Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Association Between Timp-3/Angiotensin II, Profile and Cardiac Remodeling in Patients with Essential Hypertension and Heart Failure with Mid- Range Ejection Fraction
*Corresponding author: Asparuh Nikolov, Institute for scientific research, Medical University, Pleven, Bulgaria.
Received: July 02, 2019; Published: July 16, 2019
DOI: 10.34297/AJBSR.2019.04.000753
Abstract
Background and Aims: Arterial hypertension (AH) is a leading cause for heart failure with mid-range ejection fraction (HfmrEF). The aim of our study was to:
1. Measure levels of tissue inhibitor of matrix metalloprotease-3 (TIMP-3) and Angiotensin II (AII) in sera of patients with AH and HFmrEF.
2. In sera of controls.
Material and Methods: 56 patients with AH and HfmrEF were examined, mean age 65.62±9.69; and 22 age and sex matched healthy subjects, mean age 56.4±5.53. 41 of patients were with hypertension mediated organ damage and 15 were without. Patients were divided in two subgroupssubjects with left ventricular hypertrophy (n=32); (HFmrEF+LVH) and subjects without left ventricular hypertrophy (n=24); (HFmrEF-LVH). ELISA was used for measuring AII and TIMP-3.
Results: Patients with HFmrEF-LVH showed higher levels of TIMP-3: 7.747 (1.21916.725) than HFmrEF+LVH 4.693 (2.06210.463); (KW=0.48; p=0.48) and healthy controls 6.460 (1.00712.520); (p>0.05), but not significantly. TIMP3 showed correlation with grade of AH (r=0.85; p=0.02) and stage of AH (r=-0.52; p=0.05); and PLVW (r=-0.40; p=0.03). Patients with HFmrEF+LVH showed statistically significantly higher levels of AII: 8.533 (1.47713.009) than HFmrEF-LVH 1.333 (0.4776.932) and healthy controls 1.539 (0.2745.218); (KW=3.48; p=0.04). AII correlated with TIMP-3 (r=-0.50; p=0.0001), hypertensive cerebrovascular damage (r=0.57; p=0.0009), DBP (r=0.30; p=0.05), stage with AH (r=0.47; p=0.001); CK-MB (r=0.42; p=0.002) and UA (r=0.35; p=0.02).
Conclusion: Our data suggest an association between changes in levels of TIMP-3/Angiotensin II profile and cardiac remodeling. Determination of serum TIMP-3/Angiotensin II profile may be a useful method for monitoring of development and progression of LVH.
Abbreviations: BMI- Body Mass Index; DBP- Diastolic Blood Pressure; HDL- High Density; Lipoprotein Cholesterol; HFmrEF+LVH- Mid-Range Ejection Fraction and Left Ventricular Hypertrophy; HFmrEF-LVH- Mid-Range Ejection Fraction Without Left Ventricular Hypertrophy; LDL- Low Density Lipoprotein Cholesterol; SBP- Systolic Blood Pressure
Introduction
Traditionally cardiac extracellular matrix (ECM) is thought to be a relatively inactive structure, which takes the role as scaffolding for cardiac myocytes and vessels. Recent studies show evidence that cardiac ECM is metabolically active and dynamic structure. Changes in cardiac ECM turnover are suspected of contributing to the genesis and progression of heart failure [1]. Degradation of ECM’s structural proteins occurs through the action of matrix metalloproteinases (MMPs), which are regulated by tissue inhibitor of metalloproteinases (TIMPs). TIMP-3 is the only TIMP that is ECM-bound and could exert tissue-specific effects [2,3]. However, the exact role of TIMP3 in hypertension remains to be understood. The detection of subclinical left ventricular hypertrophy (LVH) is made difficult by several factors. “Firstly, the clinical disease processes do not cause signs and symptoms; consequently, LV remodeling may remain an unrecognized and insidious process for a prolonged period of time. Secondly, LVH is not easily detectable using standard clinical means such as a patient’s history, physical exam or electrocardiography (ECG). Specialized testing tools are usually needed for that subspecialty expertise to perform and interpret” [4].
