Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Activity of Methanol Extract of Leptadenia Hastata Leaves in Alcohol-Induced Liver Injury
*Corresponding author: Akuba Barnabas Ojochegbe, Department of Science Laboratory Technology, School of Applied Sciences, Nigeria.
Received: July 03, 2019; Published: July 26, 2019
DOI: 10.34297/AJBSR.2019.04.000785
Abstract
This study investigated the hepatoprotective effects of methanol leaf extract of Leptadenia hastata (MELH) in chronic alcohol-induced liver injury in rats. The animals were given 5ml/kg ethanol then treated with the extract (250 and 500 mg/kg). This treatment was done orally and daily for 5 weeks. Twenty- four (24) hours after the last treatment, the rats were sacrificed under anaesthesia and blood samples were collected for the assay of aspartate amino transferase (AST) of alanine aminotransferase (ALT) and alkaline phosphatase (ALP) was also assayed in the serum. The levels of other biochemical markers of organ damage such as total cholesterol, triglycerides, total bilirubin and total protein were determined. The liver was excised for relative organ weight estimation as well as histological examination. The extract at both doses produced significant (P<0.05) decrease in the serum activities of ALT, AST, ALP, serum levels of total cholesterol; triglycerides and total bilirubin as well as significant (P<0.05) increase in serum concentration of total protein and albumin in comparison with the negative control that received only alcohol. The histopathological result showed protection in the extract- treated groups when compared with the group that received alcohol alone. It was concluded that methanol leaf extract of Leptadenia hastata showed promising hepato-protection activity in rats with alcohol-induced liver damage. Thus, the plant could be useful in the management of liver diseases..
Keywords: Alcohol; Liver injury; Rats; Methanol leaf extract of leptadenia hastata; Anaesthesia; Assay of aspartate amino transferase; Alanine aminotransferase; Alkaline phosphatase; Triglycerides; Cholesterol; Histopathological
Introduction
Liver is one of the largest organs in human body and the body’s main organ of detoxification. Alcohol is one of the toxic substances detoxified by the liver and it is ultimately broken down by into simple end-products for easy elimination from the body [1, 2]. During alcohol metabolism, several intermediate reactants are generated which are usually more toxic than alcohol itself and may contribute to the development of alcohol liver disease [3]. Apart from the liver, the deleterious effect of alcohol also affects other organs and systems of the body notably the central nervous system. The metabolism of alcohol is almost solely done by the liver and excessive alcohol consumption may overburden the liver leading to acute and chronic liver diseases [4, 5]. The hallmarks of alcohol liver diseases are steatosis, steatohepatitis, fibrosis, and more severe forms including cirrhosis and hepatocellular carcinoma [6]. Oxidative stress and inflammation are critical factors in etiology of ethanol-induced liver damage [6]. Reactive oxygen species (ROS) are implicated in the progression from hepatic steatosis to steatohepatitis and cirrhosis [7].
Despite tremendous strides in modern medicine, there are hardly any drugs that stimulate liver function, offer protection to the liver from damage or help regeneration of hepatic cell. Hence, there is an ever-increasing need for safe hepatoprotective agent [8]. One of the important and well documented uses of medicinal plants is their use as hepatoprotective agents. It has been reported that about 170 phytoconstituents isolated from 110 plants belonging to 55 families do possess hepatoprotective activity [9]. Liver protective herbal drugs contain a variety of chemical constituents like phenols, coumarins, curcuminoids, lignans, essential oils and terpenoids. Clinical research has also shown that herbals have genuine utility in the treatment of liver diseases. Only a small portion of hepatoprotective plants as well as formulations used in traditional medicine are pharmacologically evaluated for its efficacy [9] and one of such plants is Leptadenia hastata.
Leptadenia hastata belongs to the family asclepiadaceae widely used in Tropical Africa as vegetable [10]. The plant is medicinally important in the treatment of many ailments [10,11, 3]. Ethnobotanical information obtained from traditional medical practitioners in northern Nigeria revealed that L. hastata is used for the treatment of diabetes mellitus. The antibacterial and antimicrobial effects of L. hastata have been reported [12] and the result of its toxicity studies showed that the plant is relatively safe [13]. There is however paucity of information confirming the hepatoprotective potential of L hastata. Hence, this research evaluated the hepatoprotective potentials of L hastata with the view to validating its potentials in the management of liver diseases.
