Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
The Multifunctional Therapeutic Approach to Treatment of Alzheimer’s Disease: Molecular Bases, Pharmacology and Perspectives in Medicinal Chemistry
*Corresponding author: Aldo Sena de Oliveira, Department of Exact Sciences and Education, Federal University of Santa Catarina, Brazil.
Received: June 28, 2019; Published: July 08, 2019
DOI: 10.34297/AJBSR.2019.03.000716
Abstract
Alzheimer’s disease (AD) is a chronic neurodegenerative disease that affects a large part of the elderly population, aged over 65 years. With increasing life expectancy, AD has become the most common form of dementia, leading to progressive impairment of daily living activities and a variety of neuropsychiatric symptoms and behavioral changes. Despite advances in recent years, the chemotherapy treatment of AD still presents a challenge, since a great part of the therapies only act in the reduction of the symptoms in order to improve the quality of life of the patient. In this context, this mini review seeks to illustrate and emphasize the need to understand AD at a molecular level and to combine multi-target strategies in order to develop new drugs, safe use and with fewer adverse effects, as a way to contribute to the advancement of research in an area that represents a major global public health problem.
Keywords: Alzheimer’s disease; Cholinergic hypothesis; Glutamatergic system; Anticholinesterases.
Introduction
Alzheimer’s disease (AD), first described in 1906 in the Auguste Deter patient by the German psychiatrist Alois Alzheimer, is an irreversible neurodegenerative disease characterized by the progressive aggregation of unfolded tau protein, neuronal death, and cerebral atrophy. Getting a mapping of these changes in the brain is challenging because the changes usually precede the clinical symptoms of Alzheimer’s disease for at least one if not two decades [1]. AD is among the most prevalent disturbances that affects neurons and leads to modifications in the central nervous system (CNS), leading to a loss of cognitive ability [2]. AD is the main form of dementia that mainly affects the elderly, although the number of cases involving the middle-aged population (between 30 and 50 years) has increased in recent years. It is estimated that by 2050, 131.5 million people worldwide will be affected by AD, with the majority (68%) present in low- and middle-income countries [3]. Although the description of AD has already been made more than 100 years ago, the scientific community has not yet been able to elucidate correctly the genesis of the disease and, consequently, it has not yet been possible to find a drug that is able to cure the patient in its entirety, in addition to relieving symptoms [4].
In this way, the development of new drugs capable of impeding the neurodegenerative process has become one of the biggest challenges for the biomedicine [5]. Given the increasing number of AD registries around the world, it is necessary to develop studies with a solid approach in molecular biology, the development of methods for the early diagnosis of the disease and, especially, the development of curative therapies or , in the last resort, but they are more effective. In this mini revision, are presented some of the main questions involving the biological and pharmacological bases of the disease and some methodological approaches are presented for its chemotherapy. The intention is not to exhaust the theme, given its complexity, but to illustrate the main strategies available for the treatment of this pathology.
Molecular Bases of Alzheimer’s Disease
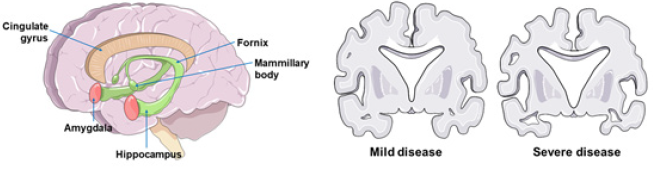
AD is a neurodegenerative disease, in which mainly atrophy of the hippocampus is observed, responsible for the clinical picture of progressive loss of formation and invocation of memories. In its terminal stage it can also affect areas of the cerebral cortex (Figure 1), thus affecting other functions, such as the ability to perform precise movements, choose words, and the sensibility of the senses. Even with this morphological difference it is not possible to accurately diagnose AD, since the healthy individual may present similar morphological changes, but from other causes other than AD [6] (Figure 1).