There is growing evidence that supports the notion that angiotensin II (AII) may directly cause cardiovascular and renal diseases, independent of its hypertensive effect. Thus, recent in vivo work, coupled with in vitro findings, has provided new insights into the molecular and cellular mechanisms of AII-mediated cardiovascular and renal diseases [5]. Angiotensin II exerts direct effects on vascular remodeling and function [6]. TIMP-3 could be implicated in the progression of vascular diseases, independently from its ability to inhibit MMPs. In this regard, in vitro and in vivo adenoviral overexpression of TIMP-3, but not TIMP-1 or -2, has shown to promote apoptosis in vascular smooth muscle cells. In vivo experiments have shown that this effect was not achieved when synthetic MMP inhibitor was used [7,8]. Arterial hypertension (AH) is a leading cause for large number of heart failure (HF) cases. Heart failure with mid-range ejection fraction (HFmrEF) is a syndrome, defined by: (1)-Left ventricular ejection fraction (LVEF%)-40-49%; (2)-Symptoms and/or signs of heart failure; (3a)-Elevated levels of natriuretic peptides; (3b)-At least one additional criterion: relevant structural heart disease (left ventricular hypertrophy/left atrial enlargement) or diastolic dysfunction- 2016 European Society of Cardiology (ESC) Guidelines for the diagnosis and treatment of acute and chronic heart failure [9].
Methods
Clinical draft
All patients were residing in the vicinity of the Pleven University Hospital. Subjects’ sera were taken from October 2016 to May 2017. All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975, as revised in 2000. Approval of local Ethics Committee was obtained and informed consent from adult research participants was obtained too. The study group consisted of 56 patients with AH and HFmrEF, mean age 65.62±9.69. Forty-one of patients were with hypertension mediated organ damage (HMOD) and 15 were without. Patients were divided in two subgroups- subjects with mid-range ejection fraction and left ventricular hypertrophy (n=32), mean age 62.5±12.58 years (HFmrEF+LVH); subjects with mid-range ejection fraction without left ventricular hypertrophy (n=24), mean age 60.4±8.4 years (HFmrEF-LVH) (Table 1). These values were compared to serum TIMP-3/Angiotensin II profile in 22 age and sex matched controls with no family history of diabetes, atherosclerosis or hypertension, mean age 56.4±5.53 years.
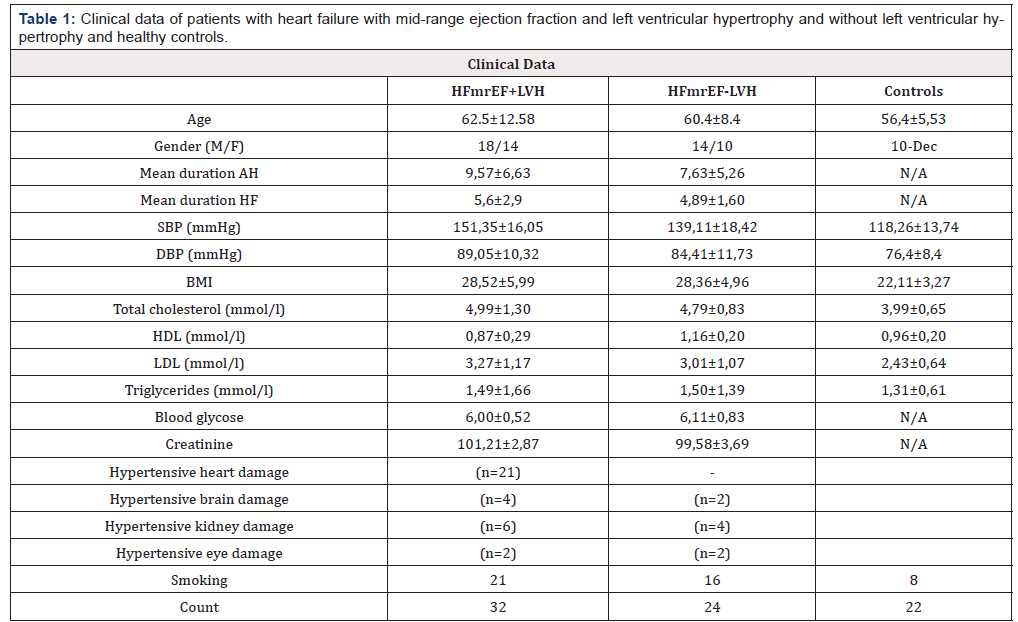
Table 1: Clinical data of patients with heart failure with mid-range ejection fraction and left ventricular hypertrophy and without left ventricular hypertrophy and healthy controls.
Note 1: Data are presented as mean±SD
Hypertension-Mediated Organ Damage Assessment: All patients were examined for HMOD via anamnesis (for established cardiovascular or premature CVD, renal, cerebrovascular or ophthalmological damage) and physical examination and pulse pressure assessment (in older people) >_60mmHg.