Materials and Methods
Chemicals and drugs
Methanol and all chemicals used in this study were of analytical grade and were purchased from Sigma Chemical Co. Ltd (USA) through a local vendor.
Animals
Adult Wistar rats of either sex weighing 120-180g were used for this study. They were kept in stainless steel cages under standard laboratory conditions. They were maintained on clean water and standard rodent feed.
Plant collection and identification
The leaves of Leptadenia hastata were collected from a natural habitat in Zaria, Kaduna State, Nigeria. The plants were identified at the Herbarium Unit of the Department of Biological Sciences, Ahmadu Bello University; Kaduna State, Nigeria.
Preparation of extracts
The leaves of Leptadenia hastata were shade-dried for five (5) days and pulverized using an electric blender. Two thousand (2000) gram of the pulverized leaves was soaked in distilled water for 72-hours. The resulting mixtures were filtered using Whatmann filter paper (Size No1) and the extract was concentrated using rotary evaporator. The extract of Leptadenia hastata shall henceforth be referred to as MELH.
Acute toxicity study
The oral median lethal dose (LD50) of the extracts was determined in rats according to the method of Lorke et al. [14].
Experimental Design
Twenty (20) adult male wistar rats were randomized into 4 groups of 5 animals each and treated as follow;
Group I: 5ml/kg Dist. H4O
Group II: 5ml/kg Ethanol
Group III: 5ml/kg Ethanol + 250 mg/kg MELH
Group IV: 5ml/kg Ethanol + 250 mg/kg MELH
The treatment was carried out for 5 weeks. The animals of alcohol control group were administered oral dose of ethanol everyday between 10:00am and 11:00am. Experimental animals of groups 3 and 4 were given orally 250 and 500 mg/kg MELH respectively after 1-hour administration of alcohol. Twenty-four (24) hours after the last treatment, all rats were fasted overnight and euthanized using chloroform, Blood samples were collected via cardiac puncture into plain bottles for biochemical analysis. The following parameters were determined.
Body Weight Measurement: The body weight of the rats was measured at the start of experiment and prior to sacrifice.
Determination of Enzymatic Liver Function Parameters: Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities were assayed using the method of Reitman and Frankel [15] as described in Randox kits while alkaline phosphatase (ALP) activity were assayed based on the methods of Kind and King [16].
Determination of Non- Enzymatic Liver Function Parameters: Total cholesterol (TC) and triacylglycerol (TAG) concentrations were determined according to the method of Trinder [17]. Total bilirubin (Tbil) and direct bilirubin were determined according to the method of Jendrasik and Grof [18]. Total protein (TP) concentration was determined by Biuret method [19] while albumin concentration was determined through the binding method as reported by Spencer & Price [20].
Organ Weight/ Histopathological Examination: After scarification of rats, the samples of liver tissues were collected from all groups and weighed then fixed in 10% neutral buffered formalin for histopathological investigation.
Statistical Analysis
Data were expressed as mean standard error of mean (SEM). Statistical comparisons were performed by one-way ANOVA, followed by Duncan’s multiple comparisons test and the values were considered statistically significant when p-value is less than 0.05.
Results
Acute Toxicity

Table 1: Observations from the Acute Toxicity Study of the Aqueous Leaf Extract of Anogeissus leiocarpus in Rats.
Note: D=death, T= No of animals treated
The results of acute toxicity studies showed no mortality or physical signs of toxicity up to a dose of 5000 mg/kg of methanol extract of Leptadenia hastata. The oral LD50 of the extract was then taken to be > 5000 mg/kg (Lorke’s method) (Table 1).
Body weight and organ weight
Table 2 shows the effect of the administration of methanol Leaf Extract of Leptadenia hastata on the body weight and liver weight of Wistar rats subjected to alcohol-induced hepatic injury. A significant (p< 0.05) difference was observed in the final body weights of the rats administered 5ml/kg ethanol compared to the distilled water- treated group (positive control). There was a significant (p< 0.05) increase in the final body weight of rats administered 500mg/kg MELH compared to the positive control. This dose produced a 16% weight gain which was comparable to that of the positive control (17.8%). There was no significant (p> 0.05) difference in the absolute and relative weights of the liver of the treated groups compared to both positive and negative controls.