Cholinergic hypothesis
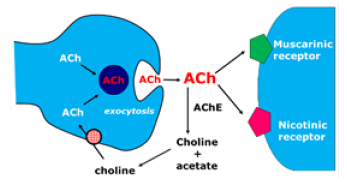
The earliest hypothesis about AD was introduced in the early 1980s and described as the cholinergic hypothesis in amnestic dysfunction in the elderly. The importance of cholinergic function in learning and memory processes has been known since the early 1970s. A relevant observation was the positive association between these two depletions and the degree of severity of the patient’s cognitive deficit in life. Subsequent studies have shown that the administration of cholinomimetic substances reduced the mnemonic difficulties presented by persons affected by the disease [7]. The cholinergic system has been implicated in mediating plasticity in the brain in response to experience or injury. There are four lines of evidence that support this hypothesis: beneficial effects of cholinergic agonists in increasing recovery and minimizing neuronal damage in invariant lesion models; cholinergic depletion has been found to compromise plasticity dependent on experience in the cortex and hippocampus and similar mechanisms are involved in mediation of lesion recovery; acetylcholine modulates the expression of neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), which play an important role in neuronal survival and plasticity in adult life. Recent work shows a fascinating interaction between acetylcholine and estrogen in supporting hippocampal plasticity in older women [8]. ACH deficiency is produced in AD by Meynert nucleus basalis atrophy, which is the source of the enzyme choline acetyltransferase (CAT).
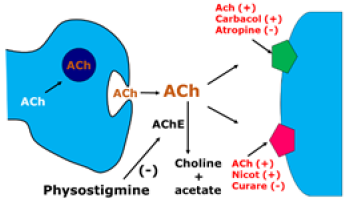
This enzyme is transported to target structures in the central nervous system (CNS): hippocampal formation, cerebral cortex and amygdala, among others. In these regions, it catalyzes the synthesis reaction of ACh from choline and acetyl coenzyme A. Once formed, ACH is released into the synaptic cleft, where it can be coupled to two types of muscarinic and nicotinic receptors. The remaining ACH is degraded by the enzyme acetylcholinesterase (ACHE) in the synaptic cleft in choline and acetate, which are the bases of its (Figure 2) [7]. In DA, there is an atrophy in nucleus basalis, resulting in a decrease in the synthesis of CAT and, consequently, of ACH. For this reason, the strategy adopted in the development of antidementia drugs is to improve cholinergic function. Synaptic levels of ACH can be increased by inhibiting ACHE, using precursors of ACH, increasing the release of ACH or stimulating postsynaptic receptors (Figure 3). There is a correlation between the severity of AD and the decline of CAT [9] (Figure 3). The use of presynaptic precursors of ACH, such as lecithin, choline and acetyl-L-carnitine, was tried without because there is a low penetration into the system central nervous system through the blood-brain barrier, short duration and low therapeutic applicability. Also, for the presynaptic region, the use of agents that increase the release of ACH, such as lop pyridine, 4-aminopyridine and befiperide, which also showed unsatisfactory therapeutic results.
Five types of muscarinic receptors were identified, among them M1, which is found in the post-synaptic membrane and is preserved in AD, and the presynaptic M2, reducing the release of ACH in the crevice and reduced in AD. Some specific M1 agonist drugs are being tested, such as xenomelia and the milameline [10]. There is a significant reduction in the number of nicotinic receptors in AD. Studies with animal models suggest that nicotinic agonists have a beneficial effect on learning and memory. In addition, there is evidence that some anticholinesterases can activate nicotinic receptors. The anticholinesterases are the most promising agents developed to date. It is the only therapeutic class of drugs that showed improvement in cognitive symptoms in AD. They act in the synaptic cleft, inhibiting the enzyme acetylcholinesterase, such as physostigmine. Physostigmine was the first ACHE inhibitor used in AD. However, their employment was limited by and the high incidence of collateral cholinergic effects (nausea, vomiting, abdominal pain, sweating and fasciculations). Inhibition of ACHE is one of the most indicated and investigated strategies for the treatment of several neurological diseases, such as Alzheimer’s disease, senile dementia, ataxia, myasthenia gravis and Parkinson’s disease. ACHE (EC 3.1.1.7) is a serine hydrolase that selectively reacts with its natural substrate, acetylcholine (ACH). Structurally this enzyme is composed of several molecular forms, which can be divided into asymmetric forms and globular forms, with the latter as monomers, dimers or catalytic tetramers, either secreted as soluble forms or anchored to the membrane by a hydrophobic domain [11].
