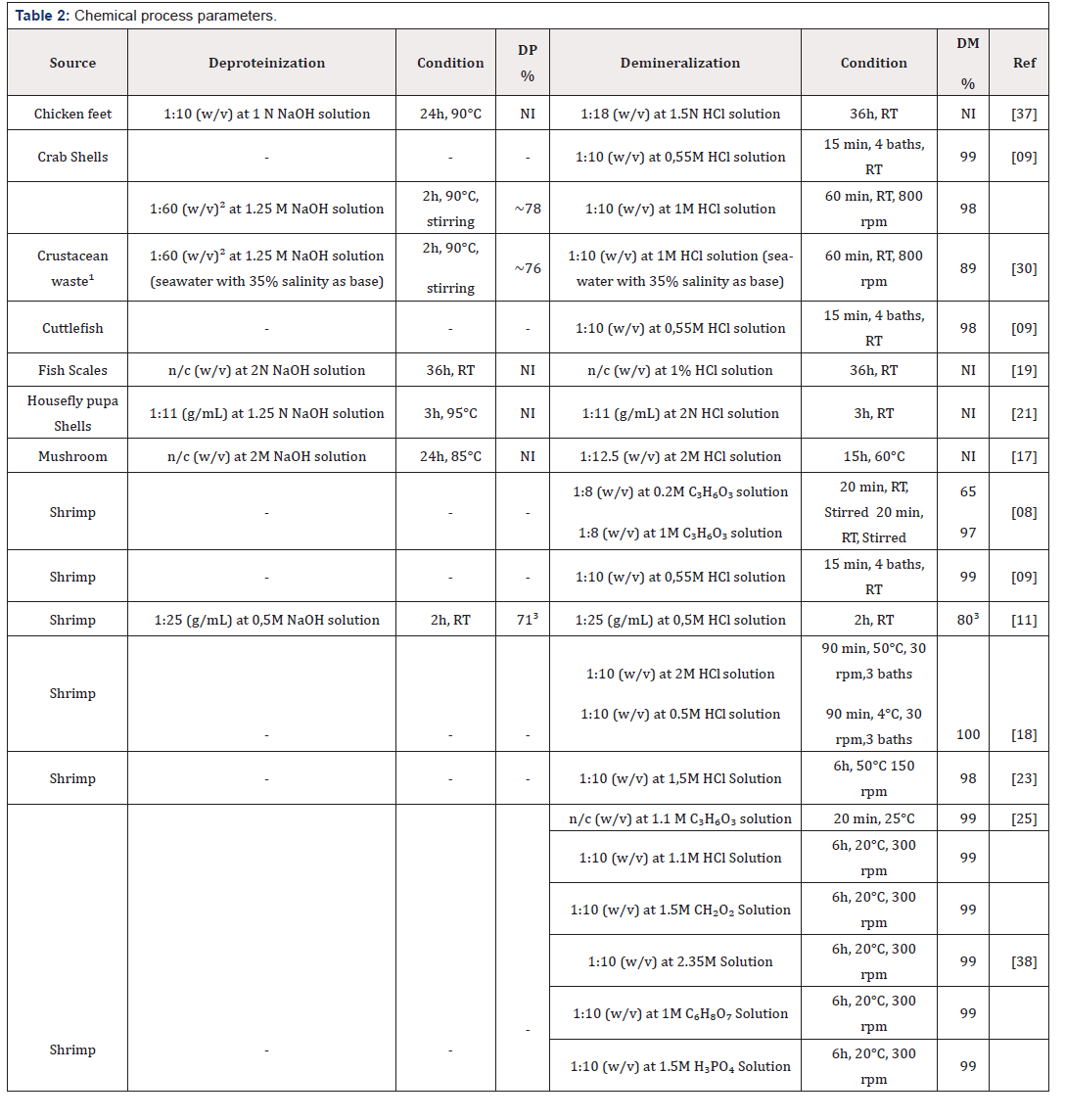
This is currently the most widely used method in both industrial and laboratory production and is also the most frequently cited in the literature. As mentioned earlier, the purpose of any extraction process is to eliminate all organic and mineral content of the raw material. For this, there are two primordial steps to obtain chitin: deproteinization and demineralization. The order of these steps in a process can be changed according to the purpose and chemicals used. In addition to these two main steps, the production can receive a step of depigmentation and deodorization, if necessary. Firstly, the raw material receives a preliminary treatment to remove impurities and coarse organic waste. At this stage, baths are carried out with deionized water and sodium hypochlorite is sometimes applied. The use of temperature is eventually employed to speed up the cleaning process. The material is still dry or not to follow the milling step, where it will provide a better reaction with subsequent steps. All types of pretreatment are adopted according to the need and condition of the waste used, as well as the species of selected raw material.