Table 2: Effect of Methanol Leaf Extract of Leptadenia hastata (MELH) on Body Weight and Organ Weight of Rats.
Note: Values shown are mean ± S.D. (n = 6). Mean values with different alphabets as superscripts down the column are significantly different at P<0.05
Enzymatic Liver Function Parameters
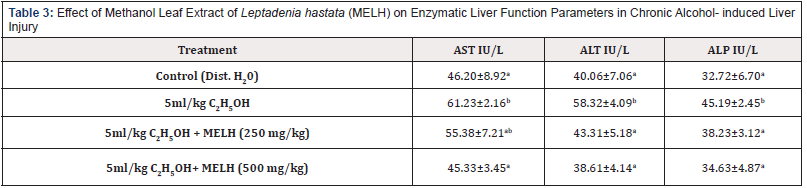
Table 3: Effect of Methanol Leaf Extract of Leptadenia hastata (MELH) on Enzymatic Liver Function Parameters in Chronic Alcohol- induced Liver Injury
Note: Values shown are mean ± S.D. (n = 6). Mean values with different alphabets as superscripts down the column are significantly different at P<0.05
The effects of the administration of methanol Leaf Extract of Leptadenia hastata on the enzyme activities of the Wistar rats subjected to alcohol- induced liver injury is presented in Table 3. 5ml/kg ethanol produced a significant (p< 0.05) increase in the serum activity of AST compared to positive control. Treatment with MELH (500mg/kg) produced a significant (p< 0.05) decrease in the activity of AST compared to the negative control (5ml/ kg ethanol-treated group). The administration of ethanol also produced significant (p< 0.05) increase in the serum activity of AST and ALP compared to positive control. However, treatment with MEHL produced a dose- dependent significant (p> 0.05) decrease in the activities of AST and ALP compared to the negative (5ml/kgethanol- treated) control.
Non-Enzymatic liver function parameters
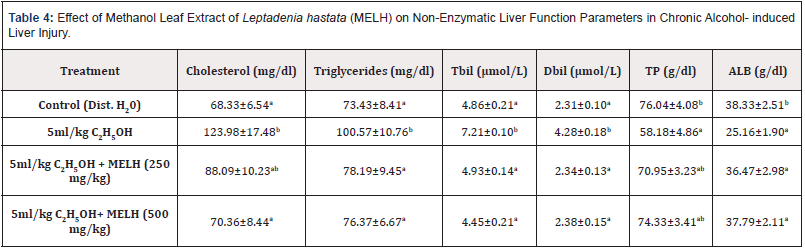
Table 4: Effect of Methanol Leaf Extract of Leptadenia hastata (MELH) on Non-Enzymatic Liver Function Parameters in Chronic Alcohol- induced Liver Injury.
Note: Values shown are mean ± S.D. (n = 6). Mean values with different alphabets as superscripts down the column are significantly different at P<0.05
Table 4 shows the effects of the administration of methanol Leaf Extract of Leptadenia hastata on the serum concentration of cholesterol, glycerides, bilirubin, total protein and albumin of Wistar rats subjected to alcohol- induced liver injury. 5ml/kg ethanol produced a significant (p< 0.05) increase in the serum concentration of cholesterol, triglycerides and bilirubin and a significant (p>0.05) decrease in total protein and albumin concentration compared to positive control. Treatment with MELH at doses of 250 and 500mg/ kg produced a significant (p< 0.05) decrease in the cholesterol, triglycerides and bilirubin concentrations and a significant (p< 0.05) increase in plasma concentrations of total protein and albumin compared to the negative control (5ml/kg ethanol-treated group).
Histological Findings
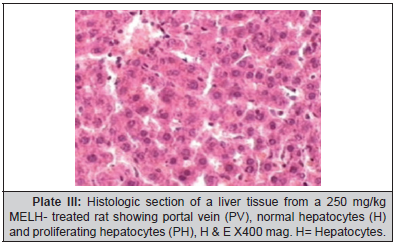
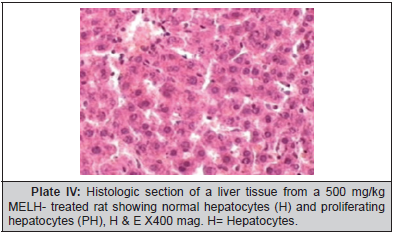
Plate 1, Plate 2, Plate 3 and Plate 4.