Several studies have been developed using the most different techniques, and these have demonstrated that the active center of ACHE is composed of several main domains: (a) a domain containing active serine, (b) an anion domain that accommodates the positive ACH and (c) hydrophobic domains linking aryl substrates, and other uncharged binders [12]. The catalytic triad consists of the amino acid residues Ser 203 - His 447-Glu 334 [12].Regarding inhibition of ACHE, some inhibitors bind to the catalytic center (competitive inhibitors), while others influence the steady-state parameters by association with an allosteric domain away from the active site. This domain is referred to as the peripheral anionic center [12].
According to the cholinergic hypothesis, people suffering from AD have low levels of acetylcholine. ACHE inhibitors slow the metabolic degradation of acetylcholine by optimizing the availability of this substrate for communication between cells. This assists in slowing the progression of cognitive dysfunction and may be effective for some patients in the early and intermediate stages of the disease. There are four drugs in this class today, approved by the Food and Drug Administration (FDA) Drug Administration): tacrine, donepezil, rivastigmine and galantamine. These treatments were released for mild to moderate AD symptoms and among them only donepezil was approved for the treatment of severe symptoms in 2006.
The Hypothesis of the Amyloid cascade
Since the discovery of AD, it has been recognized that the symptoms of the disease may be associated with the development of numerous intraneuronal and extracellular filamentous lesions in the limbic cortex, as well as in the cerebral cortex. Abnormal aggregates of cytoplasmic fibers occur both in neuronal cell bodies, involving neurofibrillary tangles, and in axons and dendrites [13]. These symptoms are collectively called dystrophic neuritis. Along with the presence of dystrophic neuritis, there is also another important histopathological sign in AD: the widespread presence of plaques and aggregates, mainly formed by the Aβ peptide, in the extracellular portion of brain tissue [13]. Tau belongs to the class of microtubule associated protein (MAP) that under normal, nonpathological conditions regulate the stability, dynamics and spatial organization of microtubules (MTs). Tau is highly expressed in the brain, believed to be due to its role in the axonal transport of organelles and vesicles, which may contain neurotransmitters. In pathological situations, in the case of AD and other diseases, the tau protein becomes highly phosphorylated, losing its stability and physiological function.
The tau when it is phosphorylated is detached from the MT, leading to the destabilization and reduction in the dynamics of the same [6]. The tau protein under normal conditions is found in axons, but in DA accumulates mainly in the cell body and dendrites of neurons, this accumulation forms paired helical filaments (PHF) that lead to the formation of tau oligomers, the ENFs The activity of tau protein is regulated, after transition, by phosphorylation and dephosphorylation mechanisms. In DA, hyperphosphorylation results from an increase in the activity of kinases, in the decrease of the activity of the phosphatases, or by both mentioned mechanisms. This process seems to be triggered by accumulation of Aβ, increasing the formation of aggregates in the dendrite region of neurons [14].
The Hypothesis of glutamatergic dysfunction
The glutamatergic system has been the object of numerous recent basic and clinical studies that suggest an important role of the disturbances of its activity, basing the ‘glutamatergic hypothesis’ on Alzheimer’s disease. Normal glutamatergic neurotransmission is replaced by pathological neurotransmission, as occurs in AD. The important differential characteristic seems to be a sustained release of low glutamate concentration (‘tonic activation’), which induces in the post-synaptic region a partial depolarization and a maintained influx of Ca2+ to the postsynaptic neuron, with increased amplitude of the compared to that recorded under normal conditions [1]. The transient release of glutamate after presynaptic activation, under these conditions, leads to a complete depolarization of the postsynaptic membrane and additional influx of Ca2+. An electrophysiological ‘signal’ is produced that does not stand out in amplitude in relation to background noise, which is not detected. Excessive intracellular Ca2+ uptake also signals pathological activities at cytoplasmic and nuclear sites. Thus, the necessary modifications related to the mechanisms of neuroplasticity do not occur and deleterious cascades are induced which may lead to neurodegeneration [1]. The use of a drug such as memantine, an uncommon antagonist of the moderately affinity NMDA receptor, appears to be able to block the pathological activation of the NMDA receptor (by providing neuroprotection), while preserving physiological activation and the acquisition or processing of cognitive information allowing neuroplasticity). Memantine, therefore, can be used in the treatment of AD, representing a new therapeutic strategy, the ‘glutamatergic strategy’, considering both its neuroprotective effect against excitotoxicity and its symptomatic effect in relation to cognitive functions, as demonstrated in clinical trials as well conducted [1].