Deproteinization is the elimination of the protein content present in the raw material. For this, alkaline solutions are used, for example NaOH and KOH. The most used and adopted for industrial production is NaOH solution. A large variation in production aspects is found in the literature. Gortari and Hours, 2013 shows that temperatures in the average of 95ºC are used in the deproteinization for commercial chitin, but also indicates that in this range can cause the depolymerization of the material and change some characteristics such as viscosity. Demineralization involves the removal of the inorganic filler (calcium carbonate and calcium phosphate) from the raw material. For this, inorganic acids, such as HCl, HNO₃ and H₂SO₄, and strong organic acids are used, for example: HCOOH and CH₃COOH. The most commonly used acid in the production of commercial chitin is HCl, due to its high efficiency in the removal of the minerals present. The staining is a strong indicator of the presence of impurities in the material. For this, the depigmentation step is carried out using acetone, sodium hypochlorite, hydrogen peroxide or potassium permanganate. Table 2 shows a comparison between some chemical processes found in the literature.
Note: NI: Not informed(¹): Crustacean waste was composed of a variety of crustacean’s shells.
RT: Room temperature(²): Weight of demineralized shells
n/c: No concentration informed(³): Chemical treatment realized after mild DP and DM by fermentation
(-): Processes in which the chemical deproteinization step was not carried out
Biotechnological Process of Obtaining
The biotechnological process is the combination of the chemical process with the use of biological methods, with application of microorganisms to the system. This technique shows to be favorable in comparison with the traditional chemical process, where large amounts of highly reactive chemical inputs are used, which can affect the final quality of the material, besides the serious effluent generated in the process. Methods involving biological pathways have also been shown to be more assertive when achieving higher purity states of chitin, with considerably lower molecular weight loss than the conventional chemical process. Chemical processes still prove to be more efficient industrially, however the use of biotechnological methods presents a new sustainable vision and new parameter of quality, offering a more suitable biomaterial for health areas. Demineralization can be performed using microorganisms. The material is deposited along with a microbial culture and a sugar source, which will provide the necessary nutrients [51].