Instrumental tests
a. Arterial blood pressure was measured using a standard anearoid sphygmomanometer, to the nearest 2 mmHg, in the dominant arm after at least 10-min rest in supine position.
b. ECG was performed for LVH assessment (Sokolow–Lyon index >35 mm, or R in aVL >_11 mm; Cornell voltage duration product >2440mm.ms, or Cornell voltage >28mm in men or >20mm in women).
c. Echocardiography was performed with General Electric (Vivid S5) with 4-MHz transducer. All measurements were obtained according to ESC 2015 criteria for Cardiac Chamber Quantification by Echocardiography [10]. Echocardiographic LVH [LV mass index: men >50 g/m2.7; women >47 g/m2.7 (height in m2.7); indexation for BSA may be used in normalweight patients; LV mass/BSA g/m2 >115 (men) and >95 (women)].
Laboratory tests
1. Serum uric acid, glucose were determined. Total cholesterol, triglyceride concentrations, HDL were measured by enzyme assay (Boehringer Mannheim, Mannheim, Germany). LDL was calculated via Friedewald formula.
2. Serum creatinine levels were measured and moderate CKD with eGFR >30–59 mL/min/1.73 m2 (BSA) or severe CKD eGFR <30 mL/min/1.73 m2 were assessed
If any HMOD was found, then patient was further consulted with a referring specialist (cardiologist, nephrologist, neurologist, ophthalmologist).
Elisa
Enzyme-linked immunosorbent assay (ELISA) was used for measuring AII and TIMP-3 levels. TIMP-3 levels were measured in serum samples using enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, DY973) according to the manufacturer’s instructions. Angiotensin II levels were measured in serum samples using enzyme-linked immunosorbent assay ALPCO Diagnostics (Salem, MA, USA, 74-ANGHU-E01) according to the manufacturer’s instructions.
Statistical analyses
The research data was processed with the computer programs EXCEL (Microsoft Corporation, Redmond, WA) and STATGRAPHICS plus (Manugistics, Rockville, MD) for WINDOWS. All results were described in tables, graphs, numerical values (mean ± SD, share indicators and correlations). For assessment and conclusions in the case of normal distribution the Student t-test, Fisher’s F-test (ANOVA) and post-hoc tests (LSD, Tukey HSD, Scheffe, Bonferroni, Newman-Keuls, and Duncan) were used, and for distribution, different from the normal – the K-W (Kruskal-Wallis)-test. The level of significance was determined as p < 0.05. In cases with different from normal distribution, median was used (M), together with first and third quartile Q1 and Q3; (twenty-fifth and seventy-fifth percentile P25 and 75P).
Results
Serum TIMP-3 levels in patients were lower than controls 5.051 (2.06210.463) vs. 6.460 (1.00712.520) (p>0.05), but not significantly. Patients with HFmrEF-LVH showed higher levels of TIMP-3: 7.747 (1.21916.725) in comparison with HFmrEF+LVH- 4.693 (2.06210.463); (KW=0.48; p=0.48) and healthy controls 6.460 (1.00712.520) (p>0.05), but not significantly. TIMP3 showed correlation with grade of AH (r=0.85; p=0.02) and stage with AH (r=-0.52; p=0.05); and PLVW (r=-0.40; p=0.03). AII levels in patients were statistically significantly higher than controls- 8.952 (1.86915.782) vs. 1.539 (0.274 5.218); (KW=2.77; p=0.05). Patients with HFmrEF+LVH showed statistically significantly higher levels of AII- 8.533 (1.47713.009) than HFmrEF-LVH 1.333 (0.4776.932) and healthy controls 1.539 (0.274 5.218); (KW=3.48; p=0.04). AII showed correlation with TIMP-3 (r=- 0.50; p=0.0001), hypertensive cerebrovascular damage (r=0.57; p=0.0009), DBP (r=0.30; p=0.05), stage with AH (r=0.47; p=0.001); CK-MB (r=0.42; p=0.002) and UA (r=0.35; p=0.02).
Discussion
The conceptual role for AII, as a local mediator of fibrosis, is suggested by studies in which circulating AII is chronically increased from either endogenous or exogenous sources [11]. Mounting evidence points towards an authentic signalling capacity for TIMPs distinct from their MMP-inhibitory activity. It also seems to play an important role in the regulation of apoptosis, cell survival, growth, migration, differentiation, angiogenesis, inflammation and overall ECM remodelling. Due to these mechanisms TIMPs could play a vital role in the process of cardiac remodelling [12,13]. A suggestion about a link between TIMPs and the renin–angiotensin-system is firstly given by Kang [14]. They identified human angiotensin- II-type-2-receptor as a novel TIMP-3 interacting partner, linking TIMP-3 with the renin–angiotensin system. Although, the pathophysiological roles and signaling mechanisms of angiotensin-IItype- 2-receptor are still largely unknown, but they were shown to be increased during hypertrophy and ischemic heart disease. Our results show decreased TIMP-3 levels and increased AII in all patients. Interestingly, the TIMP-3/AII profile is different according to presence of LVH. This can be described by the next findings: TIMP-3 levels in patients with HFmrEF-LVH are statistically significantly higher than those in HFmrEF+LVH. On the contrary- AII levels in HFmrEF-LVH are statistically significantly lower than these in HFmrEF+LVH.