The Oligomeric Hypothesis
Studies suggest that oligomers may cause cognitive impairment because they disrupt synaptic function in the absence of significant neurodegeneration in their environment [16]. PBA and its oligomers alter structure and synaptic function and compromise transmission through post-synaptic compromise. The disturbance synaptic and dendritic cells alter functional cortical neuronal networks, leading to a sub-regulation of these networks and anatomically and functionally compromising the interconnected brain areas [17]. The oligomers, besides compromising the synaptic functions, modify the neuronal activity and promote the release of neurotoxic mediators by glial cells [18].
Genetic aspects
The establishment of AD is due to the accumulation of genetic and environmental events. Each of these events contributes with small effects that result, together in the establishment of the disease with different degrees of severity. We now know that mutations in the coding genes for APP [Amyloid b (A4) precursor protein], apoE (apolipoprotein E), PSEN1 (presenilin 1) and PSEN2 (presenilin 2) are consistently associated with the establishment of AD. These genes are in different chromosomes and at least some of them must participate in a common neuropathogenic pathway, culminating in the onset of the disease. These four genes are, to this day, the most important and most consistent markers for AD. However, changes in them are not sufficient or necessary to explain all cases of AD [19].
Perspectives in Medicinal Chemistry
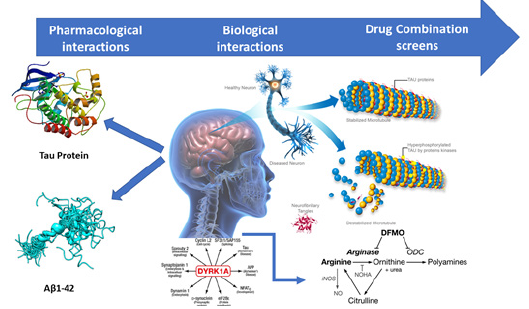
In recent years, Medicinal Chemistry has been seeking new alternatives and tools capable of bringing greater agility, safety and more efficient direction in the planning and prospecting of drug candidates. In this context, drug discovery strategies focusing on the development of ligands that target specific targets have been redistricted, since they have limited application in multifactorial diseases, where a set of biochemical events and several receptors are involved [20]. The need to circumvent the efficiency and therapeutic security has led to changes in the current paradigm of a new target, also called the reductionist approach. It is undeniable that this strategy has brought over time countless successes in the treatment of disease, paving the way for the current stage of treatment evolution . The search for better results in clinical practice, because of the inefficiency of some treatments with unique drugs and marketing decisions, led to the development of a new therapeutic strategy called polypharmacology (Figure 4).
This new design adopts the use of combinations of more than one drug in a single formulation with fixed composition or the use of drug cocktails as promising alternatives and adopted on a large scale (Figure 4). For 15 years, the literature has been reporting a series of papers that use this innovative strategy for drug planning for AD. Drugs such as donepezil, tacrine and rivastigmine have been used as structural models for molecular hybridization with synthetic or natural bioactive compounds, in an attempt to obtain ligands capable of simultaneously modulating the activity of more than one target involved in AD. Based on the different molecular hypotheses of AD, the approaches of the research groups were differentiated over the years. Different treatments are currently available in the market, both those that focus on the cholinergic and glutamatergic hypotheses, and those that help in the partial control of several symptoms, particularly agitation, depression, hallucinations and delusions, which are more frequent with the progression of the disease [21].
Treatment
AD therapy may be primary or secondary. The goal of primary therapies is to delay the manifestation of central symptoms. The currently available therapies improve the symptoms, but do not modify the disease, that is, they do not combat the basic mechanisms of AD. Secondary therapies are designed to improve problems such as depression, anxiety, sleep disorders and restlessness.
Anticholinesterases
Patients with mild to moderate AD are suitable candidates for cholinesterase inhibitor therapy, such as donepezil, galantamine and rivastigmine, which have been approved by the Food and Drug Administration (FDA). For each of these agents, clinical studies have demonstrated that prolonged use results in a modest stabilization of the cognitive and functional condition for about 6 to 12 months, compared to the absence of treatment [22]. In a particularly illuminating study, patients with AD mild to moderate were randomized to receive donepezil or placebo for up to 1 year. The end point of therapy was the loss of a predefined degree of daily functioning. A significant loss of daily functional capacity was observed after 12 months in 51% of patients treated with placebo and in only 38% of patients treated with donepezil [23]. Unfortunately, anticholinesterase agents exhibit several adverse effects, such as gastrointestinal disorders, nausea, loss of appetite, diarrhea and, less commonly, vomiting. These effects have a strong, dose-related relationship and are often manifested when the course of drugs is initiated, or the dose is increased.