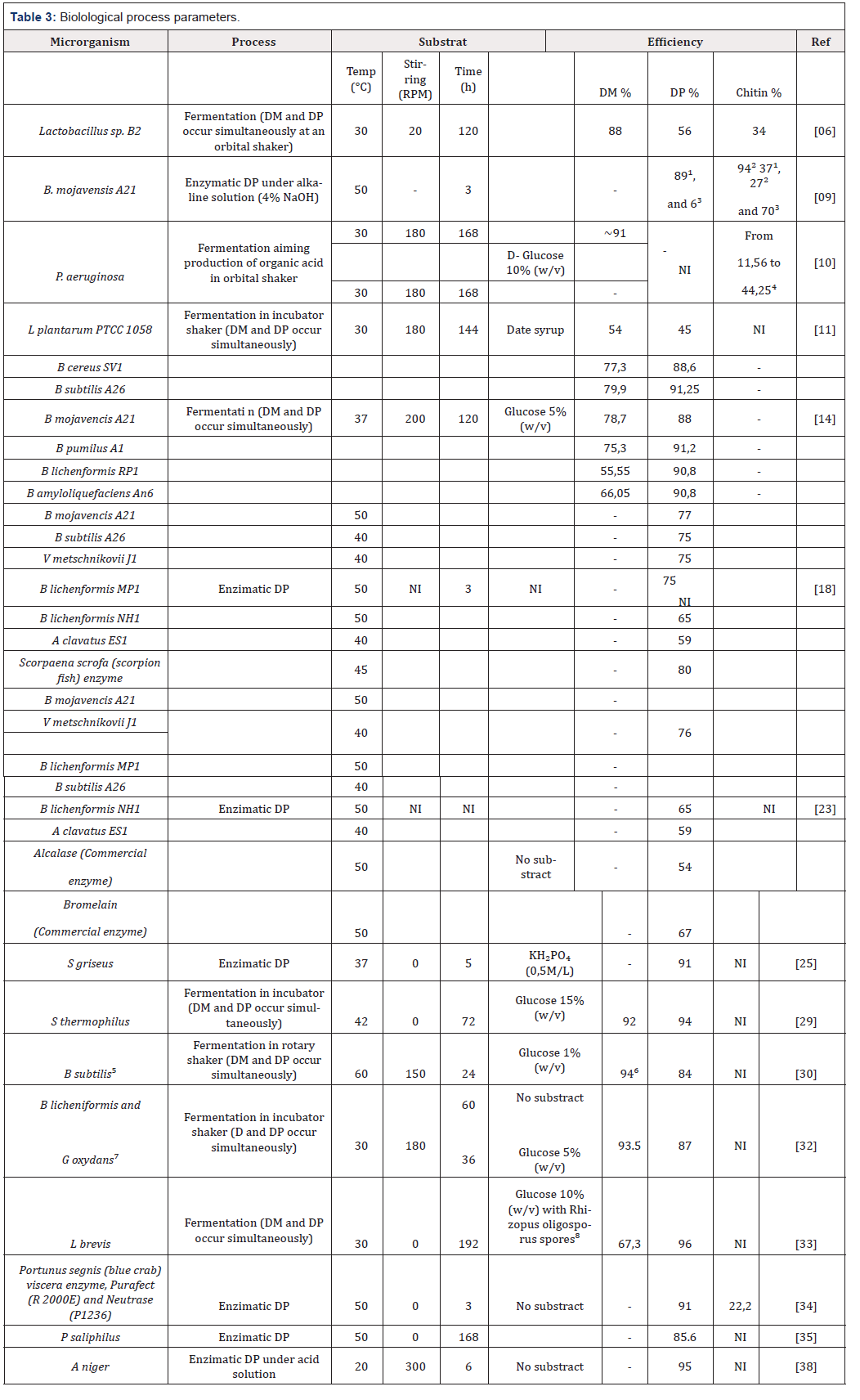
As a result of this fermentation process, there are the production of organic acids that react with the minerals and turn them into salts and precipitate. At the end of the process, the above can be removed with a simple washing process. According to Aranday-García, lactic acid from the activity of lactobacteria, provides better results and greater efficiency in demineralization. The inoculation takes place according to each treated species in a culture medium with the necessary nutrients. This medium is basically composed of sugars and fats; amino acids; sources of calcium, iron and magnesium; among other specific compounds. Agar, commercial culture media, are the most used for presenting balanced composition, such as MRS (Man Rogosa Sharpe). For the elimination of proteins, fermentative and enzymatic processes can be applied. During fermentation, digestive and microbial enzymes are produced and consume organic material. Hydrolytic enzymes (proteases) are very efficient in deproteinization and may result in the production of hydrolyzed proteins as a by-product of high added value [52].
Note: NI: Not informed ¹: Yield from shrimp shells, ²: Yield from crab shells, ³: Yield from cuttlefish bones, ⁴: Yield varies accourding to, ⁵: Medium using seawater, ⁶: DP attained after further chemical treatment, ⁷: B licheniformis followed by Goxydans.
Proteases from lacto bacteria are the most commonly used. Carotenoids are pigments found in lipids present in crustaceans and can be isolated from fermentation and enzymatic activity. Astaxanthin belongs to these carotenoids present and has great commercial value in the food area. The main disadvantage of processes involving microorganisms are the time released for fermentation and high cost of some enzymes. Often the biological process is insufficient, requiring the application of acids and alkaline solutions to confirm the reaction, but presenting a better deproteinization yield, as described in Table 3, consequently obtaining a material of significantly higher quality due to low molecular weight loss [53].