These results can be summarized with the next two ratios:
i. Profile of patients with HFmrEF-LVH- increased TIMP-3/ decreased AII
ii. Profile of patients with HFmrEF+LVH- decreased TIMP-3/ increased AII
Our results are consistent with authors [15] who found that recovery of TIMP-3 content in the failing myocardium is associated with reversing cardiac remodeling, highlighting the influence of this critical matrix constituent in maintaining normal cardiac structure and function. We report the existence of an association between changes in levels of TIMP-3/Angiotensin II profile and cardiac remodeling. Determination of serum TIMP-3/Angiotensin II profile may be a useful method for monitoring of development and progression of LVH in patients with essential hypertension and heart failure with mid-range ejection fraction. This is a pilot study. Although these findings should be confirmed in a larger study, our data suggest that changes in the TIMP-3/AII balance may play an important role in the structural, functional, and clinical manifestations of cardiac remodeling. However further examination and prospective studies are needed to clarify the MMP-independent biological functions of TIMP-3 and fully understand its effects and exact relevance on contributing to the cardiac remodeling.
Conflict of Interest
Authors declare no conflict of interest
References
- Chronic Heart Failure (2010) National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care: Partial Update. National Clinical Guideline Centre. NICE Clinical Guidelinesa No. 108 (E-book).
- Münzel T, Gori T, Keaney JF, Maack C, Daiber A (2015) Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. European Heart Journal 36(38): 2555-2564.
- Mizuno T, Yau TM, Weisel RD, Kiani CG, Li RK (2005) Elastin stabilizes an infarct and preserves ventricular function. Circulation 112(9): I81-188.
- Zile M, Desantis SM, Baicu CF, Stroud RE, Thompson SB et al. (2011) Plasma biomarkers that reflect determinants of matrix composition identify the presence of left ventricular hypertrophy and diastolic heart failure. Circ Heart Fail 4(3): 246-256.
- Flamant M, Placier S, Dubroca C, Esposito B, Lopes I, et al. (2007) Role of matrix metalloproteinases in early hypertensive vascular remodeling. Hypertension 50(1): 212-218.
- Brands MW, Banes-Berceli AK, Inscho EW, Al-Azawi H, Allen AJ, et al. (2010) Interleukin 6 knockout prevents angiotensin II hypertension: role of renal vasoconstriction and Janus kinase 2/signal transducer and activator of transcription 3 activation. Hypertension 56(5): 879-884.
- Baker AH, Zaltsman AB, George SJ, Newby AC (1998) Divergent effects of tissue inhibitor of metalloproteinase 1, -2, or -3 overexpression on rat vascular smooth muscle cell invasion, proliferation, and death in vitro. TIMP-3 promotes apoptosis. J Clin Invest 101: 1478-1487.
- George SJ, Lloyd CT, Angelini GD, Newby AC, Baker AH (2000) Inhibition of late vein graft neointima formation in human and porcine models by adenovirus-mediated overexpression of tissue inhibitor of metalloproteinase- 3. Circulation 101(3): 296-304.
- 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. European Heart Journal 37: 2129-2200.
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A (2015) Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16(3): 233–271.
- Weber KT (1997) Extracellular Matrix Remodeling in Heart Failure. A Role for De Novo Angiotensin II Generation. Circulation 96(11): 4065- 4082.
- Spinale FG (2007) Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev 87(4): 1285–1342
- Vanhoutte D, Schellings M, Pinto Y, Heymans S (2006) Relevance of matrix metalloproteinases and their inhibitors after myocardial infarction: a temporal and spatial window. Cardiovasc Res 69(3): 604-613.
- Kang KH, Park SY, Rho SB, Lee JH (2008) Tissue inhibitor of metalloproteinases- 3 interacts with angiotensin II type 2 receptor and additively inhibits angiogenesis. Cardiovascular Research 79(1): 150-160.
- Fedak P, Altamentova S, Weisel R, Nili N, Ohno N, et al. (2003) Matrix remodeling in experimental and human heart failure: a possible regulatory role for TIMP-3. Am J Physiol Heart Circ Physiol 284(2): 626-634.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.