Vitamin E
A multicenter randomized double-blind clinical trial enrolling 613 subjects (mean age 79 years) with mild to moderate Alzheimer’s disease (Mini-Mental State Examination 12-26; mean of 21) in 14 US centers [24]. All participants were already aware of cholinesterase inhibitors when they entered the study. Additionally, they received alpha-tocopherol 2,000U / day (n = 152), memantine 20mg / day (n = 155), the combination of both (n = 154) or placebo only (n = 152). Functionality was assessed as the primary outcome using the ADCS-ADL score. After an average follow-up of 2.3 years, patients receiving alpha-tocopherol had a better evolution in the functional outcome, with a 19% reduction in the progression of the deficits, corresponding to a period of 6.2 months of disease evolution. The group that received alpha-tocopherol presented an even better evolution in the measure of cognitive performance (ADAS-Cog) and a decrease in the time spent by the caregiver. Participants who received memantine did not present significant benefit in the outcomes, attributed by the authors to the predominance of patients in the light phase [24].
The enzyme DYRK1A
DYRK1A has multiple biological functions as a function of its interactions with various cellular cytoskeletal proteins, synaptic and nuclear proteins, including transcription factors and factors involved in the posttranscriptional removal of introns and ribonucleic acid (RNAs) exons [25]. The expression of DYRK1A in neurons during the fetal and postnatal period, as well as in neurons of adult individuals and in old age, suggests that the regulation of this expression is involved with neuronal development, its later maturation and aging [26]. This enzyme is expressed throughout the human organism, but is particularly abundant in the cerebellum, olfactory bulb and hippocampus. Its expression is regulated positively in the early stages of embryonic development followed by a gradual decrease to low levels in the later stages [27].
Increased immunoreactivity was observed for DYRK1A in the cytoplasm and nucleus of neurons dispersed in the entorhinal cortex, hippocampus and neocortex in neurodegenerative diseases associated with the phosphorylation of Tau proteins; including DA, SD and Pick’s disease [28]. Increased expression of DYRK1A is involved both in the formation of the NFS, via hyperphosphorylation of the Tau protein; as well as the formation of amyloid plaques via PPA cleavage and consequent formation of the Aβ peptide; resulting in neuronal loss and dementia [29].
N-methyl-d-aspartate (NMDA) receptor antagonists
Memantine has been approved by the FDA for use in the treatment of moderate to severe AD. This glutamate modulating agent, a non-competitive N-methyl-D-aspartate receptor antagonist, has been subjected to several clinical studies reporting positive results in moderate to severe dementia [30]. In clinical studies, memantine slowed the progression of symptoms. However, there is no evidence that memantine affects the biological course of AD. Studies on the use of monotherapy in the treatment of patients with mild to moderate AD demonstrated the benefits promoted by memantine [31]. However, since combined therapy with cholinesterase inhibitor and memantine did not present additional proven benefits in relation to the isolated use of cholinesterase inhibitors in cases of mild to moderate AD. The field of AD therapy is evolving rapidly. Several approaches to decrease beta-amyloid peptide loading are being evaluated, including gamma-secretase and beta-secretase inhibitors and immunological strategies. In addition, several early efforts are also being made to attack the tubulin-tau system.
Other forms of treatment
Alzheimer’s Disease (AD) shows cognitive dysfunction as core symptoms and Behavioral and Psychological Symptoms of Dementia (BPSD). Since acetylcholine nerve system derived from septum is collapsed in the AD patients, was used Olfactory Bulbectomized (OBX) mice whose cholinergic system is largely impaired in the septum [32]. Recently, Yokukansankachimpihange (YKH), a traditional Japanese Kampo medicine has used for BPSD in addition to improve cognitive dysfunction in AD patients. It was found that Atractylenolide III (Aen-III), one of the components of YKH, improved cognitive deficits and depression in the OBX mice. In summary, Aen-III as a component of YKH ameliorates cognitive dysfunctions and depression via restoring Ca2+/calmodulindependent protein kinase II (CaMKII) and Ca2+/calmodulindependent protein kinase IV (CAMKIV) signaling [32].