Final Considerations
The mastery of the techniques and study of the kinetics of each process is fundamental for the optimization of the same. The analysis of the different chitin obtaining studies by chemical means found in the literature suggests a higher efficiency in the use of consecutive and short baths instead of a long one for deproteinization as well as for the demineralization, thus dispensing with the use of solutions with large concentrations of reagents, which in turn avoids the loss of the quality of the chitin obtained in the process. Obtaining by biotechnological methods presents better results in most literary studies, however, it lacks further development, since the average time in the process is often greater than by the chemical method, leaving the employment of bacteria and enzymes less attractive for the chitin production at industrial levels. Of these bacteria, a family that proves very promising is Lactobacillus, because in the process this produces lactic acid, simultaneously promoting the demineralization of the material.
Chitin and its derivatives, as previously mentioned, have several applications in the most varied areas, but the current trend of research seems to be more focused on the biomedical area, with the development of treatments and methods that promote the regeneration of wounds and / or nervous tissues. For the application of chitin in these areas it becomes more necessary to use a material with a higher degree of purity, so it is advisable to use biotechnological methods in these cases. The fact that the degree of purity is lower in chemical processes does not deprive the application of the chitin obtained by them in other areas that do not demand such purity, these areas may be more attractive for investments, taking advantage of the faster production and greater amount provided by such methods.
References
- Anitha A (2014) Chitin and Chitosan in Selected Biomedical Applications. Progress in Polymer Science. Elsevier Ltd 39(9): 1644-1667.
- Ameh, AO, Isa MT, Adeleye TJ, Adama K (2013) Kinetics of Demineralization of Shrimp Exoskeleton in Chitin and Chitosan Synthesis. Journal of Chemical Engineering and Materials Science 4(3): 32-37.
- Arbia W (2013) Chitin Extraction from Crustacean Shells Using Biological Methods A Review. Food Technology and Biotechnolog 51(1): 12-25.
- Ameh AO (2014) Kinetics of Demineralization of Shrimp Shell Using Lactic Acid. Leonardo Electronic Journal of Practices and Technologies 13(24): 13-22.
- Azuma K (2015) Functional Biomaterials Chitin, Chitosan , and Its Derivatives for Wound Healing : Old and New Materials.
- Azuma K (2014) ‘Preparation and Biomedical Applications of Chitin and Chitosan Nanofibers’. Journal of Biomedical Nanotechnology 10(10): 2891-2920.
- Baron R (2015) ‘Kinetic Study of Solid Phase Demineralization by Weak Acids in One-step Enzymatic Bio-refinery of Shrimp Cuticles’, Process Biochemistry. Elsevier Ltd, 50(12): 2215-2223.
- Carvalho LB, Nader HB, Bezerra RS (2012) ‘Recovery of Protein, Chitin, Carotenoids and Glycosaminoglycans from Pacific White Shrimp (Litopenaeus vannamei) processing waste’, Process Biochemistry. Elsevier Ltd pp. 6-13.
- Cao W (2014) ‘Ultraviolet Irradiation and Gradient Temperature Assisted Autolysis for Protein Recovery from Shrimp Head Waste’, Food Chem. Elsevier Ltd 164: 136-141.
- Chen X (2015) ‘Effect of Treatment Methods on Chitin Structure and Its Transformation into Nitrogen-Containing Chemicals’. Chem Plus Chem 80(10): 1565-1572.
- Khor E, Lim LY (2003) Implantable applications of chitin and chitosan. Biomaterials 24(13): 2339-2349.
- Khorrami M (2012) Production of Chitin and Chitosan from Shrimp Shell in Batch Culture of Lactobacillus plantarum, Chemical & Biochemical Engineering Quarterly 26(3): 217-223.
- Puvvada, YS, Vankayalapati S, Sukhavasi S (2012) Extraction of Chitin from Chitosan from Exoskeleton of Shrimp for Application in the Pharmaceutical Industry. International Current Pharmaceutical Journal 1(9): 258-263.
- Nessa F, Masum S, Asaduzzaman M, Roy S, Hossain, et al. (2011) A Process for the Preparation of Chitin and Chitosan from Prawn Shell Waste. Bangladesh Journal of Scientific and Industrial Research 45(4): 323-330.