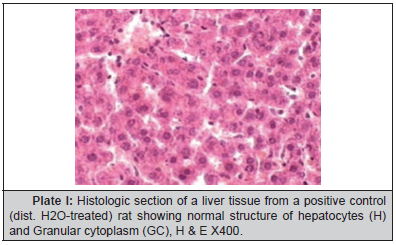
Plate I:Histologic section of a liver tissue from a positive control (dist. H4O-treated) rat showing normal structure of hepatocytes (H) and Granular cytoplasm (GC), H & E X400.
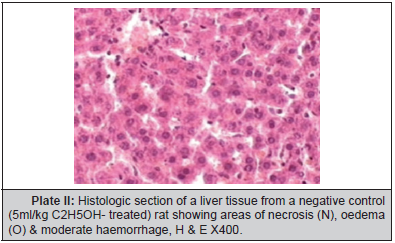
Plate II: Histologic section of a liver tissue from a negative control (5ml/kg C2H5OH- treated) rat showing areas of necrosis (N), oedema (O) & moderate haemorrhage, H & E X400.
Discussion
The liver plays a pivotal role in the biological system that is responsible for the metabolism and clearance of drugs and xenobiotics, including ROS [21]. Liver has become the central organ for detoxification as the liver cells (hepatocytes), the main components that make up the organ, contain majority of enzymes that are responsible for drug metabolism of the entire body [22]. However, when the number of xenobiotics that is encountered has exceeded the maximum metabolic capability of the liver; damaging effect of the toxins may lead to various liver ailments. Overconsumption of alcohol had been associated to a spectrum of liver injuries with varying degree of severity, with some common pathologies including steatosis, foamy degeneration, steatonecrosis, venous lesion, and cirrhosis [23].
Generally, alcohol is metabolized in the liver as a process of detoxification. The metabolism of alcohol occurs mainly via alcohol dehydrogenases (ADH), which requires the cofactor NAD+. The reduced form of NAD+ (NADH) is attenuated when the alcohol concentration is in excess, and this could cause hepatic NADH accumulation [24]. As a result, more fatty acids and triglycerides would be synthesized whereas β-oxidation of fatty acids will be impeded [24]. Accumulation of ROS and polyunsaturated fatty acids would increase the oxidative stress and toxicity to the hepatic cells [25]. In this study, the increased level of AST, ALT and ALP, as well as elevated triglycerides and bilirubin concentration following 5 weeks of ethanol administration were indications for alcohol intoxication to the liver. Apart from the, serum total protein and albumin concentrations equally decreased significantly following alcohol administration.
Results of this study showed that MELH at doses of 250 and 500 mg/kg produced significant reduction in the activities of AST and ALT towards the respective normal range and this is an indication of stabilization of plasma membrane as well as repair of hepatic tissue damage caused by ethanol. On the other hand, suppression of elevated ALP activities with concurrent depletion of raised bilirubin level and an increase in the total plasma protein content suggests the stability of biliary dysfunction in rat liver during hepatic injuries with toxicants [26]. These results indicate that the extract preserved the structural integrity of the hepatocellular membrane and liver cell architecture damaged by ethanol which was confirmed by histopathological examination.
This study revealed a significant decrease in the final body weights of the rats administered ethanol alone compared to the distilled water- treated group (positive control). This could be as a result of an increased breakdown of body fat through lipolysis. However, the extract at a dose of 500 mg/kg produced a 16% weight gain which was comparable to that of the positive control (17.8%). This observation could be attributed to a possible reversal of lipolysis.
Results also revealed the safety (acute) profile of the extract as the toxicity study showed no mortality or signs of toxicity up to a dose of 5000 mg/kg of MELH. The oral LD50 of the extract was then taken to be > 5000 mg/kg. Thus, the extract is not likely to produce toxic effects when consumed acutely.
Conclusions
In this study, methanol leaf extract of Leptadenia hastata showed promising hepato-protection activity in rats with alcoholinduced liver damage. Thus, the extract could be useful in the management of liver diseases.