The accumulation of amyloid β peptide1-42 (Aβ1-42) masses in the brains of Alzheimer’s Disease (AD) patients can be associated with neuronal loss and memory deficits. It was reported [33] that oral administration of docosahexaenoic acid (DHA, C22:6, n-3) significantly decreases Aβ burden in the brains of AD model rats and that direct in vitro incubation of DHA with Aβ1-42 curbs the progression of amyloid fibrillation. Computational analyses of the binding of DHA to Aβ1-42, as evaluated by docking studies, further corroborated the inhibitory effect of DHA on in vitro Aβ1- 42 fibrillogenic and might explain the in vivo reduction of amyloid burden observed in the brains of DHA-administered AD model rats [33].
Secondary therapies
The treatment of depression or anxiety for patients with AD should be as aggressive as for patients without AD, adhering to the best practices of geriatric pharmacology. Depression contributes to morbidity and loss of function in patients with AD. More modern agents, such as citalopram, paroxetine, sertraline and mirtazapine, are generally well tolerated by patients with dementia. The dosage may start at a lower level than that possibly used in the treatment of a young adult; dose increases should also be more spaced. The treatment of anxiety in patients with AD is more challenging, since agents commonly used in the treatment of younger patientsbenzodiazepines - produce distinct undesirable side effects in individuals with AD. Drugs such as lorazepam or alprazolam may increase confusion in patients with AD. Clonazepam, a longacting agent, may be a more appropriate choice. Buspirone is an alternative for the treatment of anxiety in patients with AD.
Final Considerations
The main known risk factor for DA, whether genetic or random, is aging. Once the life expectancy of the world population has been increased, with the best socioeconomic life expectancy, Alzheimer’s disease tends to increase in number of cases. Although AD has been discovered for more than a century, there are still several challenges in understanding its genesis and in the development of new drugs, safer and more selective, that can cure or slow the disease. Although many studies have contributed to elucidate the pathophysiological mechanisms of Alzheimer’s disease, selective neuronal loss has not yet been fully understood. The combination of strategies involving understanding the molecular aspects of AD can support the development of new drugs in the context of medicinal chemistry and pharmacology, with a view to scientific development that translates into direct benefits for public health.
References
- A Schäfer, J Weickenmeier, E Kuhl (2019) The interplay of biochemical and biomechanical degeneration in Alzheimer’s disease. Computer Methods in Applied Mechanics and Engineering 352: 369-388.
- G Mc Khann, D Drachman, M Folstein, R Katzman, D Price, et al. (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34(7): 939-944.
- T Umar, S Shalini, MK Raza, S Gusain, J Kumar, et al. (2019) European Journal Of Medicinal Chemistry 175: 2-19.
- J Hardy, N Bogdanovic, B Winblad, E Portelius, N Andreasen, et al. (2014) Pathways to Alzheimer’s disease Journal of Internal Medicine 275: 296- 303.
- J Cao, J Hou, J Ping, D Cai (2018) Advances in developing novel therapeutic strategies for Alzheimer’s disease. Molecular Neurodegeneration 13: 1-20.
- J de A Oliveira Filho, JRN Martins (2018) Ricardo Martins de Oliveira Filho | Escavador Rev da Biol 18: 24-30.
- TH Ferreira-Vieira, IM Guimaraes, FR Silva, FM Ribeiro (2016) Alzheimer’s disease: Targeting the Cholinergic System. Curr Neuropharmacol 14(1): 101-115.
- LA Craig, NS Hong, RJ McDonald (2011) Revisiting the cholinergic hypothesis in the development of Alzheimer’s disease. Neurosci Biobehav Rev 35(6): 1397-1409.
- J Raskin, J Cummings, J Hardy, K Schuh, RA Dean, et al. (2015) Neurobiology of Alzheimer’s Disease: Integrated Molecular, Physiological, Anatomical, Biomarker, and Cognitive Dimensions. Curr Alzheimer Res 12(8): 712- 722.
- BM Radu, AM M Osculati, E Suku, A Banciu, G.Tsenov, et al. (2017) All muscarinic acetylcholine receptors (M1-M5) are expressed in murine brain microvascular endothelium Sci Rep 7(1): 5083.