- Quimque MTJ, Ina Acas (2015) Structural and Morphological Analyses of Chitin and its Complex upon Manganese Ion Adsorption. Procedia Chemistry 16: 578-585.
- Flores-Albino B, Arias L, Gomez J, Castillo A, Gimeno M, et al. (2012) Chitin and L(+)-lactic Acid Production from Crab (Callinectes bellicosus) Wastes by Fermentation of Lactobacillus sp. B2 Using Sugar Cane Molasses as Carbon Source. Bioprocess Biosyst Eng 35(7): 1193-1200.
- Hajji S, Younes I, Ghorbel-Bellaaj O, Hajji R, Rinaudo M, et al. (2014) Structural Differences Between Chitin and Chitosan Extracted from Three Different Marine Sources. Int J Biol Macromol 65: 298-306.
- Jaganathan K (2016) Extraction and Characterization of Chitin from Marine Bycatch Crustaceans Employing Fermentation Method. World Journal of Pharmacy and Pharmaceutical Sciences 5(1): 1290-1301.
- Gortari MC, Hours RA (2013) Biotechnological Processes for Chitin Recovery out of Crustacean Waste: A Mini-Review, Electronic Journal of Biotechnology 16(3).
- Isa MT (2012) Extraction and Characterization of Chitin from Nigerian Sources. Leonardo Electronic Journal of Practices and Technologies (21): 73-81.
- Hamdi M (2017) Chitin Extraction from Blue Crab (Portunus segnis) and Shrimp (Penaeus kerathurus) Shells Using Digestive Alkaline Proteases from P. segnis Viscera, Int J Biol Macromol 101: 455-463
- Maruthiah T, Palavesam A (2017) Characterization of Haloalkalophilic Organic Solvent Tolerant Protease for Chitin Extraction from Shrimp Shell Waste, International Journal of Biological Macromolecules 97: 552-560.
- Moghadam A, Soraya J (2012) Extraction of Shrimp Waste pigments by Enzymatic and Alkaline Treatment: Evaluation by Inhibition of Lipid Peroxidation. Journal of Material Cycles and Waste Management 14(4): 411-413.
- Jalal AF (2012) Optimization of Chitin Extraction from Chicken Feet, Journal of Analytical & Bioanalytical Techniques 3(05): 1-5.
- Patricia S (2014) ‘Comparison of Extraction Methods of Chitin from Ganoderma lucidum Mushroom Obtained in Submerged Culture’.
- Ghorbel-Bellaaj O, Younes I, Maalej H, Hajji S, Nasri M, et al. (2012) Chitin Extraction from Shrimp Shell Waste Using Bacillus Bacteria. Int J Biol Macromol 51(5): 1196-1201.
- Younes I, Rinaudo M (2015) Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and applications, Mar Drugs 13(3): 1133-1174.
- Morganti P (2012) Application of Chitin Nanofibrils and Collagen of Marine Origin as Bioactive Ingredients, Marine Cosmeceuticals 267-289.
- Erdogan S, Kaya M, Akata I (2017) Chitin Extraction and Chitosan Production from Cell Wall of Two Mushroom Species (Lactarius vellereus and Phyllophora ribis). AIP Conference Proceedings 1809(1).
- Younes I, Hajji S, Rinaudo M, Chaabouni M, Jellouli, K, et al. (2015) Optimization of Proteins and Minerals Removal from Shrimp Shells to Produce Highly Acetylated Chitin. Int J Biol Macromol 84: 246-53
- Kumari S, Rath P (2014) Extraction and Characterization of Chitin and Chitosan from (Labeo rohit) Fish Scales. Procedia Materials Science 6: 482 - 489.
- Liu P, Liu, Shanshan, Guo, Mao, Xiang zhao, et al. (2014) Cofermentation of Bacillus licheniformis and Gluconobacter oxydans for Chitin Extraction from Shrimp Waste. Biochemical Engineering Journal 91: 10-15.