References
- Fernandez Checa JC (2003) Alcohol-induced liver disease: when fat and oxidative stress meet. Ann Hepatol 2(2): 69-75.
- Fernandez Checa JC, Kaplowitz N, Colell A, Garcı´a Ruiz C (1997) Oxidative stress and alcoholic liver disease. Alcohol Health Res World 21(4): 321-324.
- Ashak KG, Zimmerman HJ, Ray MB (1991) Alcoholic liver disease: Pathologic, pathogenic and clinical aspects. Alcohol Clin Exp Res 15(1): 45-66.
- Cederbaum AI (2001) Introduction serial review: alcohol, oxidative stress and cell injury. Free Radic Biol Med 31(12): 1524-1526.
- Cederbaum AI, Lu Y, Wu D (2009) Role of oxidative stress in alcoholinduced liver injury. Arch Toxicol 83(6): 519-548.
- Magdaleno F, Blajszczak CC, Nieto N (2017) Key Events Participating in the Pathogenesis of Alcoholic Liver Disease. Biomolecules 7(1): 9.
- Jiang JX, Torok NJ (2014) NADPH Oxidases in Chronic Liver Diseases. Adv Hepatol 7(4); 29-31.
- FM, Daly MJ (1999) Hepatic Disease, Clinical Pharmacy and Therapeutics, Churchill Livingstone, New York. pp: 195-212.
- Handa SS (1991) Plants as drugs. The Eastern Pharmacist 34: 79-85.
- Burkill HM (1985) The Useful plants of West Tropical Africa, 2nd Edition. Royal Botanic Gardens, Kew, UK 1: 960.
- Olivier Bover BEP (1986) Medicinal Plants in Tropical West Africa. 1st edition pp: 375.
- Aliero AA, Wara SH (2009) Validating the medicinal potential of Leptadenia hastata. African Journal of Pharmacy and Pharmacology 3(6): 335-338.
- Tamboura HH‚ Bayala B, Lompo M, Guissou IP, Sawadogo L (2005) Ecological distribution, morphological characteristics and acutetoxicity of aqueous extracts of Holarrhena floribunda (G Don) Durand & Schinz, Leptadenia hastata (Pers) Decne and Cassia sieberiana (dc) used by veterinary healers in Burkina Faso.
- Lorke D (1983) A new Approach to Practical Acute Toxicity Testing. Arch Toxicol 54(4): 275-287.
- Reitman S, Frankel S (1957) Method of alanine and aspartate aminotransferase determination. American Journal of Clinical Pathology 28: 56-58.
- Kind PRN, King FJ (1972) Alkaline phosphatase determination. Clinical Pathology 7: 322-360.
- Trinder P (1969) Enzymatic determination of triglycerides by GOP PAP method. Annals of Clinical Biochemistry 6: 24-27.
- Jendrassik L, Grof P (1938) Vere infachte photometric che method en zur bestm-mung des blut biluribins. Biochemische Zeitschrift. 297: 82-89.
- Doumas BT (1975) Standards for total serum protein assays-a collaboration study: Clin Chem 21(8): 1159-1166.
- Spencer K, Price CP (1971) Determination of serum albumin using bromoscresol techniques. Annals of Clinical Biochemistry 14: 105-115.
- Dancygier H, CP Strassburg (2010) Hepatic Drug Metabolism and Drug Toxicity, Springer, Berlin, Germany 2(1): 12.
- Monga SPS, U Apte, P Krishnamurthy (2007) Detoxification Functions of the Liver, Springer.
- Hall PM (2007) Alcoholic Liver Disease, Elsevier Health Sciences, Philadelphia, Pa, USA p. 6.
- Dancygier H, HK Seitz, S Mueller (2010) Alcoholic Liver Disease, Springer, Berlin, Germany 2(1): 10.
- Wu D, AI Cederbaum, (2003) Alcohol, oxidative stress, and free radical damage. Alcohol Res Health 27(4): 277-284.
- Mukherjee PK (2002) Quality control of herbal drugs, 1st edition, Business Horizons Pharmaceutical Publication, New Delhi pp. 531.
- Aliero BL, Umar MA, Suberu HA, Abubakar A (2001) A Handbook of Common Plant in North western Nigeria. Usmanu Danfodiyo University, Sokoto, Nigeria p. 78.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.