- M Racchi, M Mazzucchelli, E Porrello, C Lanni, S Govoni (2004) Acetylcholinesterase inhibitors: novel activities of old molecules. Pharmacol Res 50(4): 441-451.
- Y Bourne, PT aylor, Z Radić, P Marchot (2003) Strutural insights into ligand interactions at the acetylcholinesterase peripheral anionic site EM0042O J 22(1): 1-12.
- A Serrano-Pozo, MP Frosch, E Masliah, BT Hyman (2011) Neuropathological Alterations in Alzheimer Disease Cold Spring Harb Perspect Med 1(1): 6189.
- T Maas, J Eidenmüller, R Brandt (2000) Interaction of tau with the neural membrane cortex is regulated by phosphorylation at sites that are modified in paired helical filaments. J Biol Chem 275(21): 15733-15740.
- E Engelhardt, J Laks, JLS Cavalcanti Rev bras neurol
- E Marcello, R Epis, C Saraceno, M Di Luca (2012) Synaptic dysfunction in Alzheimer’s disease. Adv Exp Med Biol 970: 573-601.
- D Neill (2012) Ageing Res Rev
- M Sakono, T Zako, H Ueda, M Yohda, M Maeda (2006) In Polymer Preprints.
- C Fridman, SP Gregório, EDias, N Élida, P Benquique Ojopi, (2004) E Rev Psiq Clín, 31: 19-25.
- KS TDias, CT De Paula, MM Riquiel, STL Do Lago, KCM Costa, (2015) Rev Virtual Quim 7: 609-648.
- A De Falco, DS Cukierman, RA Hauser-Davis, NA Rey, Nicolás A, et al. (2016) Doença De Alzheimer: Hipóteses Etiológicas E Perspectivas De Tratamento Quim. Nova 39(1): 63-80.
- J Birks Library (London)
- RC Mohs, RS Doody, JC Morris, JR Ieni, SL Rogers, et al. (2001) A 1-year, placebo-controlled preservation of function survival study of donepezil in AD patients. Neurology 57(3): 481-488.
- MW Dysken, M Sano, S Asthana, JE Vertrees, M Pallaki, (2014) Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA 311(1): 33-44.
- J Galceran, K de Graaf, FJ Tejedor, Becker W (2003) The MNB/DYRK1A protein kinase: genetic and biochemical properties. J Neural Transm Suppl 67: 139-48.
- J Wegiel, I Kuchna, K Nowicki, J Frackowiak, K Dowjat, et al. (2004) Cell type- and brain structure-specific patterns of distribution of minibrain kinase in human brain. Brain Res 1010(1-2): 69-80.
- E Martí, X Altafaj, M Dierssen, S De La Luna, V Fotaki, et al. (2003) Dyrk1A expression pattern supports specific roles of this kinase in the adult central nervous system. Brain Res 964(2): 250-263.
- I Ferrer, M Barrachina, B Puig, M Martínez De Lagrán, E Martí, et al. (2005) Constitutive Dyrk1A is abnormally expressed in Alzheimer disease, Down syndrome, Pick disease, and related transgenic models. Neurobiol Dis 20: 392-400.
- and YWH Wegiel, Jerzy, Cheng-Xin Gong, (2004) Science 278: 236-245.
- PN Tariot, MR Farlow, GT Grossberg, SM Graham, S Mc Donald, et al. Am Med Assoc
- ER Peskind, SG Potkin, N Pomara, BR Ott, SM Graham, et al. (2006) Memantine treatment in mild to moderate Alzheimer disease: a 24-week randomized, controlled trial. Am J Geriatr Psychiatry 14(8): 704-715.
- H Izumi, Y Sasaki, Y Yabuki, Y Shinoda, N Fujita, et al. (2016) Adv Alzheimer’s Dis 5: 35-45.
- M Hashimoto, S Hossain, K Matsuzaki, A Al Mamun, H Arai, et al. (2016) Computational Analyses of Docosahexaenoic Acid (DHA, C22:6, n-3) with Alzheimer’s Disease-Causing Amyloid Peptide Aβ1-42 Reassures Its Therapeutic Utility. Adv Alzheimer’s Dis 5(2): 73-86.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.