- Aranday-Garcia R (2017) Successive Inoculation of Lactobacillus brevis and Rhizopus oligosporus on Shrimp Wastes for Recovery of Chitin and added-value Products. Process chemistry
- Muzzarelli RAA (2011) ‘Biomedical Exploitation of Chitin and Chitosan via Mechanochemical Disassembly, Electrospinning, Dissolution in Imidazolium Ionic Liquids, and Supercritical Drying’. Marine Drugs 9(9): 1510-1533.
- Izumi R (2015) ‘Favorable Effects of Superficially Deacetylated Chitin Nanofibrils on the Wound Healing Process’, Carbohydrate Polymers. Elsevier Ltd.
- Elieh Ali Komi D, Sharma L, Dela Cruz CS (2018) ‘Chitin and Its Effects on Inflammatory and Immune Responses’, Clinical Reviews in Allergy and Immunology. Clin Rev Allergy Immunol 54(2): 213-223.
- Morganti P (2014) ‘Use of chitin Nanofibrils from Biomass for an Innovative Bioeconomy Outline ’, Nanofabrication using Nanomaterials pp. 1-22.
- Kandra P, Challa MM, Kalangi Padma Jyothi H (2012) ‘Efficient Use of Shrimp Waste: Present and Future Trends’. Appl Microbiol Biotechnol 93(1): 17-29.
- Khoushab F, Yamabhai M (2010) ‘Chitin Research Revisited’. Marine Drugs 8(7): 1988-2012.
- Lee KK (2012) ‘Elevated Cell Wall Chitin in Candida Albicans Confers Echinocandin Resistance in vivo’. Antimicrobial Agents and Chemotherapy 56(1): 208-217.
- Yang G (2016) ‘Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials’. Int J Adv Res (Indore) 4(3):411- 427.
- Ongkiko A, Fernando L, Diaz L (2016) Continuous Extraction Process of Chitin from Discarded Shells of Philippine Blue Wimming Crab (Portunus pelagicus). Philippine Engineering Journal 37(2): 37-52.
- Kim M, Han Y, Jo Y, Choi M, Kang S, et al. (2016) Extraction of Chitin and Chitosan from Housefly, Musca domestica, Pupa Shells. Entomological Research 46(5): 324-328.
- Ifuku S (2015) Chitin Nanofibers: Preparations, Modifications, and Applications. Handbook of Polymer Nanocomposites. Processing, Performance and Application 165 - 178.
- Younes I (2012) Chitin and Chitosan Preparation from Shrimp Shells Using Optimized Enzymatic Deproteinization. Process Biochemistry 47(12): 2032-2039.
- Ioelovich M (2014) Crystallinity and Hydrophility of Chitin and Chitosan, Journal of Chemistry 3(3): 7-14.
- Hongkulsup, Choosit, Khutoryanskiy, Vitaliy V, Niranjan, et al. (2016) Enzyme Assisted Extraction of Chitin from Shrimp Shells (Litopenaeus vannamei). Journal of Chemical Technology and Biotechnology 91(5): 1250-1256.
- Ofem MI (2017) Properties of Chitin Reinforces Composites: A Review. Nigerian Journal of Technology 36(1): 57-71.
- Kaur S, Dhillon GS (2014) The Versatile Biopolymer Chitosan: Potential Sources, Evaluation of Extraction Methods and Applications, Crit Rev Microbiol 40(2): 155-175.
- Mao X (2017) Comprehensive Utilization of Shrimp Waste Based on Biotechnological Methods: A Review. Journal of Cleaner Production 143: 814-823.
- Mao X, Jing Zhang, Feifei Kan, Yuansong Gao, Jing Lan, et al. (2013) Antioxidant Production and Chitin Recovery from Shrimp Head Fermentation with Streptococcus thermophilus. Food Science and Biotechnology 22(4): 1023-1032.
- Pachapur VL (2016) Novel Biological and Chemical Methods of Chitin Extraction From Crustacean Waste Using Saline Water. Journal of Chemical Technology and Biotechnology 91(8): 2331-2339.
- Hamed I, Ozogul F, Regenstein (2016) Industrial Applications of Crustacean By-products (Chitin, Chitosan, and Chitooligosaccharides): A review. Trends in Food Science and Technology 48: 40-50.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.